Abstract
The presented work is a part of a research study on the volumetric, optical, and spectroscopic properties of binary mixtures containing glycerol with the four isomers of butanediol, namely: 1,2-butanediol or 1,3-butanediol or 1,4-butanediol or 2,3-butanediol. The density and refractive index measurements of pure components and their binary mixtures were carried out at atmospheric pressure and in a temperature range from 293.15 K to 318.15 K. The experimental data were then used to calculate for each system the following derived properties as a function of temperature and glycerol concentration: excess molar volumes, \(V^E\), partial molar volumes, \({\overline{V}}_i\), apparent molar volumes, \(V_{\phi i}\), partial molar volumes at infinite dilution, \({\overline{V}}_i^{\infty }\), excess partial molar volume at infinite dilution, \(\overline{V_i}^{E \infty }\), isobaric thermal expansions, \(\alpha\), excess thermal expansions, \(\alpha ^E\), and refractive index deviations, \(\Delta n _D\). Infrared spectroscopy analysis was also performed at atmospheric temperature and pressure. The experimental data obtained were fitted using the polynomial equation of Redlich-Kister. Excess molar volumes \(V^E\) for all the studied systems are negative over the entire composition range and at all the considered temperatures with deviations from ideality increasing with increasing temperature. The calculated molar excess properties were well correlated by the empirical Redlich-Kister polynomial. All measured and calculated properties reveal a significant influence of molecule structure, including the size, shape and position of the component hydroxyl groups. As intended, the infrared spectra of these binary mixtures display a high potential for hydrogen bonding. PC-SAFT EoS was successfully used to adjust the vapor pressure and liquid density of pure fluids, and was used predictively to correctly obtain the density of the mixture. Laplace’s rule was used to predict the refractive index of mixtures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The massive production of biodiesel to cover the growing need for energy while simultaneously reducing the impact of gas emissions generates relatively large volumes of the main by-product, glycerol [1, 2]. The challenge, therefore, is to provide the appropriate added value, making the entire biodiesel production chain more sustainable. For this purpose, glycerol has been the focus of much attention in recent years, and has a role in virtually every industry, either as a raw material or as an intermediate in reactions. Glycerol is often used as a lubricant, humectant and emulsifier in the cosmetics and pharmaceutical industries, as an additive and a stabilizer in the food industry, as a single user in alkyl resin and as a plasticizer in cellophane manufacture. One of glycerol’s oldest industrial uses is as a cryoprotective agent. It is also in great demand as a solvent in the chemical industry, such as its conversion into ethanol, 1,2-propanediol and 1,3-propanediol, 2,3-butanediol, n-butanol, dihydroxyacetone, succinic acid, polyols and numerous other compounds [3, 4]. All these technical applications require knowledge of the thermophysical properties of pure substances and mixtures. In thermodynamic engineering, these properties often complement phase equilibrium values. From a fundamental point of view, in addition to structural effects, they can also account for the energetic aspects governing liquid mixtures, i.e. the various molecular interactions. In addition, they are used as technical data in the design and optimization of technological processes, as well as in the resolution of numerous engineering problems [5,6,7]. These properties can also be exploited to develop new predictive models or improve existing ones. The purpose of the current paper is to provide reliable data on the density and refractive index of four binary mixtures including glycerol with 1,2-butanediol, 1,3-butanediol, 1,4-butanediol, and 2,3-butanediol, all of which are widely used in industry. Based on these experimental data, we calculated the following derived thermodynamic properties: excess molar volume \(V^E\), partial molar volume \({\overline{V}}_i\), apparent molar volume \(V_{\theta }\), thermal expansion coefficient \(\alpha\), excess thermal expansion coefficient \(\alpha ^E\), excess partial molar volume at infinite dilution \({\overline{V}}_i^{\infty }\), excess partial molar volume at infinite dilution \(\overline{V_i}^{E \infty }\), refractive index variation \(\Delta n _D\). Excess properties were correlated using the empirical Redlich-Kister equation for all binary mixtures. Detailed analysis of these excess properties revealed the likelihood of molecular interactions in the various mixtures. To this end, an infrared mode study (FT-IR) of all the binary mixtures was carried out. The results suggest the presence of intermolecular hydrogen-bonding interactions between the various hydroxyl groups in the alcohol-rich mixtures, through displacement and broadening of the (–OH) vibrational band.
