Abstract
Magnetic and magnetocaloric properties of polycrystalline (1 − x)La0.65Ca0.35MnO3/xFe2O3(0 ≤ x ≤ 0.09) composites were investigated. Our composites were elaborated using the solidstate reaction at high temperature. Polycrystalline La0.65Ca0.35MnO3 (LCMO) was synthesized using the solidstate reaction at high temperature while for Fe2O3 we used a commercial product. Xray diffraction patterns show that the parent compound La0.65Ca0.35MnO3 is a single phase without any detectable impurity and crystallizes in the orthorhombic structure with Pbnm space group. Magnetocaloric effect of our composite materials in the vicinity of the magnetic transition temperatures was investigated. The maximum of the magnetic entropy change \(\left | {\Delta S}_{M}^{\max }\right |\) is found to be 6.25, 6.37, 6.18 and 5.87 J kg−1 K−1 for x = 0, 0.03, 0.06, and 0.09 under a magnetic field change of 5 T respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, the search for new materials with enhanced magnetocaloric effect (MCE) for their utilization in room temperature magnetic refrigerators is a very active field of research due to its lower energy consumption and environmental friendly character. The magnetocaloric effect was first discovered by Warburg in 1881 [1], Deby in 1926 [2] and Giauque in 1927 [3] independently pointed out that ultra-low temperature could be reached through the reversible temperature change of paramagnetic salts with the alternation of magnetic field and first foresaw the technological potential of this effect. Magnetic refrigeration near room temperature is of special interest because of its great social effect and economical benefit. The prototype magnetic material available for room temperature magnetic refrigeration is gadolinium Gd. At the Curie temperature T c of 294 K, Gd undergoes a second-order paramagnetic–ferromagnetic transition. The MCE and the heat capacity of Gd have been studied in many research activities [4] Recently, large values of MCE are observed in the perovskite manganese oxides [5–10] The research is very interesting in the system La1−x Ca x MnO3 which is characterized by rather large values of MCE and adjustable phase transition temperatures [5, 11–13] For La0.65Ca0.35MnO3 elaborated using the solid–solid reaction method at high temperature, the \(\left | {\Delta S}_{M}^{\max }\right |\) under an applied field of 5 T reaches 6.2 J kg−1 K−1, around its Curie temperature T c = 265 K [14]. Moreover the presence of magnetic multiphases broadens the ΔS M (T) curves and enhances consequently the relative cooling power (RCP) [15–20] In this context, several magnetocaloric studies have been performed recently on composites based on manganites in order to enhance the physical properties comparing to manganites [21–25]. In our study we elaborated polycrystalline composites based on La0.65Ca0.35MnO3 manganites with Fe2O3 oxide at several mass fractions and studied the magnetic and magnetocaloric properties of (1 −x) La0.65Ca0.35MnO3/xFe2O3 with x = 0, 0.03, 0.06 and 0.09.
2 Experiments
2.1 Synthesis
The composites (1 − x)La0.65Ca0.35MnO3/xFe2O3(x = 0, 0.03, 0.06 and 0.09) were elaborated in two stages. At first we elaborate La0.65Ca0.35MnO3 sample using the ceramic route at high temperature, stoichiometric amounts of dried La2O3, CaCO3 and MnO2, with high purity equal to 99, 9 %, according to the following equation:
were intimately mixed in an agate mortar for 45 min; the obtained powder was pressed into pellets of about 1 mm thickness and 13 mm diameter and sintered at 1000 °C in air for 24 h. The sample undergoes several cycles of grinding–pelleting–annealing between 1000 and 1200 °C during 48 h for each annealing The obtained sample was characterized by X-ray powder diffraction at room temperature with CuK α radiation Structural analysis was made using the standard Rietveld technique [26, 27]. Secondly we have prepared three composites based on La0.65Ca0.35MnO3 and Fe2O3 for different mass fractions The composites were prepared by thoroughly mixing 97, 94, and 91 % of La0.65Ca0.35MnO3 with 3, 6 and 9 % of Fe2O3 respectively, and then were sintered at 1000 °C for 4 h. The microstructure was studied by scanning electron microscope (SEM). The density of the as-produced pellets was measured with a Micrometrics AccuPyc 1330 helium pycnometer. Magnetization measurements versus magnetic applied field up to 5 T were performed using a vibrating sample magnetometer in the temperature range 200–350 K. Magnetocaloric effect \(\left | {\Delta S}_{\mathrm {M}}^{\mathrm {(T)}} \right |\) at several magnetic field change up to 5 T were deduced from the M(H) curves at several temperatures.
