Abstract
(Bi,Pb)-2223 HTSs (high temperature superconductors) were synthesized from nominally pure (reference) and BN-added Bi1.7Pb0.3Ca2Sr2Cu3O y (BN) x precursors (x=0,0.10,0.15, and 0.20) by the solid state reaction method using alumina crucibles. The influence of boron nitride addition on the phase formation kinetics and transport properties of (Bi,Pb)-2223 HTSs was studied using X-ray diffraction (XRD), resistivity and critical current density measurements. BN-added compounds reveal a significant enhancement in both the high-T c 2223 phase formation and critical current density compared to the reference specimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The synthesis of a nearly pure single-phase high-T c Bi-2223 (T c ≈110 K) is a critical issue in fabrication of Bi-based superconductors. Partial substitution of Bi by Pb is the most widely used method to enhance the formation of the Bi-2223 phase [1]. Since the kinetics of the Bi-2223 phase formation is slow, it requires a long heating (150–200 h) to produce an appropriate fraction of Bi-2223 HTS material in the final product [2]. Moreover, extremely narrow stability range of 2223 results in the formation of intergrowth of the multiple phases coexisting with the 2223 phase [3]. It was also found that the high-T c 2223 phase is very sensitive to the partial pressure of oxygen and sintering in O2 as well as in ambient atmosphere suppress the formation of this HTS [4]. Thus, many factors including composition of the precursor powder, synthesis atmosphere, heat treatment conditions, and nature of doped ions significantly influence the final physical properties of the samples [5].
Our previous work shows that the precursors doped with lead borate Pb(BO2)2 and boron oxide B2O3 substantially promote the formation of (Bi,Pb)-2223 phase when their thermal processing occurs in alumina crucibles and lead to the enhancement of transport properties compared to the reference sample [6]. The purpose of the present paper is to investigate whether the BN additive has a similar positive effect on the superconducting properties of (Bi,Pb)-2223 HTS ceramics.
2 Experimental
Samples with nominal composition Bi1.7Pb0.3Ca2Sr2Cu3O y (BN) x (x=0, 0.10, 0.15, and 0.20) have been prepared by the solid state reaction method from the appropriate mixtures of Bi2O3, PbO, SrCO3, CaCO3, CuO, and BN. The mixtures were thoroughly ground and heat treated at 850 °C for 30 h in alumina crucibles with intermediate grindings. The resulting materials were pressed into pellets of 10 mm diameter and 1.5 mm thickness under hydrostatic pressure at 29 MPa. The pellets were placed in alumina crucibles, then annealed at 840 °C for 30 h in air followed by the furnace cooling to room temperature. Synthesized compounds were characterized by powder X-ray diffraction (XRD) analysis using the Dron-3M diffractometer (CuK α radiation) and the fractional amounts of high-T c phase were estimated from the XRD data. The resistivity as a function of temperature, ρ(t), and transport critical current density, J c , were measured by a standard four-probe method for bar-shaped specimens (∼10×0.5×0.5 mm3) cut from the pellets. The critical current densities were evaluated at the liquid nitrogen temperature in the self-field, with a criterion of 1 μV/cm.
3 Results and Discussion
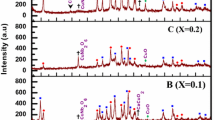
Our earlier investigations show that the rate of high-T c phase formation, and hence, the value of zero resistivity temperature (\(T_{c}^{\mathrm{off}}\)) are extremely sensitive to the thickness (height) of the nominally pure (un-doped) precursors when their thermal processing occurs for ∼50–60 h in alumina crucibles or on alumina plates. Figure 1 illustrates the effect of thickness (d) of undoped (Bi,Pb)-2223 precursors on the superconducting transition. It is evident that the \(T_{c}^{\mathrm{off}}\) value decreases with increasing d. Based on the experimental results of our group, \(T_{c}^{\mathrm{off}}(d)\) dependence can be subdivided in three main areas: (i) \(T_{c}^{\mathrm{off}}\) is about 65–75 K for d≈12–15 mm, (ii) \(T_{c}^{\mathrm{off}}\approx85\mbox{--}95~\mbox{K}\) for d≈6–8 mm, and (iii) \(T_{c}^{\mathrm{off}}>100~\mbox{K}\) for d≤3 mm. We also found that in contrast to the un-doped (Bi,Pb)-2223 samples, the superconducting transition of boron-doped (Bi,Pb)-2223 material does not correlate noticeably with the d values ranging from 2 to 15 mm. The \(T_{c}^{\mathrm{off}}\) higher than 100 K were obtained in (Bi,Pb)-2223 HTSs doped with lead borate Pb(BO2)2 and boron oxide B2O3 [6]. In the present study, BN-added (Bi,Pb)-2223 precursors were ∼12 mm in thickness. XRD patterns of the reference and BN-added fully processed specimens are presented in Fig. 2. The dominance of the low-T c 2212 phase over the high-T c 2223 phase was observed in the reference and low-content BN doped (x=0.10) samples. The lattice parameters