Abstract
This work is devoted to investigating the magnetic and magnetocaloric properties of La0.45Nd0.25Sr0.3MnO3-CuO composite prepared by the solid-state reaction with various concentration of CuO (2 wt.%, 5 wt.%, and 10 wt.%). The X-ray diffraction (XRD) and the scanning electron microscopy (SEM) techniques were used to identify the obtained structural phases and microstructural morphologies in the prepared pellets. A second-order transition was observed with critical a temperature around 294 K for 2 wt.% and 10 wt.% and 321 K for 5 wt.% of the CuO phase in the composite. Magnetic entropy changes in La0.45Nd0.25Sr0.3MnO3-CuO composites were calculated using the Maxwell approximation around the magnetic transition. The highest values of magnetic entropy change were found for 5 wt.% of CuO in La0.45Nd0.25Sr0.3MnO3-CuO pellet composites which is attributed to the localization of the copper oxide in the grains boundaries of La0.45Nd0.25Sr0.3MnO3 perovskite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnetic refrigeration based on the magnetocaloric effect (MCE) has taken a great interest over the past decade as a promising technology to replace the conventional cooling techniques. Since the discovery of the magnetocaloric effect (MCE) in 1881 [1], magnetic materials have stimulated theoretical and experimental groups to look for ferromagnetic materials with magnetic transition near room temperature [2,3,4,5]. The gadolinium metal and its derivative intermetallic compounds have been widely studied fundamentally and proved their efficiency in functional devices [6, 7]. Although, their difficult synthesis route with high cost and their easy oxidation and corrosion in the presence of water limited their use in a wide range of applications. Recently, manganite perovskite materials with the general foruma Ln1-xAxMnO3 (Ln = La, Nd… A = Ca, Sr, Br…) have attracted much attention because of their large magnetocaloric effect [8,9,10,11,12]. Several low cost and simple synthesis methods have been used to prepare rare-earth manganite perovskite such as coprecipitation, sol-gel method, solid-state reaction, and others [13,14,15,16,17]. Besides the optimization of the synthesis parameters, a number of studies have been devoted to improve the structural and magnetic, as well as the magnetocaloric, properties of rare-earth manganite perovskites and their applicability as refrigerants. Cherif et al. [18] have studied the magnetocaloric effect in Nd-doped LaSrMnO3 perovskite prepared by solid-state reaction and it was found that the magnetic entropy change increases with Nd insertion in the (La0.7-xNdx)Sr0.3MnO3 material. Shalpa et al. [19] studied the crystallographic and magnetic properties of Nd-doped La0.7Sr0.3MnO3 nanoparticles synthesized using sol-gel method with two different annealing temperatures. Cr-doped La0.7Sr0.3MnO3 pollycrystalline compounds have been synthesized by Kallel et al. [20], using solid-state reaction and large magnetic entropy change and RCP of 1.203 J/kg. K and 59 J/kg, respectively, have been obtained for La0.7Sr0.3Mn0.8Cr0.2O3. In our previous studies, the enhancement of the magnetic and magnetocaloric properties of manganite perovskites through composite such as Pr2/3Sr1/3MnO3/CuO and (La0.45Nd0.25)Sr0.3MnO3/CuO has been reported for powder composite [21, 22]. Hamad et al. [23] have shown a uniform magnetic entropy change distribution in La0.7Sr0.3MnO3/Ta2O5 composites. Nano- and polycrystalline composites with two manganite perovskite materials La0.7Ca0.3MnO3/La0.8Sr0.2MnO3 have been prepared by Pekala et al. [24]. It was found that the magnetic field dependence of magnetic entropy change is stronger for nanocrystalline composite, while a remarkable enhancemenet of the RCP is obtained for polycrystalline composite. In this work, our main aim is to investigate the structural, magnetic, and magnetocaloric properties of (1-x)(La0.45Nd0.25)Sr0.3MnO3/xCuO composite with x = 2 wt.%, 5 wt.%, and 10 wt.% with a pellet shape prepared by a simple solid-state reaction.
