Abstract
Densities of glycerol (1) + tert-butanol (2) mixtures were measured over the temperature range 293.15 to 348.15 K at atmospheric pressure, over the entire composition range, with a vibrating tube densimeter. Excess molar volumes, apparent and partial molar volumes of glycerol and tert-butanol, thermal isobaric expansivities of the mixture and partial molar expansivities of the components were calculated. The excess molar volumes of the mixtures are negative at all temperatures, and deviations from ideality increase with increasing temperature. Excess molar volumes were fitted to the Redlich–Kister equation. Partial molar volumes of glycerol decrease with increasing tert-butanol concentration. The temperature dependence of the partial molar volumes of glycerol is characterized by an inversion at x 2≈0.7. “Negative expansion” of the limiting partial volumes of glycerol was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Glycerol belongs to the class of solvents having three-dimensional networks of hydrogen bonds, like water and diols [1–14]. This network imparts to the liquid systems some specific properties such as small isothermal compressibility and isobaric thermal expansivity, relatively large free volume, and high viscosity among others. It is known that the H-bond network in water is responsible for hydrophobic effects observed in aqueous solutions of tert-butanol [15–23]. In this connection the study of the volume properties of glycerol–tert-butanol mixtures is of interest for the purpose of discovering hydrophobic effects in these media. Both the solvents are alcohols and have rather high melting points; however the intermolecular interactions in the pure solvents differ substantially.
Glycerol (Gly, 1,2,3-propanetriol) has the following physical properties: ε=41.14, μ=0.28 D, η=1.45 Pa⋅s at 293 K, T melting≈291.3 K, T boiling≈563 K (with decomposition) [24–26]. The molecule, Gly, has three hydrophilic alcoholic groups forming intra- and intermolecular H-bonds. The glycerol molecule is very flexible and it can form 126 conformers [4, 13, 14] as was shown by MD simulations. So in the crystal state, only the αα conformation forms [5], molecules of glassy glycerol have βγ conformation [6], whereas the data on quantitative composition of conformers in the liquid phase are still inconsistent [1–3, 7, 14]. The presence of only two, αα and αγ, conformers was confirmed experimentally; however the existence of others is also possible [2, 7, 14].
tert-Butanol (TBA, 2-methyl-2-propanol), ε=10.9, μ=1.66 D, η=3.316 mPa⋅s at 303 K, T melting≈298.6 K, T boiling≈355.3 K [24–26], has three hydrophobic –CH3 groups, able to undergo solvophobic effects within H-bonds networks, and one hydrophilic –OH group, which can form strong H-bonds both with Gly molecules and with other TBA molecules.
Information on the volume properties of Gly–TBA binary mixtures is absent in the literature, although the individual solvents have been extensively studied. In this connection, mixtures of these solvents over the wide range of state parameters are of interest. This work is a continuation of our previous investigations devoted to the study of volumetric properties of binary systems as functions of composition, temperature and pressure [27–37].
2 Experimental
Glycerol (stated purity 99.5 %) and tert-butanol (stated purity 99.3 %) were purified by double distillation according to references [26, 38] and were kept under vacuum. Water content was determined by the Karl Fisher method and did not exceed of 0.02 wt. % (or 4×10−5 mole fraction) for Gly and 0.015 wt. % (or 4×10−5 mole fraction) for TBA.
Mixtures were prepared gravimetrically from the degassed solvents without contact of the solution with atmospheric air. Freshly prepared solutions were used for the measurements, and the densimeter was filled by gravity feed from the sealed reservoir. For all stages of density measurements a drying column with calcined silica gel was used. Mixtures under consideration were stirred before measurements.
Densities (ρ) were determined using an Anton-Paar DMA 4500 vibrating tube densimeter. The densimeter was calibrated daily with twice-distilled water and dry air. Measurements were performed over the temperature range of 293.15–348.15 K. Densities were not measured in the whole composition range at 293.15 and 298.15 K due to freezing of the mixtures.
The uncertainties of mixture preparation and temperature measurements were less than 2×10−5 mole fraction and 0.01 K, respectively. Reproducibility and the total uncertainty of density measurements were 1×10−5 and 5×10−5 g⋅cm−3, respectively.
In Table 1 the comparison of glycerol and tert-butanol densities obtained in our work with literature data is presented. One can see satisfactory agreement between experimental and literature values. For glycerol the relative deviation of literature data of density changes from +0.6 % [39] to −0.09 % [40]. Our results are in accordance with values obtained by Ge et al. [41] (the deviation varies from +0.001 % to −0.005 % over all temperatures studied) and with values by Li et al. [40] (the deviation changes from −0.032 % to 0.091 %). The maximum relative deviation for tert-butanol, within the temperature range of 303.15–323.15 K, for which there is the largest number of experimental points, varies from +0.025 % to −0.09 %, and is −0.21 % at 348.15 K. Very good coincidence is observed for our data with ones by Kim and Marsh [22] (with temperature increasing the relative deviation decreases) and by Martınez et al. [42].
