Abstract
Magnetic and magnetocaloric properties of polycrystalline (1−x)La0.65Ca0.35MnO3/xCr2O3(0≤x ≤ 0.3) composites were investigated. Our composites were elaborated using the solid-state reaction at high temperature. Polycrystalline La0.65Ca0.35MnO3 (LCMO) was synthesized using the solid-state reaction at high temperature while for Cr2O3 we used a commercial product. X-ray diffraction patterns show that the parent compound La0.65Ca0.35MnO3 is single phase without any detectable impurity and crystallizes in the orthorhombic structure with Pbnm space group. Magnetocaloric effect of our composite materials in the vicinity of the magnetic transition temperatures was investigated. The maximum of the magnetic entropy change \(|{\Delta } S_{M}^{\max }|\) is found to be 5.5, 5.12, 4.5, and 3.12 J kg−1 K−1 for x = 0,0.1,0.2, and 0.3 under a magnetic field change of 5 T, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, the search for new materials with enhanced magnetocaloric effect (MCE) for their utilization in room temperature magnetic refrigerators is a very active field of research due to its lower energy consumption and environmental-friendly character. The magnetocaloric effect was first discovered by Warburg in 1881 [1], Deby in 1926 [2], and Giauque in 1927 [3] independently pointed out that ultra-low temperature could be reached through the reversible temperature change of paramagnetic salts with the alternation of magnetic field and first foresaw the technological potential of this effect. Magnetic refrigeration near room temperature is of special interest because of its great social effect and economical benefit. The prototype magnetic material available for room temperature magnetic refrigeration is gadolinium Gd. At the Curie temperature T C of 294 K, Gd undergoes a second-order paramagnetic–ferromagnetic transition. The MCE and the heat capacity of Gd have been studied in many research activities [4]. Recently, large values of MCE are observed in the perovskite manganese oxides [5–10]. The research is very interesting in the system La1−x Ca x MnO3 which is characterized by rather large values of MCE and adjustable phase transition temperatures [5, 11–13]. For La0.65Ca0.35MnO3 elaborated using the solid–solid reaction method at high temperature, the \(|{\Delta } S_{M}^{\max }|\) under an applied field of 5 T reaches 5.5 J kg−1 K−1, around its Curie temperature T C=265 K [14]. Moreover, the presence of magnetic multiphases broadens the ΔS M (T) curves and enhances consequently the relative cooling power (RCP) [15–20]. In this context, several magnetocaloric studies have been performed recently on composites based on manganites in order to enhance the physical properties comparing to manganites [21–25]. In our study, we elaborated polycrystalline composites based on La0.65Ca0.35MnO3 manganites with Cr2O3 oxide at several mass fractions and studied their magnetic and magnetocaloric properties of (1–x)La0.65Ca0.35MnO3/xCr2O3 with x = 0, 0.1, 0.2, and 0.3.
2 Experiments
2.1 Synthesis
The composites (1−x)La0.65Ca0.35MnO3/xCr2O3 (x = 0, 0.1, 0.2, and 0.3) were synthesized in two stages. First, we elaborated La0.65Ca0.35MnO3 sample using the ceramic route at high temperatures, stoichiometric amounts of dried La2O3, CaCO3, and MnO2, with high purity equal to 99.9 %, according to the following equation:
were intimately mixed in an agate mortar for 45 min; the obtained powder was pressed into pellets of about 1 mm thickness and 13 mm diameter and sintered at 1000 °C in air for 24 h. The sample undergoes several cycles of grinding–pelleting–annealing between 1000 and 1200 °C during 48 h for each annealing. The obtained sample was characterized by X-ray powder diffraction at room temperature with CuK α radiation. Structural analysis was made using the standard Rietveld technique [26, 27]. Secondly, we have prepared three composites based on La0.65Ca0.35MnO3 and Cr2O3 for different mass fractions. The composites were prepared by thoroughly mixing 90, 80, and 70 % of La0.65Ca0.35MnO3 with 10, 20, and 30 % of Cr2O3, respectively, and then were sintered at 1000 °C for 4 h. The microstructure was studied by scanning electron microscope (SEM). The density of the as-produced pellets was measured with a Micromeritics AccuPyc 1330 helium pycnometer. Magnetization measurements versus magnetic applied field up to 5 T were performed using a vibrating sample magnetometer in the temperature range 200–350 K., Magnetocaloric effect |ΔS M (T)| at several magnetic field change up to 5 T were deduced from the M(H) curves at several temperatures.