2 Experiment
La2O3, Nd2O3, SrO, Mn2O3, MnO2 metal oxides with high purity (> 99.9%) are used as starting materials to synthesize (La0.45Nd0.25)Sr0.3MnO3 manganite perovskite via solid-state reaction. Stoichiometric proportions of the starting precursors were mixed and grinded for 1 h then preheated at 1173 K for 8 h, and the obtained powders were grinded again and calcined at 1473 K for 12 h. Physical mixing of the prepared manganite perovskite and commercial CuO nanoparticles (purity > 99.9%) was performed for 30 min to achieve (1-x)(La0.45Nd0.25)Sr0.3MnO3/xCuO composite with x = 2 wt.%, 5 wt.%, and 10 wt.%. to prepare the pellet composites, and polyvinyl alcohol PVA (2 wt.%) solubilized in water was added to 98 wt.% of the prepared composite. The dried powders were pressed to form pellets with a diameter of 13 ± 0.1 mm and a pressure of 10 MPa, then sintered at 1473 K for 12 h. The pellets are referred as Ps-2, Ps-5, and Ps-10 for 2 wt.%, 5 wt.%, and 10 wt.% of the CuO phase, respectively.
3 Results and Discussions
3.1 X-ray Diffraction Analysis
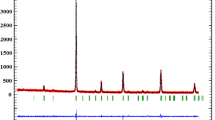
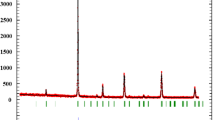
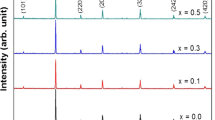
The XRD patterns of the prepared composites are presented in Fig. 1. It can be seen clearly that the obtained samples crystallize in the rhombohedral structure of the perovskite material with space group R-3c (167) and suitable for a PDF card No 01-082-1152, with presence of some peaks related to the monoclinic structure of the copper oxide (indicated with * in Fig. 1). No secondary phase to the manganite perovskite and copper oxide was detected in all composites. To confirm the crystalline phase, determine the cell parameters and the volume of cell. Rietveld refinement was performed using TOPAS Software. The obtained results are regrouped in Table 1.
3.2 SEM Analysis
In order to identify the distribution and size of particles in the obtained composites, SEM microstructural analysis is performed for all composites (1-x)(La0.45Nd0.25)Sr0.3MnO3/xCuO. Figure 2 shows the SEM micrographs of the samples. We observed two contrasted zones which prove two different chemical compositions (perovskite and copper oxide). The CuO material is located at the grain boundaries with uniform homogeneity due to the uniaxial pressure effect. This homogeneity is perfect in the Ps-5 sample; when the percent of copper oxide increase, it becomes mainly localized at the grains boundaries and the excess of CuO spreads over the surface of perovskite. The EDS (not presented) analysis of all samples gives the right chemical composition without any detection of other elements; this indicates the successful manufacture of composites.
3.3 Magnetic and Magnetocaloric Characterization
Before studying the magnetocaloric properties, the magnetization as a function of temperature under a magnetic field of 500 Oe was measured as it is presented in Fig. 2. From the (dM/dT) curve (insert Fig. 2), we can see that the prepared pellets exhibit a second-order transition to paramagnetic phase. The transition temperature remains constant (Tc = 294 K) for 2 wt.% and 10 wt.%, and we can also see that Ps-2 present a spin canted state at low temperature (below Tc) similar to that observed by Hcini et al. [25], while it increases for 5 wt.% of the CuO phase in the composite (Tc = 321 K).
The magnetic field and temperature depending of magnetization for the tree samples are measured and presented in Fig. 3. It is found that the magnetization dependence of a magnetic field up to 5 T increased linearly for temperatures above the transition temperature, which confirm the paramagnetic behavior, while the magnetizations below the transition temperature show a quick increase for low magnetic fields with a tendency to saturate for high magnetic fields that prove the ferromagnetic behavior of the composite below Tc.
The magnetic entropy change (ΔSm) is the most recommended parameter to evaluate the efficiency of magnetocaloric materials. It is calculated using the magnetic isothermal data near the vicinity of the transition temperature using the follow formula:
To study the magnetocaloric properties of the obtained (1-x)(La0.45Nd0.25)Sr0.3MnO3/xCuO pellet composites, we plotted in Fig. 4 the magnetic entropy changes. All composites have the same appearance of the entropy change as a function of the temperature; we can see that ΔSm have a maximum around the vicinity of the transition temperature and decrease with any further increase of the temperature. Thus, as the applied external magnetic field is higher, the magnetic entropy change becomes important. The maximum values of ΔSm under 5 T were found to be 3.65, 4.12, and 3.82 J/kg. K for 2 wt.%, 5 wt.%, and 10 wt.% of CuO, respectively. These results are in the same range with other perovskite or composite [26,27,28,29,30] and it is higher than some chromites [31,32,33] and ferrites oxides [34,35,36].
The higher value of the magnetic entropy change for 5 wt.% can be attributed to the good and uniform distribution of copper oxide phase in the grains boundaries compared to the non-uniform distribution of CuO neither with 2 wt.% or 10 wt.% (Fig. 5).