Experimental densities of glycerol + tert-butanol mixtures over the temperature range of 293.15–348.15 K at atmospheric pressure are summarized in Table 2.
3 Calculations and Results
The excess molar volume \(V_{m}^{\mathrm{E}}\) is determined by:

where V m is the mixture molar volume; \(V_{1}^{\mathrm{o}}\), x 1, and \(V_{2}^{\mathrm{o}}\), x 2 are the molar volumes of pure components and its corresponding mole fraction, respectively.
\(V_{m}^{\mathrm{E}}\) values were calculated directly from the experimental data using the following expression:

where M 1, ρ 1 and M 2, ρ 2 are masses weights and densities of glycerol and tert-butanol, respectively, and ρ is the mixture density. The uncertainty of determination of excess molar volumes was within ±0.04 cm3⋅mol−1.
Values of the excess molar volumes \(V_{m}^{\mathrm{E}}\) were fitted by the Redlich–Kister equation [43]:

The appropriate degree n of Eq. 3 was determined by the standard deviation, σ, calculated as:

where N is the total number of experimental points, and (n+1) is a number of coefficients (A i ) in Eq. 3. Calculated values of A i and σ at every temperature are reported in Table 3.
Apparent molar volumes of glycerol V ϕ1 and of tert-butanol V ϕ2 for each isotherm were evaluated from the experimental data with relations 5 and 6:


Partial molar volumes of components \(\bar{V}_{1}\) and \(\bar{V}_{2}\) were calculated as follows:


where w i is a mass fraction of component i in the mixture. To calculate the partial molar volumes, the (∂(1/ρ)/∂w i ) dependences were fitted with a fourth-order polynomial and differentiated.
Partial molar volumes also were calculated by differentiation of Eq. 3 on x 2 and combination of differentiation results with Eqs. 9 and 10:


This procedure results in the following equations for partial molar volumes:


The largest deviations of the partial molar volumes \(\bar{V}_{1}\) and \(\bar{V}_{2}\) calculated by Eqs. 7, 9 and 8, 10 do not exceed 0.8 % over the whole composition range.
Partial molar volumes at infinite dilution of glycerol in tert-butanol \(\bar{V}_{1}^{\infty}\) and tert-butanol in glycerol \(\bar{V}_{2}^{\infty} \) (Table 4) were obtained by linear extrapolation of the corresponding apparent molar volumes using Eqs. 13 and 14. For this purpose molar fractions, x i , were recalculated to appropriate molality values, m i . The extrapolation of V ϕ1 to m 1→0 leads to the desired quantity of \(V_{\phi 1}^{\infty} = \bar{V}_{1}^{\infty}\) and, accordingly, the extrapolation of V ϕ2 to m 2→0 gives \(V_{\phi 2}^{\infty} =\bar{V}_{2}^{\infty}\).


Extrapolation of the corresponding apparent molar volumes was done from the concentrations ranges of x 1=0.00392–0.03503 (7 points) and x 2=0.00284–0.10108 (10 points). The uncertainty of \(\bar{V}_{i}^{\infty}\) determination didn’t exceed ±0.05 cm3⋅mol−1.
In addition, limiting partial molar volumes of the mixture components were determined by Eqs. 11 and 12. At x 2=1 Eq. 11 can be written as:

and at x 2=0 Eq. 12 can be written in the form:

Limiting partial molar volumes were also calculated from the excess molar volumes using extrapolation of the “reduced volume” [44]. This expression was obtained by rearrangement of Eq. 14 and dividing it by x 1:

Linear extrapolation of the “reduced volume”, expressed as \(V_{m}^{\mathrm{E}} / x_{1}x_{2}\), to x 1→0 yields \(\bar{V}_{1}^{\infty}\), and extrapolation of the same value \(V_{m}^{\mathrm{E}} / x_{1}x_{2}\) to x 2→0 gives \(\bar{V}_{2}^{\infty}\). As one can see from Table 4, values of \(\bar{V}_{1}^{\infty}\) and \(\bar{V}_{2}^{\infty}\) calculated by Eqs. 13 and 14, 15 and 16, and 17 agree satisfactorily. The largest deviation of limiting partial molar volumes \(\bar{V}_{1}\) and \(\bar{V}_{2}\) calculated by the above equations is less that 0.2 %.
Thermal isobaric expansivities α (Table 5) were calculated as:

where values of (∂ρ/∂T) p,x were calculated from ρ=f(T) p,x fitted to a second-order polynomial followed by differentiation. To exclude the influence of changes in the number of points in the polynomial over the whole composition range (due to limited solubility of TBA in Gly at 293.15 K), the calculation of α over the temperature range of 298.15–348.5 K was carried out using the densities only at five temperatures for every composition, but for 293.15 K all six temperatures were taken into account. The uncertainty in the thermal isobaric expansivity calculations was estimated to be less than ±2×10−5 K−1.