Finally, in this study, PC-SAFT EoS was used to calculate the density for pure fluids and binary mixtures. We have used Laplace’s mixing rule + PC-SAFT EoS to predict the refractive index.
2 Experimental Section
2.1 Materials
The samples were supplied by Merck and were of analytical grade, the details about their purity are given in Table 1. The purity of the chemicals was confirmed using perkin elmer gas chromatography clarus 500 with TCD detector (Oven temperature 100–190 at 4 °C min−1; injector temperature: 220 °C; detector temperature: 230 °C) and an Agilent J &W SE-30 column of 30 m length, 0.53 mm inner diameter, and 0.5 \(\mu\)m thickness is used in the separation. The chromatogram for individual species showed a single peak signifying no impurity and hence they were used directly without any purification. Enantiomeric composition analyses of the 2,3-butanediol (mixture of meso-D- and L form) were performed using the established GC-FID analysis method [8]: GC-FID, column temperature: 95 °C–130 °C (1.0 °C min−1), injector temperature of 145 °C. Retention times (min): (+)-2 S,3 S-butanediol, 7.72; (−)-2R,3R-butanediol, 8.06; meso-butanediol, 8.53. All the analysis were repeated three times and the results are arithmetic mean values. All liquids were stored over molecular sieves (5 \(\mathring{A}\)) in dark-colored bottles to avoid water absorption. The purity of the chemicals was also checked by comparing their densities \(\rho\), and refractive indices \(n_D\) at the operating temperature with found literature values [9,10,11,12,13] as shown in Table 2. The measured densities and refractive indices are in good agreement with the reported literature values, with an average absolute percentage deviation (AAPD) of 0.017 % and 0.42 % respectively. The AAPD was obtained from Eq. 1:
where N is the number of experimental data. Exp. and Lit. are related to experimental value and literature value, respectively, and \(\theta = \rho , n_D\).
2.2 Apparatus and Procedure
Electronic densimeter Anton Paar DMA 5000 was deployed to measure densities (\(\rho\)) at several different temperatures to an accuracy of \(5 \cdot 10^{-6}\) g\(\cdot\)cm\(^{-3}\). It consists of a borosilicate glass U-tube, 2 mm in diameter and 0.2 mm thick, electronically oscillated at its characteristic frequency. The measurement of the oscillation period of the vibrating tube containing the sample is modified by the mass of the substance introduced, allowing the density of the sample to be determined at the measurement temperature maintained constant to within ± 0.01 K. The densimeter is calibrated periodically using ultrapure water and dry air as reference fluids [14]. The relative standard uncertainty in the density is estimated to be \(0.003\,\%\). Refractive index, \(n_D\), were measured with the Abbe-type refractometer Atago Nar-1t Liquid including two Amici compensating prisms, oriented to rotate in opposite directions. It comprises a built-in analog thermometer to display the temperature with an accuracy of ± 0.1 K, it is controlled by a Wise Circu thermostatic bath which is connected to the refractometer by means of a pump circulation. The refractometer is regularly calibrated using commercially available certified standard solutions. The estimated uncertainty of the refractive index is \(0.4\,\%\). Measurements were replicated at minimum three times and the results obtained were averaged with a repeatability of 0.1 %. IR spectra were collected via a Perkin Elmer spectrophotometer (model FT-IR two UATR two) at ambient temperature. Mixtures were produced by gravimetric weighing at standard ambient pressure, using an analytical balance (Ohaus, OHAUS00240) to an accuracy of 0.1 mg. The mass fraction standard uncertainty is 0.002. The following propagation error formula was used to evaluate the standard uncertainties for densities and their derivative physical properties [15]:
The corresponding uncertainty for excess molar volume was calculated using Eq. 3:
where \(x_1\), \(d_1\) and \(d_2\) denote the mole fractions, and densities of glycerol (1) and butanediol isomer (2), respectively, and d is the density of the mixtures.
3 Theoretical Models
3.1 Modeling for the Density
In this work, the perturbed chain of the statistical associating fluid theory equation of state (PC-SAFT EoS) [16, 17] was used for modeling the density of pure fluids and mixtures. This model has been widely described and used in the literature [18,19,20]. This equation of state requires five fitted parameters for each pure fluid, which are the following: the number of segments in a chain (m), the segment diameter (\(\sigma\)), the depth of the potential (\(\varepsilon / k_B\)), the association energy (\(\varepsilon ^{AB}/k_B\)), and the association volume (\(\kappa ^{AB}\)). In the above parameters, \(k_B\) is the Boltzmann constant.
