Abstract
CoFe2O4 nanoparticles were synthesized by hydrothermal method. The temperature, surfactant, capping agent and time effects on the size of CoFe2O4 nanoparticles were studied. Bis-(2-hydroxy-1-naphthaldehyde)-butanediamine Schiff-base ligand (L) used as a good capping agent to produce uniform cubic-like nanostructure. But when SDS was used as surfactant, nonuniform spherical nanoparticles were obtained. Nanoparticle was characterized using X-ray diffraction, energy-dispersive spectroscopy, scanning electron microscopy and Fourier transform infrared. The magnetic properties of the samples were investigated using VSM analyze. We found that the CoFe2O4 nanoparticles synthesized at temperature of 150 °C exhibit a ferromagnetic behavior with a saturation magnetization of 19 emu/g and a coercivity of 200 Oe. The photocatalytic behavior of CoFe2O4 was investigated using the degradation of a Rhodamine B aqueous solution under ultraviolet light irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As a kind of generic magnetic material, ferrite has been widely used in many fields, such as message recording, ferrofluids [1], microwave absorber [2], magnetic drug delivery [3], biology, medicine, magnetic sensors [4], aviation, and so on. During the past several decades, metal-oxide nanoparticles have been the subject of much interest due to their unusual optical, electronic and magnetic properties, fine mechanical and chemical stability. Magnetic properties of nanoparticles is interesting research activity driven by a fundamental interest in the novel physical properties of the nanoscale system. Also, nanostructured materials have striking potential for industrial application [5].
Among magnetic materials, magnetic ceramics have attracted special attention due to chemical stability as well as high electrical resistivity and spinel is one of the most magnetic ceramics. Base on the magnetic theory, the most important spinel’s are oxides 2, 3 or MFe2O4 [6]. Among spinel ferrites, CoFe2O4 has received special attention because of its large magneto-crystalline anisotropy, high coercivity, moderate saturation magnetization, large magnet astrictive coefficient, mechanical hardness and chemical stability [7]. The magnetic properties are very sensitive to the particle size and synthesis condition [8–10]. Also the energy of a magnetic particle is generally dependent on the magnetization direction, and, for uniaxial anisotropy and easy axis aligned with the direction of external field (H). Cobalt ferrite (CoFe2O4) is a well-known hard magnetic material with high coercivity and suitable magnetization. These attributes, along with their tremendous physical and chemical stability, make CoFe2O4 nanoparticles suitable for applications such as lithium batteries [11], ferrofluids [12] audio and video tape and high-density digital recording disks etc. There are many methods for the synthesis of nanoparticles, including: sol–gel, hydrothermal, solvothermal method, reverse micelle methods, thermal decomposition, solid-state [13] and sonochemical approach [14].
A lot of synthetic strategies for preparing nanosized cobalt ferrite have been presented. Pileni et al. [15] used oil-in-water micelle to prepare size-controlled Co-ferrite in the range of 2–5 nm. Liu et al. [16] as well as reported the nanoparticles of 2–35 nm in diameter which were prepared in normal micelle Similarly with the method of Pileni et al. Pillai [17] and Ahn [18] used water-in-oil microemulsion to obtain the nanoparticles in the diameter of 50 and 4.9 nm, respectively after heat treatment.
Properties of CoFe2O4 extremely depend on the preparation method [19]. In recent years, cobalt ferrite nanocrystals have been prepared using various methods, such as chemical co-precipitation, method, combustion method, reverse micelle methods, thermal decomposition, solvothermal method and hydrothermal method. Between these methods, hydrothermal method has been known as one of the most promising and accessible synthetic strategy for synthesis of various nanoparticles.
The main purposes of this work are to study and investigate the temperature, surfactant, capping agent and time effects on the size of CoFe2O4 nanoparticles prepared by hydrothermal method. Now, A lot of work has been done for the synthesis of nanoparticles using capping agent and surfactant [20, 21]. Bis-(2-hydroxy-1-naphthaldehyde)-butanediamine Schiff-base ligand (L) was prepared and used as a good capping agent to produce uniform cubic-like nanostructure. But when SDS was used as surfactant, nonuniform spherical nanoparticles were obtained.