3 Results and Discussion
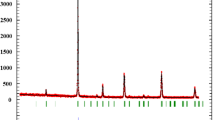
The X-ray diffraction (XRD) patterns of the parent compound La0.65Ca0.35MnO3 have been recorded at room temperature; the data have been refined using Rietveld’s profile-fitting method. Figure 1 shows the X-ray diffraction patterns at 300 K for La0.65Ca0.35MnO3 sample including the observed and calculated profiles as well as the difference profile. Our parent compound is single phase without any detectable impurity and crystallizes in the orthorhombic structure with Pbnm space group. Figure 2 shows the X-ray diffraction patterns at 300 K for our three composites (1−x)La0.65Ca0.35MnO3/xCr2O3 with x = 0.1, 0.2, and 0.3. As we can observe in this figure, there is no interaction between the parent compound and Cr2O3 oxide.
3.1 Microstructural Analysis
To investigate the microstructural properties of our synthesized samples XRD and SEM analyses were jointly used. With MAUD software, the model line broadening selected was “popa LB” combined with the isotropic model size strain. The instrument broadening was corrected using the XRD pattern of a Si standard. Figure 3 shows the SEM image obtained for the composites (1−x)La0.65Ca0.35MnO3/xCr2O3, (x = 10 and 30 %). The SEM image shows that the Cr2O3 oxide occupies the grain boundary of La0.6Ca0.35MnO3.
3.2 Magnetic and Magnetocaloric Properties
We performed magnetization measurements versus magnetic applied field up to 5 T at several temperatures in the range 200–350 K for the parent compound La0.65Ca0.35MnO3 and the three composites (1−x)La0.65Ca0.35MnO3/xCr2O3 (x = 10, 20, and 30 %). Figure 4 shows the magnetic field dependence of magnetization up to 5 T at several temperatures (1−x)La0.65Ca0.35MnO3/xCr2O3 (x = 10 and 20 %). At low temperatures, the magnetization M increases sharply with magnetic applied field for H<0.5 T and then saturates above 1 T. This result confirms well the ferromagnetic behavior at low temperatures of our composites. The hysteresis cycles exhibit a sigmoidal shape; this is due to the presence of structural distortions inside the grains. The small hysteresis losses are properties generally desired in soft magnetic materials. Figure 5 shows the hysteresis cycles for (1−x)La0.65Ca0.35MnO3/xCr2O3 (x = 10, 20, and 30 %) at different temperatures. The different values of the coercivity and the saturated magnetization for our composites are summarized in Table 1. The magnetic entropy change ΔS M (T) has been deduced from the isothermal magnetization measurements. It can be evaluated according to Maxwell’s relations, using the following equation:
where H max is the maximal value of the magnetic applied field. In practice, the relation is approximated as [19, 20]
where M i and M i+1 are the experimental values of magnetization measured at temperatures T i and T i+1, respectively, under a magnetic field H i . Figure 6 shows the variation of −ΔS M as a function of temperature at different magnetic field changes for our composites. As expected, −ΔS M reaches its maximum around the transition temperature and increases with the magnetic applied field change. At 5 T, the maximal value of the magnetic entropy change \(|{\Delta } S_{M}^{\max }|\) is found to be 5.5, 5.12, 4.5, and 3.12 J kg−1 K−1 for x = 0, 0.1, 0.2, and 0.3, respectively. We do not notice a significant effect of the mass fraction of Cr2O3 oxide on the maximum of ΔS M (T) while temperature of this maximum is shifted to higher values. From a cooling perspective, it is important to consider the refrigeration capacity which depends on both the magnetic entropy change and its temperature. The magnetic cooling efficiency of a magnetocaloric material can be evaluated through the relative cooling power (RCP) defined as