of (Bi,Pb)-2212 phase derived from XRD data are a=5.428 Å, b=5.430 Å, c=31.040 Å for undoped specimen and a=5.428 Å, b=5.407 Å, c=30.936 Å for BN-doped sample with x=0.10. Obviously, both samples have an orthorhombic structure. The lattice parameter “a” remains unchanged, but the “b” and “c” parameters decrease with BN addition. This corresponds to the contraction of about 0.8 % of the unit cell volume. This result suggests that due to their extremely small ionic radius B3+ ions may occupy substitutional or interstitial positions in the lattice. With an increase of the BN content up to x=0.15, the 2223 phase is remarkably enhanced and its increase is associated with the decrease of 2212 phase; although, a very low-T c 2201 phase appears for x=0.10 and is intensified with increasing BN concentration. The peaks corresponding to the BN could not be observed in the XRD patterns due to the minute amounts (less than 0.5 wt%) of this additive. It is observed for x=0.15 composition that all dominant peaks belong to the orthorhombic (Bi,Pb)-2223 phase with the lattice parameters of a=5.428 Å, b=5.482 Å, c=37.330 Å. A further addition of BN up to x=0.20 shows no systematic variation in the lattice parameters with respect to the x=0.15 sample. This might be attributed to the distortion of crystal lattice. The volume fractions (V) of the (Bi,Pb)-2223 phase were estimated from XRD intensity ratios of the (Bi,Pb)-2223 and Bi-2212 phases using the following equation [7]: V={I H(115)/[I H(115)+I L(115)]}×100 [%], where I H(115) and I L(115) denote the intensities of the (115) peaks of the (Bi,Pb)-2223 and (Bi,Pb)-2212 phases, respectively. In this case, the very small amount of Bi-2201 phase detected on the XRD patterns was ignored. The calculated volume fraction of (Bi,Pb)-2223 phase increases from ∼10 % for undoped specimen to ∼25 % for x=0.1 and reaches the maximum value of ∼75 % for x=0.15–0.20 in a short sintering time (60 h), which indicates that BN addition accelerates the solid state reaction rate and hence the (Bi,Pb)-2223 formation. Figure 3 represents the temperature dependence of resistivity ρ(T) for the reference and BN-added (Bi,Pb)-2223 samples. The ρ(T) dependences clearly exhibit a two-step transition, reflecting the coexistence of high-T c 2223 and low-T c 2212 phases. Splitting of the resistive transition (double superconducting transition typical of a two-phase sample) might also be due to the presence of oxygen content inhomogeneities in the HTS [8]. Onset temperature \(T_{c}^{\mathrm{on}}\) of the superconducting transition is about 115 K for all the samples. For the reference specimen zero resistivity is reached at \(T_{c}^{\mathrm{off}}=70~\mbox{K}\). \(T_{c}^{\mathrm{off}}\) increases up to 103 K with increasing x from zero to 0.15, then drops to 98 K at x=0.20. These curves show that the sample with x=0.15 is the best in the present study. It is also seen that the normal state resistivity decreases with the introduction of BN and then increases with increasing doping level. This behavior may be related to the appearance and increase of very low-T c Bi-2201 phase upon increasing the BN concentration. Presence of 2201 phase in the doped compositions deteriorates a coupling between superconducting grain boundaries which results in the reduction of \(T_{c}^{\mathrm{off}}\) at x>0.15. Figure 4 reveals the relationship between the transport J c values (77 K, zero field) and BN content. The enhancement of critical current density by increasing BN content seems to result from the increase of (Bi,Pb)-2223 phase fraction with increasing x from zero to 0.15. After passing the maximum value at x=0.15 (170 A/cm2), J c decreases for higher doping level. In agreement with the XRD and resistivity data shown in Figs. 2 and 3, lower J c value for x=0.20 (88 A/cm2) imply the deterioration of intergranular coupling due to slight increase of 2201 phase with increasing BN content. Maximum value of J c for BN-added (Bi,Pb)-2223 is lower as compared to the Pb(BO2)2 and B2O3-doped specimens (215 A/cm2 and 190 A/cm2, respectively [6]). Incorporation of low-melting Pb(BO2)2 and B2O3 (T m =500 ∘C and 450 °C, respectively) into (Bi,Pb)-2223 precursors accelerates the (Bi,Pb)-2223 phase growth [6]. In the present study, similar positive effect of BN additive on the phase formation and superconducting properties of (Bi,Pb)-2223 HTS was observed. BN starts to oxidize at 700 °C, but the rate is low up to 1000 °C, after which oxidation becomes a major problem [9]. Oxidation reaction leads to the decomposition of BN to B2O3 and N2 [10, 11]. Based on the obtained results, we assume that the appearance of boron-containing liquid phase during the annealing of BN-doped Bi1.7Pb0.3Ca2Sr2Cu3O y (BN) x precursor powders at 840–850 °C allows the enhancement of high-T c phase formation due to a higher diffusion rate of elements required to form the (Bi,Pb)-2223 phase.
