Abstract
Super paramagnetic ZnFe2O4 nanoparticles were prepared by a surfactant assisted (ethylamine) hydrothermal method along with heat treatment. The nanoparticles were characterized by X-ray diffraction, Fourier transform infrared spectroscopy, high resolution scanning electron microscopy, Transmission electron microscopy, vibrating sample magnetometer and diffuse reflectance spectra technique. From the analyses, influence of calcination temperature on the structural, vibrational, morphological, magnetic and optical properties of ZnFe2O4 nanoparticles were investigated. The ZnFe2O4 nanoparticles with an average particle size of 17 nm showed high photocatalytic activity in the degradation of methylene blue (90 %). This work demonstrates that ZnFe2O4 can be used as a potential monocomponent in visible-light photocatalysis for the degradation of organic pollutants. Furthermore, the products were super paramagnetic and could be conveniently separated within 15 min and recycled by using simple magnet, which is very beneficial for the degradation of organic pollutants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photocatalysis is a recently developed water purification technology, which utilizes semiconductor and magnetic materials as catalyst to degrade environmental pollutants [1–3]. Photocatalysis has gained much attention, because it can effectively destroy structurally stable refractory pollutants with high degradation efficiency [4–10]. In past few decades, numerous semiconductor based photo catalysts have been proposed for achieving high degradation percentage of organic pollutants [11–13]. Anyhow, the need of irradiation/activation in the UV region for these photocatalysts remains a major hurdle, as the solar spectrum contains considerably less amount of UV light. Moreover, to counter global energy crisis and environmental problems, various catalysts have been developed to exhibit photocatalytic activity in visible region and thus, the development of these visible light induced photocatalysts, has gained much interest in current photocatalysis research [14].
Ferrite based magnetic materials are among the most promising materials for photocatalysis [14]. They have been intensively studied also because of its potential applications in information storage, magnetic devices, switching devices, magnetic printing inks, ferrofluid technology, magnetic drug delivery, magnetic refrigeration, medical diagnostics, gas sensors and magnetic resonance imaging [15–20].
There is tremendous interest to develop an eco-friendly and low band gap material that can be utilized for photocatalytic degradation of the organic pollutants under visible light. Nanocrystalline ferrites having general formula MFe2O4 (M = divalent metal ion, e.g. Zn, Co, Cu, Mn, Mg, Ni, Cd, etc.) is considered to be one of the most attractive classes of materials for photocatalytic applications. Among these, the ZnFe2O4 has drawn much attention because of its high magnetic permeability, non toxicity, low eddy-current loss, high electronic conductivity and low cost [21]. It has exhibited a wide spread use in the fields of gas-sensor [22], ferrofluid technology [23], magnetic characteristic [24], high density magnetic recording [25], biomedicine [26] and photocatalysis [27]. ZnFe2O4 possesses a regular spinel structure with tetrahedral A-site occupied by Zn2+ ions and octahedral B-site occupied by Fe3+ ions. It exhibits antiferromagnetism at Neel’s temperature (TN) = 10 K [28]. The magnetic moments of ZnFe2O4 can be altered to form super paramagnetism by reducing its particle size [21]. Until now various synthesis methods have been reported for the preparation of ZnFe2O4 nanoparticles, such as sol–gel [29], co-precipitation [30], combustion [31], micro emulsion [32], citrate precursor method [33], ball milling technique [34] and hydrothermal method [35]. Liu et al., have reported a simple solution-combustion route for the one-step synthesis of octahedral ZnFe2O4 nanocrystals as monocomponent visible-light photocatalysts for the degradation of the organic dye, methylene blue [36]. Sutka et al., have demonstrated high photocatalytic activities of ZnFe2O4 nanoparticles prepared using the sol–gel auto-combustion method with different iron contents, under visible light irradiation [37]. Wang et al., have concluded that the Lacto ferrin-assisted synthesized ZnFe2O4 nanocrystals have a promising application prospect to be used as highly-efficient photocatalyst [38].
