Abstract
Nanoparticles (NP) of MnFe2 O 4 were synthesized via sol-gel method, with calcination process at 600 °C. The effects of different surfactants namely polypropylene glycol (PPG) and polysorbate 80 (PS80) in the formation of crystal structure, magnetic properties, and optical properties were studied using X-ray diffraction (XRD), vibration sample magnetometry, UV-visible diffuse reflectance spectroscopy (DRS), and photoluminescence (PL) spectroscopy. Brunauer-Emmett-Teller (BET) surface area test was also performed. XRD investigations revealed that MnFe2 O 4 sample synthesized using PS80 as surfactant (sample 2) showed pure crystalline phase of a cubic spinel structure whereas sample synthesized with PPG (sample 1) exhibited an additional phase of α-Fe2 O 3. The morphology of the samples was recorded using a scanning electron microscope (SEM). Energy dispersive X-ray (EDX) results showed that the composition of the elements was as relevant as expected from the sol-gel method. The magnetic hysteresis loops confirmed the super-paramagnetic behavior of both the samples. PL studies revealed a sharp narrow band green emission exhibited by both the samples. Catalytic activity of MnFe2 O 4 nanoparticles (NPs) was performed. The catalysts MnFe2 O 4, samples 1 and 2, were tested for the oxidation of benzyl alcohol to benzaldehyde. The conversion of benzyl alcohol reached a maximum of 82.45 % for MnFe2 O 4 sample 2, whereas for sample 1, the conversion was only 68.52 %. These Mn ferrite samples have potential applications in LED or laser diodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spinel ferrites have attracted much attention in the recent past due to their potential applications in high-density magnetic recording, magnetic fluids, data storage, drug delivery, pigments, sensors, and catalysis [1–3]. Among the various spinel ferrites, manganese ferrite (MnFe2 O 4) belongs to a group of soft magnetic materials and is used in many areas, such as microwave devices, memory chips, recording media, transformer cores, rod antennas, catalysts, and sensors [4–7]. The shape, size and morphology of the nanostructured spinel materials play a vital role in determining their structural, morphological, optical, electrical, magnetic and catalytic properties.
Several methods have been used to prepare the spinel MnFe2 O 4 nanostructures such as hydrothermal [7], solvothermal [8], ultrasonic [9], sol-gel [10], co-precipitation [11], and reverse micelle [12] methods. The size, shape, and magnetic properties of the nanoparticles synthesized depend on the preparation methods. But the control of particle size and its size distribution still remains a major challenge as the final product size depends on many processing parameters. The magnetic properties depend on the crystallite size, morphology, nature and concentration of the dopant, and cation distribution between the tetrahedral (A) and octahedral (B) sites in the spinel MFe2 O 4 structure [13]. The catalytic property of the spinel ferrites considerably depends on the distribution of metal cations among the A and B sites of the spinel MFe2 O 4 structure. Ramankutty et al. [14] have reported catalytic properties of various spinel ferrites and found that the spinel CoFe2 O 4 catalyst display better performance than other ferrites.
In this regard, the study on the effect of polymer surfactants on sol-gel synthesis will be helpful in tailoring the properties of resultant ferrite powder. Sol-gel method is a good method to synthesize the spinel MFe2 O 4 (M = Mn, Co, Ni, Cu, Zn, Mg, etc.) and has the advantages like mixing of multicomponents at an atomic level at low temperatures resulting in homogenous precursors, low cost, and leaves no by-product effluents [13].