To better illustrate the efficiency of the prepared pellets for magnetic refrigeration, the relative cooling power (RCP) is calculated using the following formula:
Figure 6 shows the variation of the RCP as a function of the concentration of CuO in the pellet for different magnetic fields up to 5 T. An increase of the RCP was observed with increasing of magnetic field and as the proportion of CuO increased and reached a value of 242 J/kg for 10 wt.% of copper oxide. The obtained values show a good agreement with other works in the literature [15, 21, 22, 30, 37].
4 Conclusion
La0.45Nd0.25Sr0.3MnO3/CuO composites have been prepared according to the solid-state reaction followed by a mechanical pression to form pellet with a diameter of 13 ± 0.1 mm. The prepared pellets showed good properties in terms of magnetic transition around room temperature and strong magnetocaloric effect. The obtained results prove the efficiency of such composite for magnetic refrigeration.
References
Warburg, E.: Magnetische Untersuchungen. Ann. Phys. 249, 141–164 (1881). https://doi.org/10.1002/andp.18812490510
Nóbrega, E.P., De Oliveira, N.A., Von Ranke, P.J., Troper, A.: Monte Carlo calculations of the magnetocaloric effect in Gd5(SixGe1-x)4 compounds. Phys. Rev. B Condens. Matter Mater. Phys. 72, (2005). https://doi.org/10.1103/PhysRevB.72.134426
Nobrega, E.P., de Oliveira, N.A., von Ranke, P.J., Troper, A.: Magnetocaloric effect in ( Gd x Tb 1 − x ) 5 Si 4 by Monte Carlo simulations. Phys. Rev. B. 74, (2006). https://doi.org/10.1103/physrevb.74.144429
Pecharsky, V.K., Gschneidner, K.A.: Giant magnetocaloric effect in Gd5 (Si2 Ge2). Phys. Rev. Lett. 78, 4494–4497 (1997). https://doi.org/10.1103/PhysRevLett.78.4494
Chen, J., Shen, B.G., Dong, Q.Y., Sun, J.R.: Giant magnetic entropy change in antiferromagnetic DyCuSi compound. Solid State Commun. 150, 1429–1431 (2010). https://doi.org/10.1016/j.ssc.2010.05.017
Luo, Q., Zhao, D.Q., Pan, M.X., Wang, W.H.: Magnetocaloric effect in Gd-based bulk metallic glasses. Appl. Phys. Lett. 89, (2006). https://doi.org/10.1063/1.2338770
Chang, L.X., Xiong, G., Wang, L., Cheng, P., Zhao, B.: A 24-Gd nanocapsule with a large magnetocaloric effect. Chem. Commun. 49, 1055–1057 (2013). https://doi.org/10.1039/c2cc35800j
Das, S., Dey, T.K.: Magnetic entropy change in polycrystalline La1-xKxMnO3 perovskites. J. Alloys Compd. 440, 30–35 (2007). https://doi.org/10.1016/j.jallcom.2006.09.051
Sun, Y., Tong, W., Zhang, Y.: Large magnetic entropy change above 300 K in La0.67Sr0.33Mn0.9Cr0.1 O3. J. Magn. Magn. Mater. 232, 205–208 (2001). https://doi.org/10.1016/S0304-8853(01)00263-3
Phan, M.H., Tian, S.B., Hoang, D.Q., Yu, S.C., Nguyen, C., Ulyanov, A.N.: Large magnetic-entropy change above 300 K in CMR materials. J. Magn. Magn. Mater. 309–311 (2003). https://doi.org/10.1016/S0304-8853(02)01151-4
Sun, Y., Xu, X., Zhang, Y.: Large magnetic entropy change in the colossal magnetoresistance material La2/3Ca1/3MnO3. J. Magn. Magn. Mater. 219, 183–185 (2000). https://doi.org/10.1016/S0304-8853(00)00433-9
Guo, Z.B., Du, Y.W., Zhu, J.S., Huang, H., Ding, W.P., Feng, D.: Large magnetic entropy change in perovskite-type manganese oxides. Phys. Rev. Lett. 78, 1142–1145 (1997). https://doi.org/10.1103/PhysRevLett.78.1142
Uskoković, V., Drofenik, M.: Four novel co-precipitation procedures for the synthesis of lanthanum-strontium manganites. Mater. Des. 28, 667–672 (2007). https://doi.org/10.1016/j.matdes.2005.07.002