were δ T FWHM is the full with at half maximum of the ΔS M (T) curve. The RCP value is found to be 238.3, 227.4, 193.7, and 145.1 J kg−1 for x = 0, 10, 20, and 30 %, respectively, under a magnetic field change of 5 T. The RCP decreases with increasing the Cr2O3 amount in our composites. In this work, we have used the Landau theory of phase transitions to modelize the magnetocaloric effect in ferromagnetic materials [28]. In a recent framework, Gibbs free energy can be expressed by
where the coefficients A, B, and C are temperature-dependent parameters usually known as Landau coefficients. For energy minimization in Eq. 4, the equation of state is given by
The corresponding magnetic entropy is obtained from differentiation of the magnetic part of the free energy with respect to temperature
A’(T), B’(T), and C’(T) are the temperature derivatives of the expansion coefficients. The same result is obtained using the equation of state and the integration of Maxwell relations. Nevertheless, in order to compare with the magnetic entropy change (ΔS (T, H)) obtained for experimental measurements, we should also calculate the temperature dependence of the magnetic entropy without magnetic field S(T, H = 0). Therefore, the theoretical magnetic entropy change ΔS (T, H) is
Here, the value of M 0 can be obtained by extrapolating the magnetization at H = 0. As shown in Fig. 7, the circles represent the calculated magnetic entropy change by using Eq. 7 and the squares represent the experimental data. V.S Amaral and J.S Amaral [28] showed that the nature of the parameter B (T) takes an important role in determining ΔS (T, H). For LCMO + 30 % Cr2O3, B (T) is observed to be negative below T C and positive above T C. This change from negative to positive suggests that the phase transition in the sample is of second order [29]. B (T) parameter increases with temperature (B’=d B/d T > 0), the magnetic entropy is then larger and the peak broadened [28]. The variations of A (T), B (T), and C (T) values for LCMO + 30 % Cr2O3 are shown in the inset of Fig. 7. It may be seen that the agreement between the theoretical and the measured data is very satisfactory, considering the fact that the present model does not take into account the influence of the Jahn–Teller effect and the exchange interactions on the magnetic properties of manganites. Nevertheless, the analysis clearly demonstrates the importance of magnetoelastic coupling and electron interaction in understanding the magnetocaloric properties of lanthanum manganites.
4 Conclusion
We can observe from DRX diffractograms that Cr2O3 does not react with the parent compound, and it forms a secondary phase located on the grain boundaries, as revealed from SEM images. The Cr2O3 slightly affects both T C and |−ΔS M |, but it induces a decrease in the remanent magnetization.