2 Experimental section
2.1 Materials and characterization
Potassium hexacyanoferrate(III), cobalt(II) acetylacetonate, 1,4-diaminobutane solution, 2-hydroxy-1-naphthaldehyde, methanol and ethanol were purchased from Merck and used without purification. Sodium dodecyl sulfate (SDS) as surfactant and ethylendiamin (en) for adjust pH and de-ionized water was used as solvent. XRD patterns were recorded by a Philips, X-ray diffractometer using Ni-filtered Cu Kα radiation. SEM images were obtained using an LEO instrument model 1455VP. FT Infrared (FT-IR) spectra were obtained as potassium bromide pellets in the range of 400–4000 cm−1 with a Nicolet-Impact 400D spectrophotometer. The magnetic properties of the samples were detected at room temperature using a vibrating sample magnetometer (VSM, Meghnatis Kavir Kashan Co., Kashan, Iran). The UV–Vis spectra of the samples were taken on a UV–Vis spectrophotometer (Shimadzu, UV-2550, Japan) + visible sources of 400 W Osram lamps.
2.2 Preparation of bis-(2-hydroxy-1-naphthaldehyde)-butanediamine Schiff-base ligand (L)
To prepare of Schiff-base ligand (L), 0.04 mol of 2-hydroxy-1-naphthaldehyde dissolved in methanol (20 ml) was added drop wise to a 1,4-diaminobutane solution (0.02 mol) in 20 ml of methanol. The solution was refluxed for 4 h. The yellow precipitate was separated by filtration, washed and dried. It was then recrystallized from methanol. The as-prepared ligand was characterized utilizing FT-IR.
2.3 Synthesis of cobaltferrit
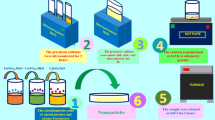
The CoFe2O4 sample was synthesized using potassium hexacyanoferrate(III) cobalt(II), acetylacetonate, ethylendiamin (en) and de-ionized water as solvents. In a typical method, 0.25 g of K3[Fe(CN)6] and 0.1 g Co(acac)2 individually dissolved in 20 ml of de-ionized water and then two solutions are mixed on magnetic stirrer at room temperature to achieved a homogeneous solution. Then we added en to adjust pH solution to 10. The obtained solution was stirred on stirring for 10 min. For studying capping agent and surfactant functions, two solution containing SDS and L-ligand was added to above solution separately; In one beaker 0.2 g L-ligand was dissolved in 50 ml methanol and added to the above solution dropwise (to obtain L-CoFe2O4). Figure abstract for this step has been shown in Fig. 1. In other beaker 0.05 g SDS dissolved in 50 ml methanol and added to the mixture. The obtained mixtures were stirred on the magnetic stirrer for 30 min. Then the mixtures were putted in two autoclaves for 12 h at 150 °C separately. The formed precipitates were collected and washed with double distilled water and ethanol and dried at 60 °C.
2.4 Photocatalytic measurements
The photocatalytic efficiency of the catalysts was investigated using a 100 ml quartz tube. 0.012 g of L-CoFe2O4 nanoparticles photocatalysts were mixed with 30 ml of Rhodamine B solution (initial concentration was 10 ppm) and finally 0/5 ml HNO3 65 % added to vessels. The resulting suspensions were stirred to obtain the maximum absorption of organic pollutant molecules on the photocatalyst surface and to make oxygen available for the reaction and to obtain most homogeneity in the mixture. Reaction carried out under the UV lamp irradiation, the mixture was placed inside the photoreactor in which the vessel was 40 cm away from the UV source of 400 W Mercury lamps. After each 10 or 15 min, sampling performed and centrifuged to separate the solid particles and after that, the samples were analyzed with the UV–Vis spectrometer.
3 Results and discussion
The CoFe2O4 nanoparticles were studied by X-ray powder diffraction (XRD). Figure 2 shows XRD patterns for CoFe2O4 nanoparticle synthesized by hydrothermal method and calcinated at 500 °C for 3 h when the Co precursor is Co(acac)2.2H2O and Fe precursor was K3[Fe(CN)6]. As shown in XRD pattern, the synthesized matter has crystal structure of CoFe2O4 (JCPDS card no. 01-1121) and according to XRD pattern, the synthesized product is quite pure. The mean size of the crystallites was estimated by using the Scherrer equation:
where DXRD is the average crystallite diameter, k is a constant equal to 0.9, β is the full-width at half-maximal, λ is the wavelength of the X-ray used and θ is the diffraction angle. It is found that the average crystallite diameter of CoFe2O4 nanoparticle was calculated to be 13.24 nm at the temperature of 500 °C.
FT-IR spectrum shows peaks at 1629 and 3434 cm−1 were assigned to the stretching vibrations of surface hydroxyl groups. The sample before calcination show peak at 2924 cm−1 due to the C–O stretching vibration of ethylene glycolic (EG), the broad absorption at around 570 and 452 cm−1 were the stretching of Me–O, which is typical for CoFe2O4 molecules [22, 23] (Fig. 3). Adsorbed water is featured by the band at 1381 cm−1. The XRD result and FT-IR result confirms the presence of nanoparticles CoFe2O4.