Due to the presence of three OH groups in glycerol and two OH groups in the butanediol isomers, it is necessary to consider some association scheme. In this manuscript, we have considered one site on each oxygen atom and one site on each hydrogen atom. Therefore, 6 sites have been used on each glycerol molecule, i.e., 3 positive sites and 3 negatives sites, and 4 sites on each molecule for butanediol isomers, i.e., 2 positive sites and 2 negative sites. According to this approach, hydrogen bonding is considered between molecules of the same type and between different molecules, that is, self-association and cross-association between molecules.
3.2 Modeling for the Refractive Index
According to the literature, the refractive index for the binary mixture (\(n_D\)) can be obtained from Laplace mixing rule [21], using Eq. 4:
where \(z_1\) and \(z_2\) are related to the weight fraction of glycerol and butanediol isomer, respectively. This mixing rule has been used successfully in our recent publications [22, 23]. The liquid density present in Eq. 4 was obtained using PC-SAFT EoS. Therefore, this theoretical approach was named Laplace mixing rule + PC-SAFT EoS.
4 Results and Discussions
4.1 Volumetric Properties
The densities, \(\rho\), and the derivative properties: excess molar volumes, \(V^E\), partial molar volumes, \({\overline{V}}_i\), apparent molar volumes, \(V_{\phi i}\), isobaric thermal expansion coefficient, \(\alpha\), and excess expansion coefficient, \(\alpha ^E\) for the glycerol (1) + butanediol isomer (2) binary mixtures at various temperatures (293.15–318.15) K and atmospheric pressure (101325 Pa) over the whole composition range, expressed as the mole fraction, \(x_1\), of glycerol, are listed in Table 3 and are illustrated in Fig. 1a–d. It can be seen that the \(V^E\) values of all mixtures are negative for all compositions and considered temperatures, and the \(V^E = f(x_1)\) curves are asymmetric parabola whose extremums correspond to \(V^ E_{min} = (-0.307, -0.315, -0.323, -0.331, -0.340, -0.350)\) cm\(^3\) \(\cdot\)mol\(^{-1}\) at \(x1= 0.5027\) for the mixture with 1,2-butanediol, \(V^ E_{min}= (-0.026, -0.039, -0.048, -0.057, -0.065, -0.071)\) cm\(^3\) \(\cdot\)mol\(^{-1}\) at \(x_1= 0.5027\) for the mixture with 1,3-butanediol, \(V^E _{min} = (-0.080, -0.079, -0.077, -0.076, -0.074, -0.072)\) cm\(^3\) \(\cdot\)mol\(^{-1}\) at \(x_1= 0.5985\) for the mixture with 1,4-butanediol and \(V^E _{min} = (-0.556, -0.571, -0.585, -0.611, -0.635, -0.658)\) cm\(^3\) \(\cdot\)mol\(^{-1}\) at \(x_1= 0.5999\) for the mixture with 2,3-butanediol isomer at temperatures \(T= (293.15, 298.15, 303.15, 308.15, 313.15, 318.15)\) K respectively for all the mixtures. This compression of the volumes of the binary mixtures (\(V^E < 0\)) could signify the formation of stronger intermolecular hydrogen bonds between glycerol and the butanediol isomers, or the existence of a greater number of this type of interaction. We also note that the absolute values of \(\vert V^E \vert\) increase with increasing temperature except for the mixture with 1,4-butanediol.