3 Results and Discussion
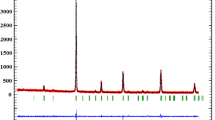
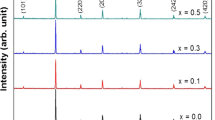
The X-ray diffraction (XRD) patterns of the parent compound La0.65Ca0.35MnO3 have been recorded at room temperature; the data have been refined using Rietveld’s profile-fitting method. Figure 1 shows the X-ray diffraction patterns at 300 K for La0.65Ca0.35MnO3 sample including the observed and calculated profiles as well as the difference profile .Our parent compound is single phase without any detectable impurity and crystallizes in the orthorhombic structure with Pbnm space group. Figure 2 shows the X-ray diffraction patterns at 300 K for our three composites (1 −x)La0.65Ca0.35MnO3/xFe2O3 with x = 0.03, 0.06, and 0.09. As we can observe on this figure, there is no interaction between the parent compound and Fe2O3 oxide.
3.1 Microstructural Analysis
To investigate the microstructural properties of our synthesized samples, XRD and SEM analyses were jointly used. With MAUD software, the model line broadening selected was “popa LB” combined with the isotropic model size–strain. The instrument broadening was corrected using the XRD pattern of a Si standard. Figure 3 shows the SEM image obtained for the composites (1 −x)La0.65Ca0.35MnO3/xFe2O3, (x = 6 and 9 %). The SEM image shows that the Fe2O3 oxide occupies the grain boundary of La0.6Ca0.35 MnO3.
3.2 Magnetic and Magnetocaloric Properties
We performed magnetization measurements versus magnetic applied field up to 5 T at several temperatures in the range 200–350 K for the parent compound La0.65Ca0.35MnO3 and the three composites (1 −x)La0.65Ca0.35MnO3/xFe2O3 (x = 3, 6, and 9 %). Figure 4 shows the magnetic field dependence of magnetization up to 5 T at several temperatures for La0.65Ca0.35MnO3 and 0.91La0.65Ca0.35MnO3/0.09Fe2O3. At low temperatures, the magnetization M increases sharply with magnetic applied field for H < 0.5 T and then saturates above 1 T. This result confirms well the ferromagnetic behavior at low temperatures of our composites. The magnetic entropy change ΔS M (T) has been deduced from the isothermal magnetization measurements. It can be evaluated according to Maxwell’s relations, using the following equation:
where H max is the maximal value of the magnetic applied field. In practice the relation is approximated as [19, 20]:
where M i and M i+1 are the experimental values of magnetization measured at temperatures T i and T i+1 respectively, under a magnetic field H i . Figure 5 shows the variation of −ΔS M as a function of temperature at different magnetic field changes for our composites. As expected, −ΔS M reaches its maximum around the transition temperature and increases with the magnetic applied field change. At 5 T, the maximal value of the magnetic entropy change \(\left | {\Delta S}_{M}^{\max }\right |\) is found to be 6.25, 6.37, 6.18, and 5.87 J kg−1 K−1 for x = 0.00, 0.03, 0.06, and 0.09 respectively. We do not notice a significant effect of the mass fraction of Fe2O3 oxide on the maximum of ΔS M (T) while temperature of this maximum is shifted to high values. From a cooling perspective, it is important to consider the refrigeration capacity which depends on both the magnetic entropy change and its temperature dependence. The magnetic cooling efficiency of a magnetocaloric material can be evaluated through the relative cooling power (RCP) defined as follows:
were δ T FWHM is the full with at half maximum of the ΔS M (T) curve. The RCP value is found to be 272.6, 255, 219.4, and 208.6 J kg−1 for x = 0, 3, 6, and 9 % respectively, under a magnetic field change of 5 T. The RCP decreases with increasing the Fe2O3 amount in our composites.
4 Conclusion
We have studied the magnetocaloric effect in composites with general formula (1 −x)(La0.65Ca0.35MnO3)/x(Fe2O3) with x = 0, 3, 6 and 9 % prepared by solid–solid reaction. The presence of the Fe2O3 oxide has no great effect on the physical properties of LCMO compound. The Curie temperature remains unchanged (T c ≈ 270 K). The maximum of the magnetic entropy \(\left | {\Delta S}_{M}^{\max }\right |\) values are not very affected by the oxide amount while the RCP decreases. The full width at half maximum δ T FWHM of the ΔS M (T) curves is ≈40 K around T c for all the samples.