Also, values of the molar isobaric expansion E p =(∂V/∂T) p,x of the mixture, partial molar expansion of glycerol \(\bar{E}_{p1} = ( \partial\bar{V}_{1} / \partial T )_{p,x}\), and tert-butanol \(\bar{E}_{p2} = ( \partial\bar{V}_{1} / \partial T)_{p,x}\), were calculated.

The partial molar expansion of glycerol \(\bar{E}_{p1}\) and tert-butanol \(\bar{E}_{p2}\) were obtained by fitting of partial molar volumes of the components by a second-order equation. Using \(\bar{V}_{i}^{\infty}\) values the \(\bar{E}_{pi}^{\infty}\) quantities were determined.
The combined uncertainty of partial molar expansion determination was about of ±5×10−3 cm3⋅mol−1 K−1.
4 Discussion
A solution volume reflects the equilibrium between various intermolecular interactions taking place. In liquid glycerol–tert-butanol mixtures the H-bond formation between –OH groups, mainly between glycerol molecules, plays a dominant role. Volume fluctuations first of all will depend on changes of both the energy and number of H-bonds. Mixture volumes will reflect the equilibrium of conformational states of all component molecules. In liquid glycerol there are several conformers present simultaneously [1–4, 13, 14], then the number and the ratio of Gly conformers will change depending on external physical conditions and molecules surrounding them in solution. In any case, Gly molecules are stabilized by optimal combination of intramolecular hydrogen bonds and intermolecular solvation of hydroxyl groups. When Gly molecules are solvated by molecules of a solvent unable to form H-bonds, all three –OH groups of Gly can only form intramolecular hydrogen bonds. It was established that in the condensed state Gly is highly associated due to intermolecular H-bonds and almost 95 % of molecules, on average, are bonded according to MD simulations [1, 2, 13]. These bonds are very stable and dissociation to monomers, dimers or trimers is negligible even at high temperature. In the vitrified state 100 % of Gly molecules form an integrated H-bonds network. High activation energy of conformational changes of Gly is the reason for slow formation of its crystal structure [1, 5, 6, 9, 12, 14], but under dilution by an another solvent the molecule association of Gly should become faster.
tert-Butanol is also able to form both strong intermolecular hydrogen bonds between –OH groups of neighboring molecules and weak C–H⋯O–H hydrogen bonds between oxygen atom of hydroxyl groups and hydrogen atoms of the methyl group. tert-Butanol can form, like many monoatomic alcohols, either zigzag H-bonded chains [45–50] or cyclic hexamers [51]. A characteristic feature of the TBA molecule is the presence of three –CH3 groups, which possibly determine the steric contribution to the formation of strong H–O⋯H–O hydrogen bonds in such chains.
Molar volumes of Gly–TBA mixtures increase with TBA concentration at all temperatures studied. Dependences of V m =f(x 2) are almost linear and do not display any extremes. With increasing temperature the ratio of molar volumes of the mixture components, V m(Gly)/V m(TBA), decreases, so it is equal to 0.765 and 0.735 at 308.15 and 348.15 K, accordingly.
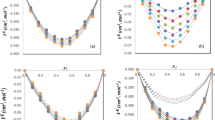
As it is shown in Fig. 1 the values of excess molar volume are negative, demonstrating more close packing in the mixture. Mixture formation is attended either by stronger intermolecular interaction between Gly and TBA molecules as compared with such interactions in pure solvents, or by formation of larger amount of such bonds. Increasing temperature leads to a lowering of steric barriers to additional formation of both hetero- and homo-molecular bonds. Thus absolute values of \(V_{m}^{\mathrm{E}}\) rise and the minimum at x 2≈0.5 becomes more pronounced.
Excess molar volumes \(V_{m}^{\mathrm{E}}\) versus x 2 for glycerol (1) + tert-butanol (2) mixture at different temperatures: (○) 298.15 K; (▲) 308.15 K; (▼) 323.15 K; (◀) 333.15 K; (▶) 348.15 K. Values of \(V_{m}^{\mathrm{E}}\) for 298.15 K were calculated by extrapolation of mixture density to x 2=1. Points are experimental data; lines are calculated by the Redlich–Kister equation
As is shown in Figs. 2(a) and 3(a), the apparent and partial molar volumes of Gly decrease at all temperatures with increasing TBA concentration in the mixture. This means that Gly in the mixture takes less volume than in the pure state. The dependences are characterized by a region of temperature inversion: Gly partial molar volume increases with temperature up to x 2≈0.7 with and decreases at higher TBA concentrations. Although increasing temperature increases the mobility of molecules in the mixture, tert-butanol addition reduces Gly association, i.e. it decreases the intermolecular hydrogen bonding and shifts the equilibrium to a more compact conformational glycerol state. It is furthered also by the lowering of the activation energy of Gly conformational changes. The type of \(\bar{V}_{1} = f(x_{2})\) function at 293.15 K (that is a little bit above the Gly melting point, i.e. at 293.15 K the unified H-bounded network in liquid glycerol is almost the same as in the crystalline state) probably resulting from a “microheterogeneous” state in the mixture. The shape of the \(\bar{V}_{1} = f(x_{2})\) dependence is also connected with changes in the tert-butanol structure, as the sharper decrease of the glycerol partial molar volume at x 2>0.7 occurs at temperatures above the TBA melting point. At 298.15 K and mixture compositions close to x 2=1, the V ϕ1 values are constant, apparently due to TBA freezing; i.e. because of switching the intermolecular interactions over to the TBA molecules themselves. With increasing temperature the average length of hydrogen bonded chains of TBA molecules shortens and its mobility increases.