Excess molar volumes of glycerol (1) + butanediol isomer (2) mixtures at T = [298.15–318.15] K and atmospheric pressure: (a) glycerol (1) + 1,2-butanediol (2) mixture, (b) glycerol (1) + 1,3-butanediol (2) mixture, (c) glycerol (1) + 1,4-butanediol (2) mixture, (d) glycerol (1) + 2,3-butanediol (2) mixture. Circles: experimental data obtained in this work. Colors: (orange) 293.15 K, (sky blue) 298.15 K, (purple) 303.15 K, (green) 308.15 K, (red) 313.15, (blue) 318.15 K. Continuous line: represent the results obtained with RK polynomial. Segmented line: represent the results obtained by Alavianmehr et al. [25] (Color figure online)
The excess molar volumes for the mixtures were calculated from the density data of the compositions and pure compounds using the Eq. 5:
where \(x_1\) and \(x_2\), \(M_1\) and \(M_2\), and \(\rho _1\) and \(\rho _2\) denote the mole fractions, molar masses, and densities of glycerol (1) and butanediol isomer (2), respectively. \(\rho\), is the density of the mixtures. The experimental \(V^E\) values were correlated by least squares polynomial regression to the Redlich-Kister equation [24]:
The empirical parameters \(A_i\) in Eq. 6 are reported in Table S1 along with the standard deviations, \(\sigma\), expressed as Eq. 7:
where N is the number of measurements and n the number of fitted parameters \(A_i\). Figure 1 shows the experimental values of excess molar volume, \(V^E\), as a function of glycerol mole fraction, \(x_1\), along with adjusted curves calculated using Eq. 6, at chosen temperature range from (293.15 to 318.15) K.
The same trend was observed in the results previously published by Alavianmehr et al. [25] concerning the binary mixture glycerol + 2,3-butanediol for the densities, \(\rho\) and the excess molar volumes, \(V^E\), at the five temperatures: 298.15 K, 303.15 K, 308.15 K, 313.15 K, and 318.15 K. The estimated average absolute percentage deviations (AAPD) for the densities equals 0.68 %. However, for the excess molar volumes, \(V^E\), \(AAPD = 45\,\%\). The difference in the enantiomeric composition of 2,3-butanediol could potentially be the source of the deviations between our results and those reported in the literature [25]. However, no such enantiomeric analysis is provided in this reference to further investigate this aspect (Fig. 1).
The partial molar volumes, \({\overline{V}}_1\) and \({\overline{V}}_2\), for glycerol (1) and butanediol isomer (2), respectively, were investigated over the entire composition range by means of the Eqs. 8, 9:
where \(V_1^0\) and \(V_2^0\) are the molar volumes of pure liquid components 1 and 2, respectively. Differentiation of \(V^E\) from Eq. 7 with respect to \(x_1\) and substitution of the results in Eqs. 8, 9 gives rise to the following Eqs. 10, 11 for the partial molar volumes \({\overline{V}}_1\) and \({\overline{V}}_2\):
Assigning \(x_1 = 0\) in Eq. 10 and \(x_2 = 0\) in Eq. 11 generates the Eqs. 12, 13, for the partial molar volume of glycerol at infinite dilution, \({\overline{V}}_1 ^{\infty }\), in the butanol and the partial molar volume of the butanol at infinite dilution, \({\overline{V}}_2 ^{\infty }\), in glycerol:
Reordering of Eqs. 12, 13 leads to Eqs. 14, 15, for the partial excess molar volume of glycerol at infinite dilution, \({\overline{V}}_1^{E \infty }\), in the butanol, and the partial molar volume of the butanol at infinite dilution, \({\overline{V}}_2^{E \infty }\), in glycerol, respectively,
Partial molar volumes, \({\overline{V}}_i\), are listed in Table 3, partial molar volumes at infinite dilution, \({\overline{V}}_i ^{\infty }\), and partial molar excess volumes at infinite dilution, \({\overline{V}}_i ^{E \infty }\), were determined at some temperatures ranging from (298.15 to 318.15) K, using the empirical parameters \(A_i\) given in Table S1. The partial properties at infinite dilution are of considerable attention since the solute-solute interactions disappear in this region and the only interactions remaining are solute-solvent [26]. Thus, the values of partial molar volumes at infinite dilution provide insight into solute-solvent interactions. The partial molar volumes at infinite dilution and partial molar excess volumes at infinite dilution are listed in Table 4.