The hydrothermal is one of the most useful techniques due to its simplicity, low cost, and nontoxic route. Furthermore, it offers the possibility to deal with a great number of experimental strategies by modification of parameters like precipitant agent concentration or temperature [39, 40]. To the best of our knowledge, there are no published reports on the use of ethylamine as a surfactant for the hydrothermal synthesis of ZnFe2O4. Sertkol et al., [35] did hydrothermal synthesis using polyethylene glycol as surfactant and they achieved room temperature magnetization of 40.3 emu/g. On comparison to this work, we have used ethylamine as a surfactant to hydrothermally synthesize ZnFe2O4 nanoparticles and were able to achieve saturation magnetization of 49.4 emu/g, a considerably more attractive super paramagnetic behavior exhibited by the nanoparticles. The structural, vibrational, morphological and magnetic properties were investigated by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), high resolution scanning electron microscopy (HR-SEM), Transmission electron microscopy (TEM), vibrating sample magnetometer (VSM) and the diffuse reflectance spectra (DRS). The photocatalytic activity was investigated by the degradation of methylene blue under visible light irradiation.
Materials and Method
Materials
Zinc nitrate hexahydrate [Zn (NO3)2·6H2O], ferric nitrate nonahydrate [Fe (NO3)3·9H2O], and ethylamine (C2H7N) were purchased from Sigma-Aldrich chemical reagent Co. (MO, USA) and were used without further purification. The de-ionized water was used in all the experiments.
Preparation of Superparamagnetic ZnFe2O4 Nanoparticles
ZnFe2O4 nanoparticles were prepared by surfactant assisted hydrothermal method. The stoichiometric quantities of zinc nitrate and ferric nitrate with a molar ratio 1:2 were first dissolved in 40 ml de-ionized water. Then 4 ml of ethylamine (EA) was added drop wise into the solution under continuous magnetic string at room temperature. Finally, the obtained precipitated solution was continuously stirred for half an hour and transferred to a 50 ml Teflon-lined autoclave. The autoclave was sealed and maintained at 160 °C for 15 h and then allowed to cool to room temperature, naturally. Finally, the brown precipitates were collected by centrifugation and the product was washed several times with de-ionized water and absolute ethanol, and dried at 60 °C for 2 h in hot air oven. The final product was grinded and calcined in air atmosphere at two different temperatures of 300 and 600 °C for 10 h and the samples were named as S1 and S2, respectively.
Characterization Studies
The phase structure of the synthesized ZnFe2O4 nanoparticles were identified by powder X-ray diffraction pattern, recorded using PAN analytical X’pert pro X-ray diffractometer with CuKα radiation (λ = 1.5406 Å). Fourier infrared spectra of the nanoparticles were recorded from 400 to 4000 cm−1 using Perkin Elmer Spectrometer by the KBr pellet technique. The morphology of the nanoparticles was investigated using a FEI company, Quanta 200 FEG scanning electron microscope with an accelerating voltage of 10 kV. The elemental analyses were performed by energy dispersive X-ray spectrometer (Quantax EDS, Bruker). Transmissions electrons microscopy (TEM) micrographs were collected on a ZEISS EM 900 TEM with an accelerating voltage of 200 kV. The optical properties were analyzed by UV–Vis Diffuse Reflectance Spectroscopy (DRS) using PG instruments-Model T90+ (2009), Japan in the wavelength range 400–800 nm. The magnetic properties were recorded by a vibrating sample magnetometer VSM Lakeshore-7410 at room temperature.
Measurement of Photocatalytic Activity
Photocatalytic activity of super paramagnetic ZnFe2O4 nanoparticles was examined by the photodegradation of methylene blue (MB) under visible light irradiation. The dye solution was obtained by adding an aqueous MB solution (20 mg/l, 100 ml) containing 50 mg of the catalyst (sample S1) in 50 ml DI water. The solution was vigorously stirred for 30 min in the dark at room temperature, and then the solution was irradiated using visible light source (500 W halogen lamp). The absorbance values were measured for the 3 ml dye solutions which were taken out from the solution after every time interval of 60 min. The same photocatalysis process was repeated for the sample S2 as catalyst. Temporal variation in the absorption of MB in the presence of ZnFe2O4 with visible light exposure was investigated using a UV–Vis Spectrophotometer (LAB India, UV 3000+) by measuring the absorbance in the wavelength range of 200–800 nm.