Though many researchers have been carried out on sol-gel synthesis of Mn ferrites, no report is available on the effects of polysorbate 80 (PS80) and polypropylene glycol (PPG) as surfactants in the sol-gel synthesis of manganese ferrites. In the present study, we have studied the effects of PS80 and PPG as surfactants in the sol-gel synthesis by analyzing the structural, morphological, optical, magnetic, and catalytic properties of the final MnFe2 O 4 powder. The prepared samples were characterized by Fourier transform infrared (FT-IR) spectroscopy, energy dispersive X-ray (EDX) spectroscopy, Bruner-Emmet-Teller (BET) surface area, UV-visible diffuse reflectance spectra (DRS), and photoluminescence (PL) spectra, and their results were discussed. The benzyl alcohol oxidation activity tests were carried out, and the product formed by the catalytic oxidation of benzyl alcohol was characterized by gas chromatography (GC). The influence of polymer surfactants on the catalytic activity of spinel MnFe2 O 4 nanoparticles is reported.
2 Experimental Part
2.1 Synthesis of Manganese Ferrite Nanoparticles
The chemicals used to synthesize MnFe2 O 4 powders were manganese nitrate, ferric nitrate, sodium hydroxide, PPG, and ketone. All chemicals were of analytical grade and used without purification. Stoichiometric amounts of metal nitrates (Mn(NO3)2⋅ 4H2O, 98 % and Fe(NO3)3⋅ 9H2O, 98 %) and 4 g of sodium hydroxide were dissolved separately in a beaker with 100 ml of deionized water and stirred for 15 min to obtain clear solution. The polymer solution was prepared by mixing 20 wt.% of PPG and 10 ml of iso-propyl methyl ketone in deionized water using a magnetic stirrer. After a constant stirring for some time, polymer solution was added to sols, drop by drop. The subsequent mixture was then heated to 90 ∘C and subjected to continuous stirring till a gel was obtained. The gel was then kept in hot air oven for 2 days to evaporate water. The resultant solid was then calcinated at 600 ∘C for 2 h at a heating rate of 10 ∘C/min. Afterwards, it was crushed for 30 min in a mortar to form a powder sample and labeled as sample 1. In another experiment, sample 2 was synthesized following the same procedure but replacing PPG with PS80.
2.2 Characterization Techniques
The phase structure analysis of products was identified using X-ray diffractometer (PANalytical X’pert pro) of wave length (λ= 0.15406 nm) in a wide range of 2 𝜃 (20 ∘ <2 𝜃 <70 ∘). The magnetic saturation magnetization and coercivity of manganese ferrite powder were measured by a vibrating sample magnetometer (VSM)-Lakeshore 7304 with a maximum field of 15,000 Gauss at room temperature. The morphology and the dispersabilility of the calcinated powder were observed by SEM (FEI QUANTA 200 FEG), equipped with EDX for elemental chemical analysis. The surface functional groups were analyzed by Perkin Elmer FT-IR spectrometer. The surface area was derived from N2 adsorption-desorption isotherms using liquid nitrogen at 77 K using an automatic adsorption instrument (Quantachrome Corp. Nova-1000 gas sorption analyzer). The UV-visible diffuse reflectance spectrum (DRS) was recorded using Cary100 UV-visible spectrophotometer to estimate the energy band gap (E g). The photoluminescence (PL) properties were recorded using a Varian Cary Eclipse Fluorescence Spectrophotometer. BET surface area technique was performed using N2 adsorption/desorption studies.