Ravi, S., Karthikeyan, A.: Effect of calcination temperature on La0.7Sr0.3MnO3 nanoparticles synthesized with modified sol-gel route. Phys. Procedia. 54, 45–54 (2014). https://doi.org/10.1016/j.phpro.2014.10.035
Kharrat, A.B.J., Boujelben, W.: Magnetic, magnetocaloric and correlation with critical behavior in Pr0.8Sr0.2MnO3 compound prepared via solid-state reaction. J. Low Temp. Phys. 197, 357–378 (2019). https://doi.org/10.1007/s10909-019-02223-5
Gibbons, K.E., Jones, M.O., Blundell, S.J., Mihut, A.I., Gameson, I., Edwards, P.P., Miyazaki, Y., Hyatt, N.C., Porch, A.: Rapid synthesis of colossal magnetoresistance manganites by microwave dielectric heating. Chem. Commun. 159–160 (2000). https://doi.org/10.1039/a907677h
López-Trosell, A., Schomäcker, R.: Synthesis of manganite perovskite Ca0.5Sr0.5MnO 3 nanoparticles in w/o-microemulsion. Mater. Res. Bull. 41, 333–339 (2006). https://doi.org/10.1016/j.materresbull.2005.08.015
Cherif, K., Zemni, S., Dhahri, J., Dhahri, J., Oumezzine, M., Ghedira, M., Vincent, H.: Magnetocaloric effect in the doped perovskite manganese oxide La 0.7-xNdxSr0.3MnO3 (x = 0.42, 0.56 and 0.7). J. Alloys Compd. 396, 29–33 (2005). https://doi.org/10.1016/j.jallcom.2004.12.008
Shlapa, Y., Solopan, S., Bodnaruk, A., Kulyk, M., Kalita, V., Tykhonenko-Polishchuk, Y., Tovstolytkin, A., Belous, A.: Effect of synthesis temperature on structure and magnetic properties of (La,Nd)0.7Sr0.3MnO3 Nanoparticles. Nanoscale Res. Lett. 12, (2017). https://doi.org/10.1186/s11671-017-1884-4
Kallel, N., Dhahri, J., Zemni, S., Dhahri, E., Oumezzine, M., Ghedira, M., Vincent, H.: Effect of Cr doping in La0.7Sr0.3Mn1-xCrxO3 with 0 ≤ x ≤ 0.5. Phys. Status Solidi. 184, 319–325 (2001). https://doi.org/10.1002/1521-396X(200104)184:2<319::AID-PSSA319>3.0.CO;2-K
Fkhar, L., Mounkachi, O., El Maalam, K., Hamedoun, M., Mahmoud, A., Boschini, F., El kenz, A., Ali, M.A., Hlil, E.K., Xiao, Y., Benyoussef, A.: Large magnetic entropy change in Pr2/3Sr1/3MnO3-CuO composite at room temperature. J. Supercond. Nov. Magn. 32, 3579–3585 (2019). https://doi.org/10.1007/s10948-019-5136-y
El Maalam, K., Balli, M., Habouti, S., Dietze, M., Hamedoun, M., Hlil, E.K., Es-Souni, M., El Kenz, A., Benyoussef, A., Mounkachi, O.: Composite (La0.45Nd0.25)Sr0.3MnO3/5CuO materials for magnetic refrigeration applications. J. Magn. Magn. Mater. 449, 25–32 (2018). https://doi.org/10.1016/j.jmmm.2017.09.076
Hamad, M.A.: Magnetocaloric effect in La0.7Sr0.3MnO3/Ta2O5 composites. J. Adv. Ceram. 2, 213–217 (2013). https://doi.org/10.1007/s40145-013-0062-0
Pekała, M., Pekała, K., Drozd, V., Staszkiewicz, K., Fagnard, J.F., Vanderbemden, P.: Magnetocaloric and transport study of poly- and nanocrystalline composite manganites La 0.7Ca 0.3MnO 3/La 0.8Sr 0.2MnO 3. J. Appl. Phys. 112, (2012). https://doi.org/10.1063/1.4739262
Hcini, S., Boudard, M., Zemni, S., Oumezzine, M.: Effect of Fe-doping on structural, magnetic and magnetocaloric properties of Nd0.67Ba0.33Mn1-xFexO3 manganites. Ceram. Int. 40, 16041–16050 (2014). https://doi.org/10.1016/j.ceramint.2014.07.140
Tang, W., Lu, W., Luo, X., Wang, B., Zhu, X., Song, W., Yang, Z.: Particle size effects on La0.7Ca0.3MnO3: size-induced changes of magnetic phase transition order and magnetocaloric study. J. Magn. Magn. Mater. 322, 2360–2368 (2010). https://doi.org/10.1016/j.jmmm.2010.02.038