As is shown in Figs. 2(b) and 3(b), the apparent and partial molar volumes of TBA increase both with temperature and with tert-butanol concentration. Thus decreasing the concentration of the hydrophilic glycerol leads to some changes of TBA packing in the mixture and to its increasing volume. Probably, this is the consequence of leveling of contributions from interactions between –OH groups of glycerol and tert-butanol to the total intermolecular interactions in the mixture.
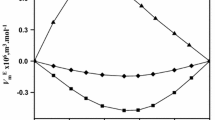
Partial molar volumes at infinite dilution \(\bar{V}_{i}^{\infty}\) are important allowing exclusion of the solute–solute contact interactions. However all changes of \(\bar{V}_{i}^{\infty}\) values are mainly determined by alterations of solvent packing due to a solute’s influence. As is seen in Fig. 4, limiting partial molar volumes of TBA, \(\bar{V}_{2}^{\infty}\), in glycerol increase with temperature like many organic solvents, and the limiting partial molar volume of Gly \(\bar{V}_{1}^{\infty}\) in TBA decreases; this means that glycerol undergoes “compression” with increasing temperature. Such phenomena as “negative expansion” was revealed earlier in various mixtures: carbamide–methanol [52, 53], water–ethanol [54], water–iso-propanol [19, 55], water–iso-butanol [56], water–tert-butanol [20, 23, 57, 58], water–tert-pentanol (up to ≈298 K) [59, 60], and ethylene glycol–tert-butanol [61]. Decreasing limiting partial molar volumes of mono-protic alcohols in water with temperature are observed over a confined temperature range. Such behavior probably indicates conformational rearrangement. When there is no strong external intermolecular hydrogen bond formed by the Gly molecule in TBA, as compared to the intermolecular bonds in pure Gly, then the intramolecular H-bond to Gly molecules becomes dominant and cause the molecule to fold.
Limiting partial molar volumes \(\bar{V}_{i}^{\infty}\) of glycerol and tert-butanol for glycerol (1) + tert-butanol (2) mixtures at different temperatures: (□) Gly surrounded by its own molecules; (■) Gly surrounded by TBA molecules; (○) TBA surrounded by its own molecules; (●) TBA surrounded by Gly molecules
Figure 5 demonstrates that the α=f(x 2) dependences are similar at all temperatures: isobaric thermal expansivities in the glycerol–tert-butanol system increase both with TBA concentration and temperature. Small α values and weak temperature dependence are typical for glycerol resulting from H-bonds network formation. Dependences of α=f(x 2) at 293.05 and 298.15 K attract attention. The distinctive kind of the dependence at 293.15 K is connected with the partial solubility of TBA in glycerol. The increase of α at x 2≈0.5, as was mentioned above, is probably caused by “microheterogeneity” of the liquid system and according to the dependence at 298.15 K this phenomena remains at this temperature also.
From Figs. 6 and 7(a) it is evident that the partial molar isobaric expansion of glycerol decreases in general with increasing molar fraction except at 293.15 K. At that temperature the \(\bar{E}_{p1}\) value changes slowly up to x 2≈0.7, and then the sign reverses and sharply decreasing values are observed. The concentration dependence of the partial molar isobaric expansion of glycerol (Fig. 7(a)) has a region of temperature inversion. The partial molar isobaric expansions of tert-butanol \(\bar{E}_{p2} \) (Figs. 6 and 7(b)) are positive and increase with increasing TBA concentration over the whole compositions range, except at 293.15 K.