2.3 Catalytic Test
The oxidation of benzyl alcohol using MnFe2 O 4 samples as catalysts was carried out in a batch reactor operated under atmospheric conditions. Ten moles of oxidant (H2 O 2) were added along with 1 g of nanosized catalysts (samples 1 and 2), and the contents were heated at 80 ∘C in an acetonitrile medium for 10 h in a three-necked round-bottom flask equipped with a reflux condenser and thermometer. The oxidized products after the catalytic reaction were collected and studied using Agilent GC spectrometer. DB wax column (capillary column) of length 30 mm was used for the study, and helium was used as the carrier gas. The yields of the carbonyl compounds formed were calculated by the following formulae
3 Results and Discussion
3.1 Structural Analysis
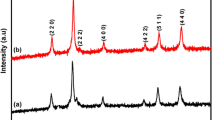
The XRD patterns of the MnFe2 O 4 powder synthesized via sol-gel method using PS80 as surfactant is shown in Fig. 1b. The prominent peaks observed at 2 𝜃 values of 30 ∘, 35 ∘, 43 ∘, 53 ∘, 57 ∘, and 62 ∘ are assigned to (220), (311), (400), (422), (511), and (440) planes, respectively. All these peaks confirm the cubic spinel-type lattice of MnFe2 O 4 which matches well with the standard XRD pattern (JCPDS card no. 73-1964). This indicates that PS80 as surfactant led to the formation of MnFe2 O 4 particles of good crystallinity with no impurity phase. However, the XRD pattern of the sample synthesized with the surfactant PPG (Fig. 1a) shows the peaks corresponding to spinel structure and additional peaks due to a second phase identified as α-Fe2 O 3. The shift of peaks at higher angles is attributed to small anisotropic distortions of the structure [15].
3.2 Fourier Transform Infrared Spectroscopy Studies
Figure 2 shows the FT-IR spectrum of Mn ferrite samples. The bands at 3455 and 3353 cm −1 are assigned to the O–H stretching vibration of the adsorbed water molecules [16]. Metal oxide (M-O) stretching vibrations of Mn ferrite are located in the region between 400 and 700 cm −1 [17]. In ferrites, according to the geometrical configuration of the oxygen nearest neighbors, the metal ions are situated in tetrahedral and octahedral sub-lattices. The studies on vibrational spectra of ferrites indicated that high-frequency bands at 569 and 542 cm −1 are attributed to the intrinsic vibration of tetrahedral sites and low-frequency bands at 430 and 454 cm −1 are attributed to the octahedral sites [17]. Peaks at 1383 cm −1 may be due to asymmetric stretching of NO\(_{3}^{\mathrm {-}}\). The peaks at 1116 and 1119 cm −1 may be due to (C–O–C) symmetric vibration due to dehydration of OH group from the polymers. The absence of peaks in the range of 1000–1300 and 2000–3000 cm −1 in the sample synthesized with the surfactant PS80 confirmed that the O–H mode, C–O mode, and C–H stretching mode of organic sources did not exist [18].
3.3 Morphological Analysis
The SEM images shown in Fig. 3a, b show the size, shape, and agglomeration of the MnFe2 O 4 samples synthesized from PPG and PS80 as surfactants, respectively. The SEM image (Fig. 3b) of MnFe2 O 4 PS80 (sample 2) reveals that the morphology of the particles were dispersed uniformly with a wider range of particle sizes (50 nm to 1 µm), whereas grain growth was apparent in the sample synthesized from PPG (sample 1) (Fig. 3a). The particles were agglomerated due to interaction between magnetic nanoparticles. Heat treatment would have also caused agglomeration, resulting in bigger grains. This is a typical characteristic feature of spinel ferrites [19]. Analysis of the two SEM micrographs presents the fact that PS80 acted as a better surfactant than PPG, producing magnetic nanoparticles.
3.4 Energy Dispersive X-ray Analysis
The composition of the samples has been determined using the EDX technique, and the patterns obtained are shown in Fig. 4, which revealed the presence of Fe, Mn, and O peaks of Mn ferrites. A small peak appearing at 2.1 KeV for both the samples indicates the presence of Au used in sputter coating, while preparing the sample for the analysis.
3.5 BET Surface Area
The adsorbance capability of MnFe2 O 4 samples was determined using BET surface area technique using N2 adsorption/desorption studies. The surface area of sample 2 was found to be 62.85 m 2/g, far greater than that of sample 1 (38.35 m 2/g). The smaller particle sizes of sample 2, as observed from the SEM image (Fig. 3b) could be the reason behind this. It is believed that the catalytic properties of sample 2 would be better than that of sample 1 because of its high surface area.