Results and Discussion
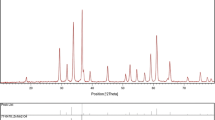
The phase and crystal structural analyses were carried out by XRD. The powder XRD patterns of ZnFe2O4 samples are shown in Fig. 1a, b. All the diffraction peaks at 2θ (29.95, 35.34, 36.28, 42.93, 53.20, 56.69, 62.27) confirm the formation of single phase ZnFe2O4 FCC spinels structure belonging to Fd3 m space group and are consistent with lattice parameter of α = 8.412 Å (JCPDS card No. 89–1009). Further, no other peaks of any impurities and secondary phase are observed. From Fig. 1, it is obvious that with the increase in calcination temperature, the intensity of diffraction peaks increases and the peaks become sharper as well as narrower. This reveals the improvement in the crystallinity and the formation of larger grains in the ZnFe2O4 samples due to the grain growth phenomenon which is faster at elevated temperatures [41]. The full width half maximum (FWHM) of the XRD peaks was used to calculate the crystal size of ZnFe2O4 nanoparticles. The calculated average crystallite sizes were 17 and 20 nm for sample S1 and S2, respectively.
FITR spectra of the annealed ZnFe2O4 samples S1 and S2 are shown in Fig. 2. In the FTIR spectrum, broad band present at around 3435 cm−1 relates to O–H stretching vibration. The weak band observed at 1601 cm−1 corresponds to stretching vibration of C=C, which is due to the presence of ethylamine on surface of the ZnFe2O4 nanoparticles. The vibrational bands present at around 541 and 456 cm−1 are attributed to stretching vibrations of Fe–O and Zn–O, which are the characteristics of ZnFe2O4 [42].
The morphology and particle size of the synthesized nanoparticles were investigated using SEM and TEM. The SEM micrograph of the ZnFe2O4 nanoparticles is shown in Fig. 3a, b. It is observed that the nanoparticles have almost uniform spherical shape with less aggregation. Figure 3d, e shows the TEM image of the annealed ZnFe2O4 nanoparticles. It can be seen that the extent of aggregation in the nanoparticles are relatively higher in sample S1 than sample S2. Furthermore, average particle size of sample S1 (17 nm) is observed to be smaller than S2 (20 nm). This increase in particle size of S2 might be due to the heat treatment. The calculated particle size using scherrer’s formula from the obtained XRD data are in good agreement with the TEM analysis.
The EDS spectra of the respective sample are shown in Fig. 3c, f. The EDS measurement was performed to identify elemental composition of calcined nanoparticles. The EDS analyses of samples S1 and S2 confirm (Fig. 3c, f) the presence of Zn, Fe and O elements. The absence of any other peaks of impure element suggest the high purity of the synthesized products.
The magnetic properties of synthesized ZnFe2O4 nanoparticles were measured by VSM at room temperature. It is evident from the results shown in Fig. 4 that the prepared ZnFe2O4 nanoparticles posses typical super paramagnetic behavior with zero coercivity and remanence. This indicates that the fine crystallite sizes of magnetic nanoparticles were formed during the hydrothermal method of synthesis. The saturation magnetization of samples S1 and S2 are 49.4, 47.5 emu/g, respectively. These values are smaller than the value of 88.4 emu/g as observed in bulk counterpart of the samples [43]. A higher saturation magnetization observed in S1 sample than S2 sample can be ascribed to the heat treatment in air causing reduction in oxygen vacancies, leading to reduction in magnetic moment [44] and the redistribution of cations in the nanocrystalline ZnFe2O4 [45]. Furthermore, the prepared nanoparticles were almost completely separated within 15 min with the help of a bar magnet as shown in inset Fig. 4. Therefore, in addition to good photocatalytic activity of the nanoparticles, their excellent feasible recovery property can bring the hope for its practical application in photocatalysis. However, very small amount of particles remains suspended in the solution and could not be well separated. This may be due to the fact that the heat treatment given to the particles during the synthesis may have affected their magnetic properties.