The apparent molar volumes of glycerol in butanol, \(V_{\phi 1}\), and the apparent molar volume of butanol in glycerol, \(V_{\phi 2}\), are given by the following equations:
where the molar volume of the binary system, V, is expressed by:
Combining the Eqs. 16, 17, 18 mains to Eqs. 19, 20, for the apparent molar volumes \(V_{\phi 1}\) and \(V_{\phi 2}\):
The apparent molar volumes, \(V_{\phi i}\), are summarized in Table 3. The apparent and partial molar volumes of glycerol increase at all temperatures as the composition of glycerol in the mixture increases, in contrast to the apparent and partial molar volumes of butanediols which tend to decrease with the composition of glycerol and the rise of temperature. This means that the butanediols take up less volume in the mixture than in the pure component. The behavior of the partial molar volumes and the apparent molar volumes shows a reduction in the total volume of the compositions compared with simple linear additions. The decrease of the volume could be assigned to the strong hydrogen bonding that occurring from the dipole-dipole interactions between the butanediols and glycerol [27].
Thermal expansivities, \(\alpha\), were calculated from the density and \(V^E\) results, to allow better understanding of change in the solution structure during blending. Thermal expansion coefficients for pure components and compositions, respectively are expressed by Eqs. 21, 22:
Excess thermal expansibilities, \(\alpha ^E\), were calculated using Eq. 23:
where \(\Phi _i\) is the volume fraction of the pure component i, defined as Eq. 24:
The values of the thermal expansibilities \(\alpha\), and those of excess thermal expansivity, \(\alpha ^E\), at various temperatures, are reported in Table 3. The coefficients of thermal expansion \(\alpha\) for all the binary systems increase both with increasing \(x_1\) concentration and temperature. Whereas the values of excess thermal expansion coefficients \(\alpha ^E\) are negative for the three mixtures of glycerol with 1,2-butanediol, with 1,3-butanediol and with 2,3-butanediol but positive in the case of the mixture with isomer 1,4-butanediol.
4.2 Optical properties
Values of refractive indices, \(n_D\), and the refractive indices deviations at all the temperatures are given in Table 5 along with glycerol mole fraction, \(x_1\). Figure 2a–d shows that the \(\Delta n_D\) values are positive for all compositions and at all studied temperatures for all the mixtures where the magnitude of \(\Delta n_D\) increase with increasing temperature except for the mixture with 1,3-butanediol where negative values are noted and the magnitude decreases as temperature increases. The negative values of the excess refractive indices indicate the presence of specific molecular interactions between the components of the mixture, leading to changes in the polarization of light. This is in contrast to what might be expected on the basis of the refractive indices of the individual components of the mixture alone. It is also possible that the nanoscale structure of the mixture is complex, resulting in local variations in density affecting the behavior of light. Negative excess refractive indices are an interesting phenomenon that can be studied in detail using advanced experimental techniques and complex theoretical models. The curves \(\Delta n_D =f(x_1)\) are slightly asymmetrical with maximums estimated to \(\Delta n_{D,max} \cdot 10^{-2} = (0.183, 0.205, 0.223, 0.269, 0.319, 0.358)\) at \(x_1= 0.4063\) for the mixture with 1,2-butanediol, \(\Delta n_{D,max} \cdot 10^{-2} = (0.307, 0.347, 0.402, 0.452, 0.492, 0.522)\) at \(x_1= 0.3994\) for the mixture with 1,4-butanediol, \(\Delta n_{D,max} \cdot 10^{-2} = (0.153, 0.191, 0.216, 0.241, 0.266, 0.291)\) at \(x_1= 0.5022\) for the mixture with 2,3-butanediol and with minimums situated at \(x_1= 0.5002\) as \(\Delta n_{D,min} \cdot 10^{-2} = (-0.338, -0.363, -0.401, -0.425, -0.450, -0.476)\) for the mixture with 1,3-butanediol.
Refractive index deviations vs mole fractions of glycerol for the glycerol (1) + butanediol isomer (2) mixtures at T = [298.15–318.15] K and atmospheric pressure: (a) glycerol (1) + 1,2-butanediol (2) mixture, (b) glycerol (1) + 1,3-butanediol (2) mixture, (c) glycerol (1) + 1,4-butanediol (2) mixture, (d) glycerol (1) + 2,3-butanediol (2) mixture. Circles: experimental data obtained in this work. Colors: (orange) 293.15 K, (sky blue) 298.15 K, (purple) 303.15 K, (green) 308.15 K, (red) 313.15, (blue) 318.15 K. Lines: represent the results obtained with RK polynomial (Color figure online)
4.3 Spectroscopic Properties
The spectroscopic IR has been used for the analysis of molecular interactions and the identification of the bonds present. The infrared spectra of each mixture have been recorded at room temperature at three glycerol compositions (\(x1=0.25\) or 0.5 and 0.75). Infrared spectra are presented in transmittance (fraction of the transmitted intensity with respect to the incident intensity) expressed in percentage and the abscissa axis as a function of the wave number (inverse of the wavelength), on axis directed to the left.