In Fig. 8 one can see the temperature dependences of the limiting partial molar isobaric expansions of glycerol \(\bar{E}_{p1}^{\infty}\) in tert-butanol and in its pure state, where the value is numerically equal to \(E_{p1}^{\mathrm{o}}\), and of tert-butanol in glycerol \(\bar{E}_{p2}^{\infty}\) and in its pure state (numerically equal to \(E_{p2}^{\mathrm{o}}\)). The value of the limiting molar expansion \(\bar{E}_{pi}^{\infty}\) eliminates the influence of interactions between solute molecules. Values \(E_{p2}^{\mathrm{o}}\) and \(\bar{E}_{p2}^{\infty}\) are positive and increase with temperature, and \(\Delta\bar{E}_{p2}^{\infty}/ \Delta T\) is less than \(\Delta E_{p2}^{\mathrm{o}} / \Delta T\). This implies that increases of \(E_{p2}^{\mathrm{o}}\) and \(\bar{E}_{p2}^{\infty}\) of tert-butanol with temperature are mainly determined by changes in its molecules but not by intermolecular interactions. The values of molar isobaric expansion of glycerol \(E_{p1}^{\mathrm{o}}\) are positive and much lower compared to \(E_{p2}^{\mathrm{o}}\) of TBA, and increase weakly with temperature. The value of \(\bar{E}_{p1}^{\infty}\) for infinitely dilute Gly in TBA is negative and decreases further with increasing temperature. The different temperature dependences of glycerol’s \(E_{p1}^{\mathrm{o}}\) and \(\bar{E}_{p1}^{\infty}\) values show that the behavior of Gly is strongly affected by its surroundings. In the absence (or weakening) of intermolecular bonds appearently glycerol’s properties are determined by intramolecular bonds.
Temperature dependences of limiting partial molar isobaric expansions \(\bar{E}_{pi}^{\infty}\) of components in glycerol (1)–tert-butanol (2) mixtures: (□) Gly surrounded by its own molecules; (■) Gly surrounded by TBA molecules; (○) TBA surrounded by its own molecules; (●) TBA surrounded by Gly molecules
5 Conclusions
Volume properties of glycerol–tert-butanol liquid mixtures illuminate that under temperature and composition changes in the system, processes take place due to rearrangement of intermolecular H-bonds resulting in conformational changes.
The mixture formation is accompanied by closer packing. With increasing temperature the absolute values of excess molar volumes increase.
Glycerol partial molar volumes, in the mixtures with TBA, are smaller than its volume in the pure state.
With increasing temperature the limiting partial molar volume of glycerol decreases, i.e. for Gly in the mixture “negative expansion” is observed, indicating reorganization in the solvent. The temperature influence on volume properties of glycerol molecules vary with changes in their molecular environment.
References
Towey, J.J., Soper, A.K., Dougan, L.: The structure of glycerol in the liquid state: a neutron diffraction study. Phys. Chem. Chem. Phys. 13, 9397–9406 (2011)
Champeney, D.C., Joarder, R.N., Dore, J.C.: Structural studies of liquid D-glycerol by neutron-diffraction. Mol. Phys. 58, 337–347 (1986)
Chelli, R., Gervasio, F.L., Gellini, C., Procacci, P., Cardini, G., Schettino, V.: Density functional calculation of structural and vibrational properties of glycerol. J. Phys. Chem. A 104, 5351–5357 (2000)
Chelli, R., Procacci, P., Cardini, G., Della Valle, R.G., Califano, S.: Glycerol condensed phases Part I. A molecular dynamics study. Phys. Chem. Chem. Phys. 1, 871–877 (1999)
van Koningsveld, H.: The crystal structure of glycerol and its conformation. Recl. Trav. Chim. Pays-Bas. 87, 243–254 (1968)
Dawidowski, J., Bermejo, F.J., Fayos, R., Perea, R.F., Bennington, S.M., Criado, A.: Coherent neutron scattering response from glassy glycerol. Phys. Rev. E 53, 5079–5088 (1996)
Garawia, M., Dorea, J.C., Champeney, D.C.: Structural studies of liquid D-glycerol II. Molecular conformation and long range correlations. Mol. Phys. 62, 475–487 (1987)
Dashnau, J.L., Nucci, N.V., Sharp, K.A., Vanderkooi, J.M.: Hydrogen bonding and the cryoprotective properties glycerol/water mixtures. J. Phys. Chem. B 110, 13670–13677 (2006)
Jain, P., Levchenko, A., Yu, P., Sen, S.: Molecular dynamics in supercooled glycerol: results from 13C NMR spectroscopy. J. Chem. Phys. 130, 194506–194506-5 (2009)