As well as to confirm the purity of the CoFe2O4 nanoparticle, elemental analysis energy dispersive X-ray spectroscopy (EDS) was taken. Figure 4 shows the typical EDS curve of the CoFe2O4 nanoparticle synthesized by hydrothermal method which obviously depicts lines of Co, O and Fe elements.
The scanning electron microscopy (SEM) images of CoFe2O4 nanoparticle at different temperatures, time and capping agent was taken and used for synthesis condition optimization. First, we have synthesized CoFe2O4 in three temperatures of 120, 150 and 180 (Fig. 5). According to Fig. 5b, it can be seen that more uniform particle size has been synthesized and the optimum temperature is 150 °C. In the next step, we need to optimize the time in temperature 150 °C, therefore reaction is carried out in three different times of 12 and 18 and 24 h and then the synthesized materials were studied by SEM. From obtained SEM images we understood that the best time for the synthesis of the nanoparticles is 12 h (Fig. 6a). So the best temperature and time obtained 12 h and 150 °C. After the temperature and time optimization, the effect of surfactant and capping agent on the particle size and morphology was investigated. Here, we use the SDS and ligand-L as surfactant and capping agent respectively. As shown in SEM photographs, when we use of SDS particles, have spherical morphology and particle size distribution is smaller (Fig. 7 b), but when we use the ligand-L, it is clear that the obtained nanoparticles are cubic-like and particle size has been increased (Fig. 7a).
For studying magnetization treatment of the cobalt ferrite nanoparticles which synthesized with hexacyanoferrate(III) cobalt(II) acetylacetonate and en, Hysteresis loops are mentioned in magnetic curves at three temperatures (Fig. 8). Results indicate that CoFe2O4 nanoparticles synthesized at 150 °C show ferromagnetic behavior and have a saturation magnetization of 19 emu/g which is smaller than the bulk value (74.08 emu/g) [24, 25] and reminisce of 6 emu/g and a coercivity of 200 Oersted. As shown, hysteresis loop for nanoparticles at 150 °C is narrower in comparison to other two samples. So these circumstances were selected as optimum condition and further inquiries continued on that.
Light absorption by the material and the migration of the light-induced electrons and holes are the important factors to controlling a photocatalytic activity reaction, this feature relevant to the electronic structure characteristics of the material.
Figure 9 display UV–Vis spectrum (DRS) for L-CoFe2O4. The nanoparticles are photoresponsive in the UV ranges, and as shown, L-CoFe2O4 has absorption in the UV area. The first wavelength of absorption used to calculate the optical band gap. Calculated band gap in onset (λ) for L-CoFe2O4 revealed that this nanostructure have good potential to acting as appropriate photocatalyst (Fig. 10).
Photocatalyst degradation of Rhodamine B by L-CoFe2O4 synthesized by hydrothermal was performed under ultraviolet irradiation. Change in the concentration of dye studied by UV absorption spectroscopy. The Rhodamine B destruction percentage in time of t (DP(t)) was calculated as follows:
where A0 and At are the absorbance value of the solution at 0 and t minute, respectively [26]. No dye degradation after 60 min without using UV light irradiation or nanostructured photocatalysts, so the contribution of self-degradation was insignificant. As time passed, dye decomposed and the color of the solution became brighter. The changes in the concentration of dye after 60 min under UV irradiation are depicted in Fig. 11. As time increased, more and more dye were adsorbed on the nanoparticles catalyst and degraded until the solutions were transparent (Fig. 12). Just after 40 min degradation percentage for L-CoFe2O4 was obtained 66 %. Over time, the rate degradation decreased because of decrement of available dye molecules surrounds the catalyst nanoparticles. According to photocatalytic calculations by Eq. (1), the Rhodamine B degradation by L-CoFe2O4 was about 82 % presents after 60 min under irradiation of UV light. So as prepared nanostructure photocatalyst presented very good photocatalytic activity.
4 Conclusion
CoFe2O4 nanoparticle was synthesized by method hydrothermal and characterized by XRD, SEM,EDS, DRS, VSM and IR techniques. The effects of time, temperature, surfactant and capping agent were studied. In order to optimize morphology and size, after completing each step, SEM image were taken from the synthesized nanoparticles. CoFe2O4 synthesized by hydrothermal method studied for photocatalitic activity. Rhodamine B degraded by catalysts CoFe2O4. Results proved that optimum temperature is 150 °C and the best time for the synthesis of the nanoparticles is 12 h. Previous investigations proved that heterogeneous photocatalytic processes consists of diffusion, adsorption and reaction steps and suitable distribution of the pore will improve the diffusion of reactants, which increase the rate of photocatalytic reaction. In this study, the enhanced photocatalytic activity may be attributed to favorable distribution of the pore, mono dispersive and good distribution of CoFe2O4 nanoparticles [27].