As shown by Fig. S1A–D, the IR spectra are relatively similar in the four mixtures for the same mole fraction of glycerol. The analysis reveals a (O–H) elongation band for pure 1,2-butanediol at (3326 cm\(^{-1}\)); for pure 1,3-butanediol at (3302 cm\(^{-1}\)), for pure 1,4-butanediol at (3294 cm\(^{-1}\)) and at (3348 cm\(^{-1}\)) for pure 2,3-butanediol typical of intermolecular OH functions. Increasing the concentration of glycerol in the mixture leads to a shift of this band towards lower wavenumbers, reaching (3280 cm\(^{-1}\)) for pure glycerol. This shift could highlight the predominance of intermolecular interactions between 1,2-butanediol and glycerol in the dialcohol-rich region. Peak intensity increases with increasing glycerol composition.
4.4 Effect of Isomeric Position
The variation of volumetric properties of binary systems containing glycerol with butanediol isomers at \(T= 298.15\) K follows the sequence: (Glycerol + 2,3-Butanediol) > (Glycerol + 1,2-Butanediol) > (Glycerol + 1,4-Butanediol) > (Glycerol + 1,3-Butanediol). However, an opposite sequence is observed for the absolute values of the refractive indices, which leads us to consider the impact of positional isomers: the closer the hydroxyl groups in the butanediol isomer, the stronger the intermolecular interactions with glycerol.
4.5 Modeling of Density and Refractive Index
In this study, we have used the experimental density data (obtained in this work) and the vapor pressure data from DIPPR [28] to calculate the fitted parameters of PC-SAFT EoS in the temperature range of 293.15 K to 318.15 K. The absolute average deviation for property X and the objetive function to obtain the fitted parameters required in PC-SAFT EoS are given Eqs. 25 and 26, respectively:
where P is the vapor pressure for pure fluid, \(X = \rho , n_D\), and Theo. is related to the theoretical value. The fitted parameters are published in Table 6. According to Table 6, PC-SAFT EoS correctly fit the compressed liquid density and the vapor pressure. The overall deviations are 0.06 % and 0.08 % for vapor pressure and liquid density, respectively.
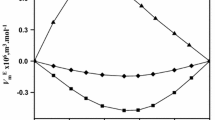
On the other hand, in this work, we have used a predictive approach to calculate the density and refractive index of binary mixtures, i.e., using PC-SAFT EoS and Laplace mixing rule + PC-SAFT EoS, respectively. It is a great advantage of not requiring fitted parameters in binary mixtures. According to the Table 7, the overall deviation was 0.28 % and 0.15 % for density and refractive index. We have also used PC-SAFT to predict the experimental density data for glycerol + 2,3-butanediol mixture obtained by Alavianmehr et al [25]; the deviation was 0.80 %. Figures 3 and 4 show the behavior of the liquid density and refractive index for glycerol + 2,3-butanediol at different temperatures, respectively. According to Fig. 3, the liquid density obtained by us and Alavianmehr et al [25] is well predicted by PC-SAFT EoS. From the Fig. 4, it is observed that PC-SAFT EoS + Laplace mixing rule underpredicts the refractive index for a part of the glycerol mole fraction range and depending on temperature. Fig. 5 shows a graph of deviations of the refractive index for the glycerol + 2,3-butanediol mixture at different compositions and temperatures. The deviations are between approximately 0 and 0.2 %. Furthermore, it is consistent that in mole fractions 0 and 1, the deviations are 0 %, because PC-SAFT + Laplace mixing rule is not capable of predicting the refractive index of pure fluids.