References
Warburg, E.: Ann. Phys 13, 141 (1881)
Debye, P.: Ann. Phys 81, 1154 (1926)
Giauque, W.F., Amer, J.: Chem. Soc 49, 1864 (1927)
Yu, B.F., Gao, Q., Zhang, B., Meng, X.Z., Chen, Z.: Int. J. Refrigeration 26, 622 (2003)
Zhensheng, P.: J. Rare Earths 22, 232 (2004)
Gschneidner, K.A. Jr., Pecharsky, V.K., Tsokol, A.O.: Rept. Prog. Phys 68, 1479 (2005)
Cheikhrouhou-Koubaa, M., Koubaa, W., Cheikhrouhou, A.: J. Alloys compd 470, 42 (2009)
Koubaa, W., Cheikhrouhou-Koubaa, M., Cheikhrouhou, A.: J. Phys. Chem. Solids 70, 326 (2009)
Cheikhrouhou-Koubaa, M., Koubaa, W., Cheikhrouhou, A.: J. Phys Procedia 2, 989 (2009)
Phan, M.H., Yu, S.C.: J. Magn. Magn Mater 308, 325 (2007)
Schiffer, P.E., Ramirez, A.P., Bao, W., Cheong, S.W.: Phys. Rev. Lett 75, 3336 (1995)
Tang, W., Lu, W., Luo, X., Wang, B., Zhu, X., Song, W., Yang, Z., Sun, Y.: J. Magn. Magn. Mater 322, 2360 (2010)
Krichene, A., Boujelben, W., Cheikhrouhou, A.: J. Alloys Comp 550, 75 (2013)
Koubaa, M., Cheikhrouhou-Koubaa, W., Cheikhrouhou, A, Haghiri-Gosnet, A.M.: Physica B403, 2477 (2008)
CaballeroFlores, R., Franco, V., Conde, A., Knipling, K.E., Willard, M.A.: Appl. Phys. Lett. 98, 102505 (2011)
Smaili, A., Chahine, R.: Appl. Phys. Lett 81, 824 (1997)
de Oliveira I.G, Von Ranke, P.J., Nobrega, E.P.: J. Magn. Magn. Mater 261, 112 (2003)
Chaturvedi, A., Stefanoski, S., Phan, M.H., Nolas, G.S., Srikanth, H.: Appl. Phys. Lett. 99, 162513 (2011)
Paticopoulos, S.C., Caballero-Flores, R., Franco, V., Blasquer, J.S., Conde, A., Knipling, K.E., Willard, M.A.: Solid State Commun 152, 1590 (2012)
Romero Muniz, C., Franco, V., Conde, A.: Appl. Phys. Lett. 102, 082402 (2013)
Pekala, M., Pekala, K., Drozd, V., Staszkie Wicz, K., Fagnard, J.F., Vanderbemden, P.: J. Appl Phys. 112, 023906 (2012)
Panwar, N., Coondoo, I., Agarwal, S.K.: Mater. Lett 64, 2638 (2010)
Lim, S.P., Tang, G.D., Li, Z.Z., Qi, W.H., Ji, D.H., Li, Y.F., Chen, W., Hou, D.L.: J. Alloys Comp 509, 2320 (2011)
Yang, H., Cao, Z.E., Shen, X., Xian, T., Feng, W.J., Jiang, J.L., Feng, Y.C., Wei, Z.Q., Dai, J.F.: J. Appl. Phys 106, 104317 (2011)
Yuan, G.L., Liu, J.M., Liu, Z.G., Du, Y.W., Chan, H.L.W., Choy, C.L.: J. Chem. Mater. Phys 75, 161 (2002)
Rietveld, H.M.: J. Appl. Cryst 2, 65 (1969)
Roisnel, T., Rodriguez-Carvayal, J.: Computer progam Fullprof, LLB-LCSIM (2003)
Acknowledgments
This work has been supported by the Tunisian Ministry of Higher Education, Scientific Research and Information and Communication Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marzouki-Ajmi, A., Cheikrouhou-Koubaa, W. & Cheikhrouhou, A. Magnetic and Magnetocaloric Study of Polycrystalline (1−x)La0.65Ca0.35MnO3/xFe2O3 Composites. J Supercond Nov Magn 28, 103–108 (2015). https://doi.org/10.1007/s10948-014-2805-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-014-2805-8