Marcus, Y.: Some thermodynamic and structural aspects of mixtures of glycerol with water. Phys. Chem. Chem. Phys. 2, 4891–4896 (2000)
Westh, P., Rasmussen, E.L., Koga, Y.: Intermolecular Interactions in ternary glycerol–sample–H2O: towards understanding the Hofmeister series (V). J. Solution Chem. 40, 93–105 (2011)
Zelent, B., Nucci, N.V., Vanderkooi, J.M.: Liquid and ice water and glycerol/water glasses compared by infrared spectroscopy from 295 to 12 K. J. Phys. Chem. A 108, 11141–11150 (2004)
Callam, C.S., Singer, S.J., Lowary, T.L., Hadad, C.M.: Computational analysis of the potential energy surfaces of glycerol in the gas and aqueous phases: effects of level of theory, basis set, and solvation on strongly intramolecularly hydrogen-bonded systems. J. Am. Chem. Soc. 123, 11743–11754 (2001)
Chelli, R., Procacci, P., Cardini, G., Califano, S.: Glycerol condensed phases. Part II: a molecular dynamics study of the conformational structure and hydrogen bonding. Phys. Chem. Chem. Phys. 1, 879–885 (1999)
Perron, G., Desnoyers, J.E.: Heat capacities and volumes of interaction between mixtures of alcohols in water at 298.15 K. J. Chem. Thermodyn. 13, 1105–1121 (1981)
Alary, I.F., Simard, M.A., Dumont, J., Jolicoeur, C.: Simultaneous flow measurement of specific heats and thermal expansion coefficients of liquids: aqueous t-BuOH mixtures and neat alkanols and alkanediols at 25 °C. J. Solution Chem. 11, 755–776 (1982)
Tamura, K., Osaki, A., Koga, Y.: Compressibilities of aqueous tert-butanol in the water-rich region at 25 °C: partial molar fluctuations and mixing schemes. Phys. Chem. Chem. Phys. 1, 121–126 (1999)
Franks, F., Smith, H.T.: Precision densities of dilute aqueous solutions of the isomeric butanols. J. Chem. Eng. Data 13, 538–541 (1968)
Sakurai, M., Nakamura, K., Nitta, K.: Volumetric properties of dilute aqueous alcohol solutions at different temperatures. Bull. Chem. Soc. Jpn. 67, 1580–1587 (1994)
Sakurai, M.: Partial molar volumes in aqueous mixtures of nonelectrolytes. I. t-Butyl alcohol. Bull. Chem. Soc. Jpn. 60, 1–7 (1987)
Hvidt, A., Moss, R., Nielsen, G.: Volume properties of aqueous solutions of tert-butyl alcohol at temperatures between 5 and 25 °C. Acta Chem. Scand. 32, 274–280 (1978)
Kim, E.S., Marsh, K.N.: Excess volumes for 2-methyl-2-propanol–water at 5 K intervals from 303.15 to 323.15 K. J. Chem. Eng. Data 33, 288–292 (1988)
Egorov, G.I., Makarov, D.M.: Densities and volume properties of (water + tert-butanol) over the temperature range of (274.15 to 348.15) K at pressure of 0.1 MPa. J. Chem. Thermodyn. 43, 430–441 (2011)
Rabinovich, V.A., Havin, Z.Ya.: In: Potekhin, A.A., Efimov, A.I. (eds.) Short Chemical Handbook, 3th edn. Khimiya Press, Moscow (1991)
Lide, D.R. (ed.): Handbook of Chemistry and Physics, 82nd edn. CRC Press, New York (2001)
Weissberger, F., Proskauer, E.S., Riddik, J.A., Toops, E.E.: Organic Solvents. Physical Properties and Methods of Purification. Interscience, New York (1955)
Egorov, G.I., Syrbu, A.A., Kolker, A.M.: Volume properties of the H2O–DMF mixture at the pressure 0.101 MPa in the temperature range 278.15–323.15 K. Russ. J. Gen. Chem. 72, 693–696 (2002)
Egorov, G.I., Afanas’ev, V.N., Kolker, A.M.: VTx properties of the system water–2-propanol in the range 275.15–338.15 K. Russ. J. Gen. Chem. 74, 171–173 (2004)
Egorov, G.I., Makarov, D.M.: The bulk properties of ethylene glycol–dimethylsulfoxide mixtures over the temperature range 278–323 K at p=0.1 MPa. Russ. J. Phys. Chem. A 82, 1778–1784 (2008)
Egorov, G.I., Makarov, D.M.: The bulk properties of the water–dimethylsulfoxide system at 278–323.15 K and atmospheric pressure. Russ. J. Phys. Chem. A 83, 693–698 (2009)
Egorov, G.I., Makarov, D.M., Kolker, A.M.: Volumetric properties of the water–ethylene glycol mixtures in the temperature range 278–333.15 K at atmospheric pressure. Russ. J. Gen. Chem. 80, 1577–1585 (2010)
Egorov, G.I., Gruznov, E.L., Kolker, A.M.: p–V m –T–x properties of water–acetone mixtures over the temperature range 298–323 K and pressures from 1 to 1000 bar: isothermal compressibility, volume expansion coefficients and inner pressure of water–acetone mixtures. Russ. J. Phys. Chem. A 70, 197–204 (1996)