Liquid densities for glycerol (1) + 2,3-butanediol (2) mixture at different temperatures. Circles: experimental data obtained in this work. Diamond: experimental data obtained from Alavianmehr et al [25]. Colors: (orange) 293.15 K, (sky blue) 298.15 K, (purple) 303.15 K, (green) 308.15 K, (red) 313.15, (blue) 318.15 K. Lines: represent the results obtained with PC-SAFT EoS (Color figure online)
Refractive indices for glycerol (1) + 2,3-butanediol (2) mixture at different temperatures. Circles: experimental data obtained in this work. Colors: (orange) 293.15 K, (sky blue) 298.15 K, (purple) 303.15 K, (green) 308.15 K, (red) 313.15, (blue) 318.15 K. Lines: represent the results obtained with PC-SAFT EoS + Laplace mixing rule (Color figure online)
Percentage deviation for refractive index at each composition and temperature for glycerol (1) + 2,3-butanediol (2) mixture at different temperatures. Circles: values of the percentage deviations. Colors: (orange) 293.15 K, (sky blue) 298.15 K, (purple) 303.15 K, (green) 308.15 K, (red) 313.15, (blue) 318.15 K (Color figure online)
5 Conclusions
In this work, some thermophysical properties (density and refractive index) covered the whole composition range of glycerol + butanediol isomer have been measured at several temperatures between 293.15 K to 318.15 K and atmospheric pressure. Some derivatives have been calculated such as excess molar volumes, partial molar volumes, apparent molar volumes, thermal isobaric expansibilities, excess thermal expansibilities, partial molar volumes at infinite dilution. The results suggest the presence of strong interactions between unlike molecules across intermolecular hydrogen bonding confirmed by spectroscopic infrared measurements. The thermophysical properties of the studied mixtures show that changes in temperature, in composition in structure and in the position of hydroxyl groups have a significant influence on the molecular environment. Liquid density of binary mixtures was correctly predicted with PC-SAFT EoS (overall deviation = 0.28 %). Furthermore, the deviation in refractive index obtained with Laplace’s rule is low (overall deviation = 0.15%).
Data Availability
All data generated or analyzed during this study are included in this article and its supplementary information files.
References
P.C. Shukla, G. Belgiorno, G. Di Blasio, A.K. Agarwal, Alcohol as an alternative fuel for internal combustion engines (Springer, Singapore, 2021)
A. Basile, F. Dalena, Alcohols and bioalcohols: characteristics, production, and uses (Nova Science Publishers, Inc., New York, 2015)
M. Pagliaro, M. Rossi, The future of glycerol (Royal Society of Chemistry, Cambridge, 2010)
C.J. Mota, B.P. Pinto, A.L. de Lima, Glycerol: a versatile renewable feedstock for the chemical industry (Springer, Cham, 2017)
A. Mustain, E.D. Setiawati, R. Tetrisyanda, G. Wibawa, Experimental and predicted values of bubble point pressure for binary and ternary systems consisting of 1-butanol, 2-methyl-1-propanol, glycerol, and water. J. Chem. Eng. Data 67, 941–947 (2022)
V.B. Vicente, G.V. Olivieri, R.G. dos Santos, R.B. Torres, Volumetric properties of binary mixtures of glycerol+ alkanols (c1–c4): Experimental study and application of the peng–robinson–stryjek–vera equation of state. J. Chem. Thermodyn. 168, 106728 (2022)
M. Tyczyńska, A. Dentkiewicz, M. Jóźwiak, Thermodynamic and thermal analyze of n, n-dimethylformamide+ 1-butanol mixture properties based on density, sound velocity and heat capacity data. Molecules 28, 4698 (2023)
E.V. Anslyn, D.A. Dougherty, Modern physical organic chemistry (University science books, Sausalito, 2005)
G.I. Egorov, D.M. Makarov, Volumetric properties of binary mixtures of glycerol + tert-butanol over the temperature range 293.15 to 348.15 k at atmospheric pressure. J. Solut. Chem. 41, 536–554 (2012)
M.-L. Ge, J.-L. Ma, B. Chu, Densities and viscosities of propane-1, 2, 3-triol + ethane-1, 2-diol at t=(298.15 to 338.15) k. J. Chem. Eng. Data 55, 2649–2651 (2010)