3.6 Photoluminescence Studies
PL spectra can give information on sub band gap defect states of spinel ferrite nanoparticles. A visible region peak observed at 496 and at 497 nm for samples 1 and 2, respectively, indicated the presence of defects and oxygen vacancies in both the samples. A green emission peak observed at 532 and 530 nm is also attributed to the oxygen vacancies [20, 21]. Such a prominent and sharp narrow band green emission observed for both the samples depict that these materials can be used for laser diodes or LED applications. The PL intensity of sample 1 for the green emission is approximately 1.4 times greater than that of sample 2. So, the Mn ferrite sample synthesized using PPG as surfactant is found to have more defects and oxygen vacancies. But sample 1 with such defects may be preferred for luminescence devices, as its PL intensity is far higher (Fig. 5).
3.7 VSM Measurements
Magnetic properties of MnFe2 O 4 samples were recorded at room temperature using VSM and the results of hysteresis curves of the samples are shown in Fig. 6. The obtained values of saturation magnetization (M s), remnant magnetization (M r) and coercivity (H c) are given in Table 1. Super paramagnetic behavior was observed in both the samples, in concordance with the previous studies of Mn ferrite nanoparticles. For the sample synthesized using PS80 (sample 2), the saturation magnetization M s was 31.9 emu/g and coercivity was 70 Oe. Whereas, M s and H c values of the sample synthesized using PPG (sample 1) reduced to 15.4 emu/g and 60 Oe respectively. The presence of an antiferromagnetic phase (α-Fe2 O 3) in sample 1 contributed to the decrease of its saturation magnetization value [15]. The presence of more oxygen vacancies in sample 1, as observed from PL studies, could be another reason. Generally, coercivity H c of a magnetic material is a measure of its magnetocrystalline anisotrophy [22]. It seems to originate from the exchange anisotrophy due to spin disorder at the particle interface. This effect is expected to be larger for smaller particles due to the increase in surface-to volume ratio [23]. This could be the reason for the decrease of H c value to 60 Oe for sample 1, as it contained bigger grains compared with sample 2 (Fig. 3a, b).
3.8 Catalytic Test
The catalytic oxidation of benzyl alcohol was studied using MnFe2 O 4 samples as catalysts, and their performances were investigated. The oxidized product was analyzed using GC; the conversion of benzyl alcohol reached a maximum of 82.45 % for MnFe2 O 4 synthesized by using PS80 (sample 2) as surfactant, whereas for MnFe2 O 4 synthesized by using PPG as surfactant (sample 1), the conversion was only 68.52 % (Table 2). In the present work, the Mn ferrites synthesized via sol-gel method using PS80 as surfactant performed as a good catalyst with higher yield of benzaldehyde with good selectivity than the MnFe2 O 4 ferrites synthesized using PPG surfactant. The smaller particle size of sample 2 with high surface area is the prime reason for its increased catalytic activity.
4 Conclusions
-
1.
MnFe2 O 4 nanoparticles were successfully synthesized via sol-gel method using PPG and PS80 as surfactants. Powder XRD results indicated that pure single-phase crystalline was obtained by using PS80 as surfactant. Whereas sample synthesized by using PPG had an impurity phase of Fe2 O 3. The SEM images show that the morphology of the product is consist of well-defined nanoparticle structure with agglomeration.
-
2.
VSM results show the super paramagnetic behavior of both the samples. The catalytic oxidation of benzyl alcohol was studied using MnFe2 O 4 samples as catalysts, and their performances were investigated. Surface area of both the samples was studied by BET method. The surface area of sample MnFe2 O 4 synthesized by using PS80 was found to be 62.85 m 2/g, far greater than that of sample MnFe2 O 4 synthesized by using PPG (38.35 m 2/g).
-
3.
The conversion of benzyl alcohol reached a maximum of 82.45 % for MnFe2 O 4 synthesized by using PS80, whereas for sample MnFe2 O 4 synthesized by using PPG, the conversion was only 68.52 %. This is due to the smaller particle size with large surface area of sample synthesized by using PS80.