Egorov, G.I., Syrbu, A.A., Kolker, A.M.: The p–V m –x properties of water–acetamide mixtures at 298.15 K over the pressure range of 1–1000 bar. J. Phys. Chem. A 73, 1949–1951 (1999)
Egorov, G.I., Kolker, A.M.: Effect of pressure and temperature on volume properties of water–N,N-dimethylformamide mixtures. J. Mol. Liq. 106, 239–248 (2003)
Egorov, G.I., Makarov, D.M.: Compressibility coefficients of water–2-propanol mixtures over the temperature and pressure ranges 278–323.15 K and 1–1000 bar. Russ. J. Phys. Chem. A 82, 1037–1041 (2008)
Egorov, G.I., Makarov, D.M.: The compressibility of water–dimethyl sulfoxide mixtures over the temperature and pressure ranges 278–323.15 K and 1–1000 bar. Russ. J. Phys. Chem. A 83, 2058–2065 (2009)
Egorov, G.I., Makarov, D.M., Kolker, A.M.: Densities and volumetric properties of ethylene glycol + dimethylsulfoxide mixtures at temperatures of (278.15 to 323.15) K and pressures of (0.1 to 100) MPa. J. Chem. Eng. Data 55, 3481–3488 (2010)
Gordon, A.J., Ford, R.A.: The Chemist’s Companion. A Handbook of Practical Data, Techniques, and References. Wiley, New York (1972)
Xu, L., Hu, X., Lin, R.: Volumetric properties of glycerol with N,N-dimethylformamide and with water at 25 and 35 °C. J. Solution Chem. 32, 363–370 (2003)
Li, Q.-S., Su, M.-G., Wang, S.: Densities and excess molar volumes for binary glycerol + 1-propanol, + 2-propanol, + 1,2-propanediol, and + 1,3-propanediol mixtures at different temperatures. J. Chem. Eng. Data 52, 1141–1145 (2007)
Ge, M.-L., Ma, J.-L., Chu, B.: Densities and viscosities of propane-1,2,3-triol + ethane-1,2-diol at T=(298.15 to 338.15) K. J. Chem. Eng. Data 55, 2649–2651 (2010)
Martınez, S., Garriga, R., Perez, P., Gracia, M.: Densities and viscosities of binary mixtures of butanenitrile with butanol isomers at several temperatures. J. Chem. Eng. Data 45, 1182–1188 (2000)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Torres, R.B., Marchiore, A.C.M., Volpe, P.L.O.: Volumetric properties of binary mixtures of (water + organic solvents) at temperatures between T=288.15 K and T=303.15 K at p=0.1 MPa. J. Chem. Thermodyn. 38, 526–541 (2006)
Narten, A.H., Sandler, S.I.: X-ray diffraction study of liquid tertiary butyl alcohol at 26 °C. J. Chem. Phys. 71, 2069–2073 (1979)
Bowron, D.T., Finney, J.L., Soper, A.K.: The structure of pure tertiary butanol. Mol. Phys. 93, 531–543 (1998)
Kusalik, P.G., Lyubartsev, A.P., Bergman, D.L., Laaksonen, A.: Computer simulation study of tert-butyl alcohol. 2. Structure in aqueous solution. J. Phys. Chem. B 104, 9533–9539 (2000)
Fukasawa, T., Tominaga, Y., Wakisaka, A.: Molecular association in binary mixtures of tert-butyl alcohol–water and tetrahydrofuran–heavy water studied by mass spectrometry of clusters from liquid droplets. J. Phys. Chem. A 108, 59–63 (2004)
Wojtkow, D., Czarnecki, M.A.: Effect of temperature and concentration on the structure of tert-butyl alcohol/water mixtures: near-infrared spectroscopic study. J. Phys. Chem. A 109, 8218–8224 (2005)
Yoshida, K., Yamaguchi, T., Kovalenko, A., Hirata, F.: Structure of tert-butyl alcohol–water mixtures studied by the RISM theory. J. Phys. Chem. B 106, 5042–5049 (2002)
Nath, P.P., Sarkar, S., Krishna, P.S.R., Joarder, R.N.: Intermolecular structure of liquid D-tert-butanol by neutron-diffraction data. Appl. Phys. A 74, S348–S351 (2002)
Hamilton, D., Stokes, R.H.: Apparent molar volumes of urea in several solvents as functions of temperature and concentration. J. Solution Chem. 1, 213–221 (1972)
Abrosimov, V.K., Ivanov, E.V.: Water, structure, state and solvation, achievements of last years. In: Kutepov, A.M. (ed.) Water in Nonaqueous Solutions: State and Solvation, pp. 277–346. Nauka, Moscow (2003)
Nakajima, T., Komatsu, T., Nakagawa, T.: Apparent molal volumes and adiabatic compressibilities of n-alkanols and α,ω-alkane diols in dilute aqueous solutions at 5, 25, and 45 °C. I. Apparent molal volumes. Bull. Chem. Soc. Jpn. 48, 783–787 (1975)
Sakurai, M.: Partial molar volumes in aqueous mixtures of nonelectrolytes. II. Isopropyl alcohol. J. Solution Chem. 17, 267–276 (1988)
Franks, F., Smith, H.T.: Volumetric properties of alcohols in dilute aqueous solutions. Trans. Faraday Soc. 64, 2962–2972 (1968)