References
F. Tourinho, R. Franck, R. Massart, J. Mater. Sci. 25, 3249 (1990)
R.C. Che, L.M. Peng, X.F. Duan, Q. Chen, X.L. Liang, Adv. Mater. 16, 401 (2004)
C. Brazel, Pharm. Res. 26, 644 (2009)
N.E. Osman, N. Thapliyal, W. Alwan, R. Karpoormath, T. Moyo, J. Mater. Sci.: Mater. Electron. 26, 5097 (2015)
S.R. Ahmed, S.B. Ogale, G.C. Papaefthymiou, R. Ramesh, P. Kofinas, Appl. Phys. Lett. 80, 1616 (2002)
H. Shokrollahi, J. Magn. Magn. Mater. 320, 463 (2008)
G.A. El-Shobaky, A.M. Turky, N.Y. Mostafa, S.K. Mohamed, J. Alloys Compd. 493, 415 (2010)
J. Huo, M. Wei, Mater. Lett. 63, 1183 (2009)
N. Li, M. Zheng, X. Chang, G. Ji, H. Lu, L. Xue, L. Pan, J. Cao, J. Solid State Chem. 184, 953 (2011)
G.B. Ji, S.L. Tang, S.K. Ren, F.M. Zhang, B.X. Gu, Y.W. Du, J. Cryst. Growth 270, 156 (2004)
Y.-Q. Chu, Z.-W. Fu, Q.-Z. Qin, Electrochim. Acta 49, 4915 (2004)
J. Wagner, T. Autenrieth, R. Hempelmann, J. Magn. Magn. Mater. 252, 4 (2002)
M. Masjedi-Arani, M. Salavati-Niasari, J. Ultrason. Sonochem. 29, 226 (2016)
M. Masjedi-Arani, M. Salavati-Niasari, J. Mater. Sci. Mater. Electron. 26, 2316 (2015). doi:10.1007/s10854-015-2686-z
N. Moumen, P. Veillet, M.P. Pileni, J. Magn. Magn. Mater. 149, 67 (1995)
C. Liu, B. Zou, A.J. Rondinone, Z.J. Zhang, J. Am. Chem. Soc. 122, 6263 (2000)
V. Pillai, D.O. Shah, J. Magn. Magn. Mater. 163, 243 (1996)
Y. Ahn, E.J. Choi, S. Kim, H.N. Ok, Mater. Lett. 50, 47 (2001)
D. Peddis, C. Cannas, A. Musinu, A. Ardu, F. Orrù, D. Fiorani, S. Laureti, D. Rinaldi, G. Muscas, G. Concas, G. Piccaluga, Chem. Mater. 25, 2005 (2013)
M. Masjedi-Arani, N. Mir, E. Noori, T. Gholami, M. Salavati-Niasari, J. Superlattices Microstruct. 62, 30 (2013)
M. Masjedi-Arani, M. Salavati-Niasari, D. Ghanbari, G. Nabiyouni, J. Ceram. Int. 40, 495 (2014)
C. Cannas, A. Ardu, A. Musinu, D. Peddis, G. Piccaluga, Chem. Mater. 20, 6364 (2008)
B. Gillot, F. Jemmali, A. Rousset, J. Solid State Chem. 50, 138 (1983)
M.P. Gonzalez-Sandoval, A.M. Beesley, M. Miki-Yoshida, L. Fuentes-Cobas, J.A. Matutes-Aquino, J. Alloys Compd. 369, 190 (2004)
Z. Jia, D. Ren, R. Zhu, Mater. Lett. 66, 128 (2012)
H. Khojasteh, M. Salavati-Niasari, A. Abbasi, F. Azizi, M. Enhessari, J. Mater. Sci. Mater. Electron. 1 (2015). doi: 10.1007/s10854-015-3884-4
Jb Zhong, Jz Li, Fm Feng, Y. Lu, J. Zeng, W. Hu, Z. Tang, J. Mol. Catal. A Chem. 357, 101 (2012)
Acknowledgments
Authors are grateful to the council of Iran National Science Foundation and University of Kashan for supporting this work by Grant No. 159271/392.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbasi, A., Khojasteh, H., Hamadanian, M. et al. Synthesis of CoFe2O4 nanoparticles and investigation of the temperature, surfactant, capping agent and time effects on the size and magnetic properties. J Mater Sci: Mater Electron 27, 4972–4980 (2016). https://doi.org/10.1007/s10854-016-4383-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4383-y