References
Warburg, E.: Ann. Phys. 13, 141 (1881)
Debye, P.: Ann. Phys. 81, 1154 (1926)
Giauque, W.F.: J. Am. Chem. Soc. 49, 1864 (1927)
Yu, B.F., Gao, Q., Zhang, B., Meng, X.Z., Chen, Z.: Int. J. Refrig. 26, 622 (2003)
Zhensheng, P.: J. Rare Earths 22, 232 (2004)
Gschneidner, KA. Jr, Pecharsky, V.K., Tsokol, A.O.: Rep. Prog. Phys. 68, 1479 (2005)
Cheikhrouhou-Koubaa, W., Koubaa, M., Cheikhrouhou, A.: J. Alloys Compd. 470, 42 (2009)
Koubaa, M., Cheikhrouhou-Koubaa, W., Cheikhrouhou, A.: J. Phys. Chem. Solids 70, 326 (2009)
Cheikhrouhou-Koubaa, W., Koubaa, M., Cheikhrouhou, A.: J. Phys. Procedia 2, 989 (2009)
Phan, M.H., Yu, S.C.: J. Magn. Magn. Mat. 308, 325 (2007)
Schiffer, P.E., Ramirez, A.P., Bao, W., Cheong, S.W.: Phys. Rev. Lett. 75, 3336 (1995)
Tang, W., Lu, W., Luo, X., Wang, B., Zhu, X., Song, W., Yang, Z., Sun, Y.: J. Magn. Magn. Mat. 322, 2360 (2010)
Krichene, A., Boujelben, W., Cheikhrouhou, A.: J. Alloys Compd. 550, 75 (2013)
Koubaa, M., Cheikhrouhou-Koubaa, W., Cheikhrouhou, A., Haghiri-Gosnet, A.M.: J. Phys. B 403, 2477 (2008)
Caballero-Flores, R., Franco, V., Conde, A., Knipling, K.E., Willard, M.A.: Appl. Phys. Lett. 98, 102505 (2011)
Smaili, A., Chahine, R.: Appl. Phys. Lett. 81, 824 (1997)
de Oliveira, I.G., Von Ranke, P.J., Nobrega, E.P.: J. Magn. Magn. Mat. 261, 112 (2003)
Chaturvedi, A., Stefanoski, S., Phan, M.H., Nolas, G.S., Srikanth, H.: Appl. Phys. Lett. 99, 162513 (2011)
Paticopoulos, S.C., Caballero-Flores, R., Franco, V., Blasquer, J.S., Conde, A., Knipling, K.E., Willard, M.A.: Solid State Commun. 152, 1590 (2012)
Romero Muniz, C., Franco, V., Conde, A.: Appl. Phys. Lett. 102, 082402 (2013)
Pekala, M., Pekala, K., Drozd, V., Staszkie Wicz, K., Fagnard, J.F., Vanderbemden, P.: J. Appl. Phys. 112, 023906 (2012)
Panwar, N., Coondoo, I., Agarwal, S.K.: Mater. Lett. 64, 2638 (2010)
Lim, S.P., Tang, G.D., Li, Z.Z., Qi, W.H., Ji, D.H., Li, Y.F., Chen, W., Hou, D.L.: J. Alloys Compd. 509, 2320 (2011)
Yang, H., Cao, Z.E., Shen, X., Xian, T., Feng, W.J., Jiang, J.L., Feng, Y.C., Wei, Z.Q., Dai, J.F.: J. Appl. Phys. 106, 104317 (2011)
Yuan, G.L., Liu, J.M., Liu, Z.G., Du, Y.W., Chan, H.L.W., Choy, C.L.: Mater. Chem. Phys. 75, 161 (2002)
Rietveld, H.M.: J. Appl. Cryst. 2, 65 (1969)
Roisnel, T., Rodriguez-Carvayal, J.: Computer progasm Fullprof, LLB-LCSIM, May (2003)
Amaral, V.S., Amaral, J.S.: J. Magn. Magn. Mater. 272–276, 2104 (2004)
Anwar, M.S., Kumar, S., Ahmed, F., Arshi, N., Kim, G.W., Koo, B.H.: J. Korean Phys. Soc. 60, 1587 (2012)
Acknowledgments
This work has been supported by the Tunisian Ministry of Higher Education, Scientific Research and information and communication technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marzouki-Ajmi, A., Cheikhrouhou-Koubaa, W., Koubaa, M. et al. Structural, Magnetic and Magnetocaloric Study of Polycrystalline (1−x)La0.65Ca0.35MnO 3 /xCr2O3 Composites. J Supercond Nov Magn 28, 1065–1070 (2015). https://doi.org/10.1007/s10948-014-2892-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-014-2892-6