de Visser, C., Perron, G., Desnoyers, J.E.: The heat capacities, volumes, and expansibilities of tert-butyl alcohol–water mixtures from 6 to 65 °C. Can. J. Chem. 55, 856–862 (1977)
Hyncica, P., Hnedkovsky, L., Cibulka, I.: Partial molar volumes of organic solutes in water. XIII. Butanols (aq) at temperatures T=298 K to 573 K and at pressures up to 30 MPa. J. Chem. Thermodyn. 38, 418–426 (2006)
Sakurai, M., Nakagawa, T.: Densities of dilute solutions of water in n-alkanols at 278.15, 288.15, 298.15, 308.15, and 318.15 K. Partial molar volumes of water in n-alkanols. J. Chem. Thermodyn. 16, 171–174 (1984)
Sakurai, M.: Partial molar volumes in aqueous mixtures of nonelectrolytes. III. t-Pentyl alcohol. J. Solution Chem. 18, 37–44 (1989)
Egorov, G.I., Makarov, D.M.: Volumetric properties of the binary mixture of ethylene glycol + tert-butanol at T=(278.15,288.15,298.15,308.15,323.15,333.15,348.15) K under atmospheric pressure. J. Mol. Liq. (in press)
Riddick, J.A., Bunger, W.B., Sakano, T.K.: Organic Solvents: Physical Properties and Methods of Purification; Techniques of Chemistry. Wiley-Interscience, New York (1986)
Soujanya, J., Satyavathi, B., Vittal Prasad, T.E.: Experimental (vapour + liquid) equilibrium data of (methanol + water), (water + glycerol) and (methanol + glycerol) systems at atmospheric and sub-atmospheric pressures. J. Chem. Thermodyn. 42, 621–624 (2010)
Adamenko, I.I., Bulavin, L.A., Ilyin, V., Zelinsky, S.A., Moroz, K.O.: Anomalous behavior of glycerol–water solutions. J. Mol. Liq. 127, 90–92 (2006)
Sanz, M.T., Blanco, B., Beltran, S., Cabezas, J.L., Coca, J.: Vapor liquid equilibria of binary and ternary systems with water, 1,3-propanediol, and glycerol. J. Chem. Eng. Data 46, 635–639 (2001)
Verhoeye, L., Lauwers, E.: Vapor–liquid equilibrium of the system 2-propanol–water–1,2,3-propanetriol at 760 mm of Hg. J. Chem. Eng. Data 14, 306–309 (1969)
Sadek, H., Habez, A.M., Khalil, F.X.: Conductance of KIO3 in glycerol–water mixtures. Electrochim. Acta 14, 1089–1096 (1969)
Murthy, M.N., Subrahmahyan, S.V.: Behaviour of excess heat capacity of aqueous non-electrolytes. Indian J. Pure Appl. Phys. 15, 485–489 (1977)
Uosaki, Y., Kitaura, S., Moriyoshi, T.: Static relative permittivities of water + ethane-1,2-diol and water + propane-1,2,3-triol under pressures up to 300 MPa at 298.15 K. J. Chem. Eng. Data 51, 423–429 (2006)
Darbari, G.S., Singh, R.P., Verma, G.S., Rajagopalan, S.: Acoustic absorption in mixtures of glycerol and water below 1 MHz. II. Nuovo Cimento B 52, 1–17 (1967)
Nain, A.K.: Densities and volumetric properties of binary mixtures of aniline with 1-propanol, 2-propanol, 2-methyl-1-propanol, and 2-methyl-2-propanol at temperatures from 293.15 to 318.15 K. Int. J. Thermophys. 28, 1228–1244 (2007)
Anson, A., Garriga, R., Martinez, S., Perez, P., Gracia, M.: Densities and viscosities of binary mixtures of 1-chlorobutane with butanol isomers at several temperatures. J. Chem. Eng. Data 50, 677–682 (2005)
Kenttaemaa, J., Tommila, E., Martti, M.: Some thermodynamic properties of the system t-butanol + water. Ann. Acad. Sci. Fenn. Ser. A2 93, 1–20 (1959)
TRC Thermodynamic Tables. Non-Hydrocarbons. Thermodynamic Research Center, Texas A&M University, College Station, TX, d-5030 (1966)
Langa, E., Mainar, A.M., Pardo, J.I., Urieta, J.S.: Excess enthalpy, density, and speed of sound for the mixtures β-pinene + 2-methyl-1-propanol or 2-methyl-2-propanol at several temperatures. J. Chem. Eng. Data 52, 2182–2187 (2007)
Kubota, H., Tanaka, Y., Makita, T.: Volumetric behavior of pure alcohols and their water mixtures under high pressure. Inter. J. Thermophys. 8, 47–70 (1987)
Harris, K.R., Newitt, P.J., Back, P.J., Woolf, L.A.: Thermodynamic property measurements for 2-methyl-2-propanol + water from the freezing surface to 75 °C. High Temp., High Press. 30, 51–62 (1998)
Acknowledgement
This work was supported by the Russian Foundation for Basic Research (project 09-03-97501a).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egorov, G.I., Makarov, D.M. Volumetric Properties of Binary Mixtures of Glycerol + tert-Butanol over the Temperature Range 293.15 to 348.15 K at Atmospheric Pressure. J Solution Chem 41, 536–554 (2012). https://doi.org/10.1007/s10953-012-9813-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9813-8