The optical properties of ZnFe2O4 nanoparticles were investigated by UV–Vis diffuse reflectance spectroscopy as shown in Fig. 5. The optical absorption was estimated from the optical reflectance data using the Kubelka–Munk function [46]. The optical band gaps of S1 and S2 samples are 2.2 and 2.0 eV, which were calculated from the graph of (αhυ) 2 versus hυ as shown in inset of Fig. 5. It is clearly visible from Fig. 5 that the estimated band gap of ZnFe2O4 nanoparticles decreases with increasing temperature. The absorption edges of the ZnFe2O4 nanoparticles have shown a red shift, compared with their bulk counterparts which have the band gap value of 1.9 eV [47]. The shift in band gap energy of the ZnFe2O4 nanoparticles with the increasing crystallite size is due to the quantum confinement effect arising from the small size regime [48, 49].
The photocatalytic activities of the as-synthesized super paramagnetic ZnFe2O4 photocatalyst for the degradation of the MB dye were performed under visible light irradiation at room temperature. Figure 6a, b shows the UV–Vis spectra of the MB dye solution at different time intervals of the photodegradation process carried out using ZnFe2O4 nanoparticles prepared at 300 (S1) and 600 °C (S2), respectively. It is observed from Fig. 6 that MB has a characteristic absorption peak at 664 nm, which decreases with the increase of irradiation time. Figure 6c displays the photodegradation rates of dyes for the samples S1 and S2. A high percentage (90 %) of MB was degraded in 360 min using sample S1, whereas only 83 % of MB degradation was observed when the same photodegradation process was carried out using sample S2 as catalyst. This might be due to the fact that sample S1 may have higher surface energy than S2 because of the presence of smaller sized nanoparticles (17 nm) than S2, which has 20 nm sized nanoparticles. Essentially, high rates of adsorption of dye molecules onto the photocatalyst’s surface result in the enhancement of photodegradation process. As the probability of adsorption increases with the increase of surface energy, therefore a high photocatalytic activity is exhibited by the sample S1.
The absorption spectra of MB solution after irradiated by the visible light for various duration under the presence of ZnFe2O4 catalyst: a S1, b S2, c The correlation between the concentration of MB solution and the irradiation time, d Fitted pseudo first-order kinetics for the degradation of MB over for the ZnFe2O4 nanoparticles S1 and S2
The first order kinetics rate for the photodegradation reaction is derived from the equation,
Here k is the apparent reaction constant. Co is the initial concentration of MB and C is the concentration of MB at different reaction time. Kinetics of photodegradation is analyzed by plotting 1n(C/Co) as a function of time (Fig. 6d). The rate constant evaluated for sample S1 and S2 from the slope of 1n(C/Co) versus time graph, are 0.250172 and 0.21131 min−1 respectively.
Usually, well crystalline particles with high absorption in the visible region show higher photocatalytic activity. But interestingly, in this study S1 particle having 2.2 band gap, resembling amorphous material has shown high degradation efficiency than S2. The difference arises from the fact that sample S2 was calcined at 600 °C which is considerably a higher temperature than 300 °C, the temperature at which sample S1 was given the heat treatment. The results suggest that the calcination of catalyst at elevated temperature reduces its activity in spite of its improvement in crystallinity. Calcination increases the crystallite size but reduces the surface energy which results in the loss of surface active sites. This loss of active sites is mainly responsible for the decrease of photocatalytic activity.