B. Hawrylak, K. Gracie, R. Palepu, Thermodynamic properties of binary mixtures of butanediols with water. J. Solut. Chem. 27, 17–31 (1998)
Q.-S. Li, Y.-M. Tian, S. Wang, Densities and excess molar volumes for binary mixtures of 1, 4-butanediol+ 1, 2-propanediol,+ 1, 3-propanediol, and+ ethane-1, 2-diol from (293.15 to 328.15) k. J. Chem. Eng. Data 53, 271–274 (2008)
Y. Matsumoto, H. Touhara, K. Nakanishi, N. Watanabe, Molar excess enthalpies for water+ ethanediol,+ 1, 2-propanediol, and+ 1, 3-propanediol at 298.15 k. J. Chem. Thermodyn. 9, 801–805 (1977)
Anton Paar GmbH, Digital densimeter: Instuction manual DMA 5000, (2009)
K.R. Hall, D.J. Kirwan, O.L. Updike, Reporting precision of experiments. Chem. Eng. Educ. 9, 24–30 (1975)
J. Gross, G. Sadowski, Perturbed-chain saft: an equation of state based on a perturbation theory for chain molecules. Ind. Eng. Chem. Res. 40, 1244–1260 (2001)
J. Gross, G. Sadowski, Application of the perturbed-chain saft equation of state to associating systems. Ind. Eng. Chem. Res. 41, 5510–5515 (2002)
M. Almasi, A. Hernández, Experimental and theoretical studies of ethylene glycol dimethyl ether and 2-alkanol mixtures. Int. J. Thermophys. 44, 109 (2023)
A. Hernández, A.Z. Zeqiraj, F.R. Aliaj, Densities, sound speeds, and refractive indices of 1-propanol+ cyclohexane (or cyclohexene or cyclohexanone) binary mixtures at various temperatures under atmospheric pressure: experimental and modeling study. Int. J. Thermophys. 44, 102 (2023)
R. Abidi, M. Hichri, C. Lafuente, A. Hernández, Surface tensions for binary mixtures of alkyl levulinate+ alkanol: measurement and modeling. Int. J. Thermophys. 44, 33 (2023)
B. Giner, C. Lafuente, A. Villares, M. Haro, M.C. Lopez, Volumetric and refractive properties of binary mixtures containing 1, 4-dioxane and chloroalkanes. J. Chem. Thermodyn. 39, 148–157 (2007)
N.L. Benkelfat-Seladji, F. Ouaar, A. Hernández, N. Muñoz-Rujas, I. Bahadur, N.C.-B. Ahmed, E. Montero, L. Negadi, Measurements and modeling of physicochemical properties of pure and binary mixtures containing 1, 2-dimethoxyethane and some alcohols. J. Chem. Eng. Data 66, 3397–3416 (2021)
N.L. Benkelfat-Seladji, F. Ouaar, A. Hernández, I. Bahadur, N. Muñoz-Rujas, S.K. Singh, E. Montero, N.C.-B. Ahmed, L. Negadi, Density, speed of sound, refractive index of binary mixtures containing 2-ethoxyethanol and some alcohols: measurement and correlation. J. Chem. Thermodyn. 66, 106762 (2022)
O. Redlich, A.T. Kister, Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
M.M. Alavianmehr, R. Ahmadi, N. Aguilar, M. El-Shaikh, S.M. Hosseini, S. Aparicio, Thermophysical and molecular modelling insights into glycerol+ alcohol liquid mixtures. J. Mol. Liq. 297, 111811 (2020)
H. Iloukhani, M. Almasi, Densities, viscosities, excess molar volumes, and refractive indices of acetonitrile and 2-alkanols binary mixtures at different temperatures: experimental results and application of the prigogine-flory-patterson theory. Thermochim. Acta 495, 139–148 (2009)
A.S. Alkindi, Y.M. Al-Wahaibi, A.H. Muggeridge, Physical properties (density, excess molar volume, viscosity, surface tension, and refractive index) of ethanol+ glycerol. J. Chem. Eng. Data 53, 2793–2796 (2008)
T.E. Daubert, R.P. Danner, Physical and thermodynamic properties of pure chemicals data compilation (Taylor & Francis, Bristol, 2004)
Acknowledgments
A.H acknowledges the economic support given by the UCSC.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
YC: Experimental investigation, Writing. AH: Theoretical investigation, Writing. FA: Experimental investigation, Writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chabouni, Y., Hernández, A. & Amireche, F. Volumetric, Optical and Spectroscopic Properties of Binary Mixtures of Glycerol with Butanediol Isomers. Int J Thermophys 44, 161 (2023). https://doi.org/10.1007/s10765-023-03272-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03272-5