Xi, S., Lu, W., Sun, Y.: Magnetic properties and magnetocaloric effect of La 0.8Ca 0.2MnO 3 nanoparticles tuned by particle size. J. Appl. Phys. 111, 0–9 (2012). https://doi.org/10.1063/1.3699037
Tozri, A., Dhahri, E., Hlil, E.K.: Magnetic transition and magnetic entropy La0.8Pb 0.1MnO3 andLa0.8Pb0.1Na 0.1MnO3. Mater. Lett. 64, 2138–2141 (2010). https://doi.org/10.1016/j.matlet.2010.06.051
Phan, T.L., Ho, T.A., Thang, P.D., Tran, Q.T., Thanh, T.D., Phuc, N.X., Phan, M.H., Huy, B.T., Yu, S.C.: Critical behavior of Y-doped Nd0.7Sr0.3MnO3manganites exhibiting the tricritical point and large magnetocaloric effect. J. Alloys Compd. 615, 937–945 (2014). https://doi.org/10.1016/j.jallcom.2014.06.107
Fan, J., Pi, L., Zhang, L., Tong, W., Ling, L., Hong, B., Shi, Y., Zhang, W., Lu, D., Zhang, Y.: Magnetic and magnetocaloric properties of perovskite manganite Pr 0.55Sr0.45MnO3. Phys. B. 406, 2289–2292 (2011). https://doi.org/10.1016/j.physb.2011.03.056
Biswal, H., Singh, V., Nath, R., Angappane, S., Sahu, J.R.: Magnetic and magnetocaloric properties of LaCr1-xMnxO3 (x = 0, 0.05, 0.1) Hrudananda. Ceram. Int. 3, (2019). https://doi.org/10.1016/j.ceramint.2019.07.311
Bora, T., Ravi, S.: Bipolar switching of magnetization and tunable exchange bias in NdCr1− x Mn x O3 (x= 0.0–0.30). J. Appl. Phys. 3, (2014). https://doi.org/10.1063/1.4891682
Kumar, S., Coondoo, I., Vasundhara, M., Patra, A.K., Kholkin, A.L., Panwar, N.: Magnetization reversal behavior and magnetocaloric effect in SmCr0.85Mn0.15O3 chromites. J. Appl. Phys. 21, (2017). https://doi.org/10.1063/1.4974737
Anwar, M.S., Ahmed, F., Koo, B.H.: Enhanced relative cooling power of Ni1−xZnxFe2O4 (0.0≤x≤0.7) ferrites. Acta Mater. 71, 100–107 (2014). https://doi.org/10.1016/j.actamat.2014.03.002
El Maalam, K., Fkhar, L., Hamedoun, M., Mahmoud, A., Boschini, F., Hlil, E.K., Benyoussef, A., Mounkachi, O.: Magnetocaloric properties of zinc-nickel ferrites around room temperature. J. Supercond. Nov. Magn. 30, 2–6 (2017). https://doi.org/10.1007/s10948-016-3961-9
Oumezzine, E., Hcini, S., Baazaoui, M., Hlil, E.K., Oumezzine, M.: Structural, magnetic and magnetocaloric properties of Zn0.6-xNixCu0.4Fe2O4 ferrite nanoparticles prepared by Pechini sol-gel method. Powder Technol. 278, 189–195 (2015). https://doi.org/10.1016/j.powtec.2015.03.022
Fkhar, L., Mahmoud, A., Boschini, F., Hamedoun, M., Benyoussef, A., Hlil, E.K., Ali, M.A., Mounkachi, O.: Structural, magnetic, and magnetocaloric properties in rare earth orthochromite (Sm, Nd, and La)CrO3 for cooling product. J. Supercond. Nov. Magn. 15–17 (2019). https://doi.org/10.1007/s10948-019-05260-z
Acknowledgments
This work was supported by the MESRSFC (Ministère de l’Enseignement Supérieur, de la Recherche Scientifique et de la Formation des Cadres) in the Framework of the national program PPR under contract no. PPR/2015/57. A. Mahmoud is grateful to the Walloon region for a Beware Fellowship Academia 2015-1, RESIBAT n° 1510399.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fkhar, L., Lamouri, R., Mahmoud, A. et al. Enhanced Magnetic and Magnetocaloric Properties of La0.45Nd0.25Sr0.3MnO3/CuO Composite. J Supercond Nov Magn 33, 2543–2549 (2020). https://doi.org/10.1007/s10948-020-05509-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-020-05509-y