Conclusion
Super paramagnetic ZnFe2O4 nanoparticles were successfully prepared by surfactant assisted hydrothermal method. The powder XRD analysis revealed the high purity and single phase of ZnFe2O4 nanoparticles. The average crystallite sizes of 17 and 20 nm were obtained for the ZnFe2O4 nanoparticles, calcined at 300 and 600 °C, respectively. The formation of octahedral and tetrahedral spinel phases of ZnFe2O4 were confirmed by the FTIR spectra. The optical band gap energies for the synthesized nanoparticles at 300 and 600 °C were observed to be 2.2 and 2.0 eV, respectively. A catalytic efficiency of 90 % for the photodegradation of MB was achieved in 360 min. Furthermore, all the prepared nanoparticles exhibited super paramagnetic behavior and could be easily separated using a magnet. The present work successfully explores the potential use of ZnFe2O4 nanoparticles as a monocomponent visible-light photocatalyst in environmental remediation.
References
M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas, and A. M. Mayes (2008). Nature 452, 301.
Manoj Pudukudy and Zahira Yaakob (2014). Appl. Surf. Sci. 292, 520.
Manoj Pudukudy and Zahira Yaakob (2013). Superlattices Microstruct. 63, 47.
B. Neppolian, C. Wang, and M. Ashokkumar (2014). Ultrason. Sonochem. 21, 1948.
Manoj Pudukudy, Ain Hetieqa, and Zahira Yaakob (2014). Appl. Surf. Sci. 319, 221.
T. Ochiai, H. Nanba, T. Nakagawa, K. Masuko, K. Nakata, T. Murakami, R. Nakano, M. Hara, Y. Koide, T. Suzuki, M. Ikekita, Y. Morito, and A. Fujishima (2012). Catal. Sci. Technol. 2, 76.
Manoj Pudukudy, Zahira Yaakob, Ramesh Rajendran, and Thushara Kandaramath (2014). Reac. Kinet. Mech. Cat. 112, 527.
S. Ganesh Babu, R. Vinoth, B. Neppolian, D. D. Dionysiou, and M. Ashokkumar (2015). J. Hazard. Mater. 291, 83.
Manoj Pudukudy and Zahira Yaakob (2014). Solid. State. Sci. 30, 78.
S. Ganesh Babu, R. Vinoth, P. Surya Narayana, Detlef Bahnemann, and B. Neppolian (2015). APL Materials 3, 104415.
Manoj Pudukudy and Zahira Yaakob (2014). Chem. Pap. 68, 1087.
Ramesh Rajendran, Manoj Pudukudy, Subramaniam Sohila, Zahira Yaakob, Muhammad Syukri, and Abd Rahaman (2014). J. Mater. Sci. : Mater. Electron. 25, 4755.
Manoj Pudukudy and Zahira Yaakob (2015). J Clust Sci. 26, 1187.
R. Rameshbabu, Niraj Kumar, A. Karthigeyan, and B. Neppolian (2016). Mater. Chem. Phys. 181, 106.
J. Popplewell and L. Sakhnini (1995). J. Magn. Magn. Mater. 149, 72.
R. S. Molday and D. Mackenzie (1982). J. Immunol. Methods. 52, 353.
L. Gunther (1990). Phys. World. 3, 28.
H. H. Kung and M. C. Kung (1985). Adv. Catal. 33, 159.
C. W. Jung and P. Jacobs (1995). Magn. Reson. Imaging. 13, 661.
C. V. G. Reddy, S. V. Manorama, and V. J. Rao (2000). J. Mater. Sci. Lett. 19, 775.
M. G. Naseri, E. B. Saion, M. Hashim, A. H. Sharri, and H. A. Ahangar (2011). Solid. State. Commun. 151, 1031.
G. Zhang, C. Li, F. Cheng, and J. Chen (2007). Sens. Actuators. B: Chem. 120, 403.
Ibrahim Sharifi, H. Shokrollahi, and S. Amiri (2013). J. Magn. Magn. Mater. 324, 903.
F. Li, H. Wang, L. Wang, and J. Wang (2007). J. Magn. Magn. Mater. 309, 295.
A. Moser, K. Takano, D. T. Margulies, M. Albrecht, Y. Sonobe, Y. lkeda, S. Sun, and E. E. Fullerton (2002). J. Phys. D: Appl. Phys. 35, R157.