-
4.
PL studies shows a sharp narrow emission bands observed in green region for both the samples. Therefore, this could be used for LED-based applications.
-
5.
Compared with other synthetic methods, sol-gel method is a facile, low-cost pathway to novel MnFe2 O 4 nano-architectures.
References
Horvath, M.P. J. Magn. Magn. Mater. 215(171) (2000)
Adam, J.D., Davis, L.E., Dionne, G.F., Schloemann, E.F., Stitzer, S.N.: IEEE Trans. Microwav. Theory Tech. 50, 721 (2002)
Zhou, Z.H., Xue, J.M., Wang, J., Chan, H.S.O., Yu, T., Shen, Z.X.: J. Appl. Phys. 91, 6015 (2002)
Arulmurugan, R., Jeyadevan, B., Vaidyanathan, G., Sendhilnathan, S.: J. Magn. Magn. Mater. 288, 470 (2005)
Fujioka, H., Ikeda, T., Ono, K., Ito, S., Oshima, M.: J. Cryst. Growth 241, 309 (2002)
Salah, L.M., Moustafa, A.M., Farag, I.S.A.: Ceram. Int. 38, 5605 (2012)
Phumying, S., Labuayai, S., Swatsitang, E., Amornkitbamrung, V., Maensiri, S.: Mater. Res. Bull. 48, 2060 (2013)
Guo, P, Zhang, G, Yu, J, Li, H., Zhao, X.S.: Physicochem. Eng. Aspects 395, 168 (2012)
Chen, D., Liu, H., Li, L.: Mater. Chem. Phys. 134, 921 (2012)
Li, J., Yuan, H., Li, G., Liu, Y., Leng, J.: J. Magn. Magnc. Mater. 322, 3396 (2010)
Amighian, J., Mozaffari, M., Nasr, B.: Phys. Status. Solidi C 3, 3188 (2006)
Liu, C, Zou, B, Rondinone, AJ, Zhang, ZJ: J. Phys. Chem. B 104 (2000)
Sivakumar, M., Kanagesan, S., Chinnaraj, K., Sureshbabu, R., Nithiyanantham, S.: J. Inorg. Organomet. Polym. Mater. 23, 439 (2013)
Ramankutty, C.G., Sugunan, S.: Appl. Catal. A Gen. 218, 39 (2001)
Rivas, P., Sagredo, V., Rossi, F., Pernechele, C., Solzi, M., Pena, O.: IEEE Trans. Magn. 49, 4614 (2013)
Li, K., Cheng, R., Wang, S., Zhang, Y.: J. Phys. Condens. Matter. 10, 4315 (1998)
Cannas, C., Ardu, A., Musinu, A., Peddis, D., Piccaluga, G.: Chem. Mater. 20, 6364 (2008)
Brabers, V.A.M.: Phys. Status Solidi B. 33, 563 (1969)
Gharagozlou, M.: J. Alloy. Compd. 486, 660 (2009)
Bhargava, R, Sharma, PK, Dutta, RK, Kumar, S, Pandey, A.C., Kumar, N.: Mater. Chem. Phys. 120, 393 (2010)
Yang, J., Gao, M., Yang, L., Zhang, Y., Lang, J., Wang, D., Wang, Y., Liu, H., Fan, H.: Appl. Surf. Sci. 255, 2646 (2008)
Joy, P.A., Date, S.K.: J. Magn. Magn. Mater. 222, 33 (2000)
Kasapoglu, N., Baykal, A., Koseoglu, Y., Toprak, M.S.: Scr. Mater. 57, 441 (2007)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jacintha, M., Neeraja, P., Sivakumar, M. et al. Comparative Study of MnFe2 O 4 Nanoparticles Synthesized by Sol-gel Method with Two Different Surfactants. J Supercond Nov Magn 30, 237–242 (2017). https://doi.org/10.1007/s10948-016-3714-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-016-3714-9