Superconducting transition as a function of thickness of un-doped Bi1.7Pb0.3Ca2Sr2Cu3O y sintered at 840–850 °C. 1—heat treatment of powder (d≈12 mm, 30 h) and pellet (∼1.5 mm thick, 30 h) in alumina crucible; 2—heat treatment of powder (d≈7 mm, 30 h) and pellet (∼1.5 mm thick, 30 h) in alumina crucible; 3—heat treatment of powder (d≈2 mm, 30 h) and pellet (∼1.5 mm thick, 30 h) on alumina plate
4 Conclusion
Phase evolution and transport properties of BN-added (Bi,Pb)-2223 HTSs prepared by the heat treatment of Bi1.7Pb0.3Ca2Sr2Cu3O y (BN) x (x=0–0.2) precursors in alumina crucibles were investigated. Addition of boron nitride accelerates the formation of high-T c (Bi,Pb)-2223 phase and leads to the increase in the critical current density compared to the reference sample. We could conclude that choosing the optimum doping level it could be possible to prepare BN-added (Bi,Pb)-2223 HTS materials with even higher fraction of the high-T c phase as well as with much better critical current density in a short heating time of ∼50–60 h.
References
Takano, M., Takada, J., Oda, K., Kitaguchi, H., Miura, Y., Ikeda, Y., Tomii, Y., Mazaki, H.: Jpn. J. Appl. Phys. 27, L1041 (1988)
Yau, J.K.F., Wong, Y.L.: Physica C 339, 79 (2000)
Eltsev, Y., Lee, S., Nakao, K., Tajima, S.: Supercond. Sci. Technol. 23 (2010). doi:10.1088/0953-2048/23/5/055007
Watanabe, K., Kojima, M.: Supercond. Sci. Technol. 11, 392 (1998)
Özkurt, B., Ekicibil, A., Aksan, M.A., Özçelik, B., Yakinci, M.E., Kiymac, K.: J. Low Temp. Phys. 149, 105 (2007)
Margiani, N.G., Metskhvarishvili, I.R., Adamia, Z.A., Medoidze, T.D., Papunashvili, N.A., Dzanashvili, D.I., Chubabria, M.I.: J. Supercond. Nov. Magn. 26, 965 (2013)
Lee, M.S, Song, K.Y.: Supercond. Sci. Technol. 23, 851 (2002)
Vidal, F., Viera, J.A., Maza, J., Mosqueira, J., Carballeira, C.: In: Drechsler, S.-L., Mishonov, T. (eds.) High-T c Superconductors and Related Materials, p. 298. Kluwer Academic, Norwell (2001)
Bengisu, M.: Engineering Ceramics. Springer, Berlin (2001)
He, Z., Ma, J., Qu, Y., Feng, X.: J. Eur. Ceram. Soc. 22, 2143 (2002)
Ho, I.C., Hsieh, H.L.: J. Electron. Mater. 23, 471 (1994)
Acknowledgement
This work was financially supported by the Shota Rustaveli National Science Foundation of Georgia (Grant No. GNSF/ST09_844_7-121).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Margiani, N.G., Mumladze, G.A., Papunashvili, N.A. et al. Effect of BN-Added Precursors on Phase Formation and Transport Properties of (Bi,Pb)-2223 HTS. J Supercond Nov Magn 27, 397–400 (2014). https://doi.org/10.1007/s10948-013-2330-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-013-2330-1