J. Bai and J. P. Wang (2005). Appl. Phys. Lett. 87, 152502.
J. Qiu, C. Wang, and M. Gu (2004). Mater. Sci. Eng. B. 112, 1.
M. Wang, A. I. Zhihui, and L. Zhang (2008). J. Phys. Chem. C. 112, 13163.
M. H. Habibi and A. H. Habibi (2013). J. Therm. Anal. Calorim. 113, 843.
P. Yaseneva, M. Bowker, and G. Hutchings (2011). Phys. Chem. Chem. Phys. 13, 18609.
A. Shanmugavani, R. KalaiSelvan, Samar Layek, and C. Sanjeeviraja (2014). J. Magn. Magn. Mater. 354, 363.
F. Grasset, N. Labhsetwar, D. Li, D. C. Park, N. Saito, H. Haneda, O. Cador, T. Roisnel, S. Mornet, E. Duguet, J. Portier, and J. Etourneau (2002). Langmuir. 18, 8209.
I. Soibam, S. Phanjoubam, H. B. Sharma, H. N. K. Sarma, and C. Prakash (2009). Physica B. 404, 3839.
S. Dasgupta, J. Das, J. Eckert, and I. Manna (2006). J. Magn. Magn. Mater. 306, 9.
M. Sertkol, Y. Koseoglu, A. Baykal, H. Kavas, and A. C. Basaran (2009). J. Magn. Magn. Mater. 321, 157.
C. Liu, Y. Ni, L. Zhang, F. Guo, and T. Wu (2014). RSC Adv. 4, 47402.
A. Sutka, R. Parna, J. Kleperis, T. Kaambre, I. Pavlovska, V. Korsaks, K. Malnieks, L. Grinberga, and V. Kisand (2014). Phys. Scr. 89, 044011. 8 pp.
X. Wang, L. Chen, Q. Fan, J. Fan, G. Xu, M. Yan, M. J. Henderson, J. Courtois, and K. Xiong (2015). J. Alloys Compd. 652, 132.
R. Rameshbabu, R. Ramesh, S. Kanagesan, A. Karthigeyan, and S. Ponnusamy (2013). J. Mater. Sci.: Mater. Electron. 24, 4279.
Niraj Kumar, P. Dineshkumar, R. Rameshbabu, and A. Sen (2015). Mater. Lett. 158, 309.
Y. P. Sui, X. F. Huang, Z. Y. Ma, W. Li, F. Qiao, K. Chen, and K. J. Chen (2003). J. Phys.: Condens. Matter. 15, 5793.
V. A. M. Brabers (1969). Phys. Stat. Sol. 33, 563.
G. F. Goya and H. R. Rechenberg (1999). J. Magn. Magn. Mater. 196–197, 191.
S. Ayyappan, S. P. Raja, C. Venkateswaran, J. Philip, and B. Raj (2010). Appl. Phys. Lett. 96, 143106.
F. S. Li, H. B. Wang, L. Wang, and J. B. Wang (2007). J. Magn. Magn. Mater. 309, 295.
Q. Li, R. C. Xie, Y. W. Li, E. A. Mintz, and J. K. Shang (2007). Environ. Sci. Technol. 41, 5050.
X. Y. Li, Y. Hou, Q. D. Zhao, W. Teng, X. J. Hu, and G. H. Chen (2011). Chemosphere. 82, 581.
C. H. B. Ng and W. Y. Fan (2006). J. Phys. Chem. B. 110, 20801.
B. Liu and H. C. Zeng (2004). J. Phys. Chem. B. 108, 5867.
Acknowledgments
The authors would like to thank SRM University for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rameshbabu, R., Neppolian, B. Surfactant Assisted Hydrothermal Synthesis of Superparamagnetic ZnFe2O4 Nanoparticles as an Efficient Visible-Light Photocatalyst for the Degradation of Organic Pollutant. J Clust Sci 27, 1977–1987 (2016). https://doi.org/10.1007/s10876-016-1057-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1057-0