Abstract
The effects of alpine glaciers on the hydrology, physical features, and biogeochemistry of lakes have been investigated over contemporary time scales. However, the influence of these factors on algal communities over longer time scales remains unclear, yet is critical to paleolimnological interpretation of environmental change in alpine regions. We examined sedimentary algal pigments and fossil diatom assemblages in two proximal lakes with equivalent local climates, one glacier-fed and one snow-fed, in the central Rocky Mountains (USA) to determine how glacier meltwater has altered algal records over the last 3,000 years. Differences between the records of the two lakes intensified during the Medieval Climate Anomaly and the Little Ice Age, with the glacier-fed lake exhibiting an overall increase in fossil algal pigment concentrations and greater diatom assemblage turnover. Starting 1,000 years ago, the glacier-fed lake in this study showed evidence of nitrogen enrichment from glacier meltwater, as indicated by increasing relative abundances of Asterionella formosa and, to a lesser extent, Fragilaria crotonensis. Since the Little Ice Age, diatom species richness declined in the glacier-fed lake, and further decreased following the 1950s, while assemblage turnover increased. These results demonstrate that glaciers can strongly alter the algal sedimentary record and should be considered when interpreting high-elevation lake records.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fossil algal records are a key tool for deciphering climate-driven change in montane regions through the Holocene (Stone and Fritz 2006; Stevens et al. 2006; Hobbs et al. 2010). These records can provide higher temporal resolution than pollen-based reconstructions because algae have shorter generation times than terrestrial vegetation, and so respond more rapidly to climate and environmental changes. Sedimentary archives using fossil algae and pigments can also extend climate inferences beyond what is possible from tree-ring reconstructions, which are often limited to the past millennium. The use of lake sediments to reconstruct past environmental settings requires the appropriate integration of the ecological characteristics of biological indicators (chemical optima, habitat preference, within-lake ecological processes) as well as site-specific features (landscape, basin morphology, climate, hydrology) of a given lake.
In alpine regions, glaciers are a key landscape feature linked to climate, with changes in glacier meltwaters strongly affecting the hydrology (Schindler and Donahue 2006), chemistry (Thies et al. 2007; Saros et al. 2010) and water transparency (Hylander et al. 2011) of lakes. In turn, these physical and chemical changes affect aquatic production (Vinebrooke et al. 2010; Slemmons and Saros 2012) and diversity (Koenings et al. 1990; Milner et al. 2009). While the effects of glacier meltwater on lake hydrology and turbidity are long recognized (Irwin 1974; Jansson et al. 2003; Hylander et al. 2011), the influence of meltwater on the nutrient chemistry of lakes has only recently been documented (Robinson and Kawecka 2005; Saros et al. 2010; Hobbs et al. 2011). The observed effects show a high degree of spatial and likely temporal variability. At present, lakes in the Rocky Mountains of the US that are fed by both glacial and snowpack meltwaters (GSF) have nitrate (N) concentrations up to 200 times greater than counterpart snow-fed (SF) lakes (Saros et al. 2010), have higher rates of primary production, are limited by P rather than co-limited by N and P, and have a lower species richness (Slemmons and Saros 2012).
The effects of elevated anthropogenic N deposition on alpine SF lakes over the past 150 years are well documented (Saros et al. 2005a, 2011; Holtgrieve et al. 2011). While the mechanisms behind elevated nitrate concentrations in GSF lakes have been explored (Williams et al. 2006; Wynn et al. 2007; Baron et al. 2009), the source of this N is unresolved. This raises the question of whether N-enriched meltwater is a recent phenomenon apparent only with elevated anthropogenic N deposition during the twentieth century (NADP 2011), or has alternatively occurred at other times prior to this. The concentrating action of glaciers on atmospheric constituents (Daly and Wani 2005) suggests that GSF lakes may have experienced N-enriched meltwaters even with pre-industrial N deposition rates. A 270-year chemical analysis from the Upper Fremont Glacier in the Central Rocky Mountains (Wind River Range) indicates slight N enrichment intermittently throughout the record, with sections from AD 1717–1740 and AD 1890 showing elevated N concentrations (>20 µg L−1), with particularly high enrichment from AD 1985 to present (>100 µg L−1; Schuster et al. 2000). Amounts of N extracted from numerous ice cores (GISP2, 20D, Mount Logan, Sentik Glacier and South Pole) correlate to snow accumulation rate throughout the last 800 years (Yang et al. 1996), indicating atmospheric deposition was the source of elevated N concentrations in glacial ice. Alternatively, others have suggested a bedrock source of this N, made available via microbial redox transformations of nitrogenous compounds in subglacial environments (Skidmore et al. 2000; Boyd et al. 2011) or photolysis and nitrogen processing in the glacial ice (Naftz et al. 2011).
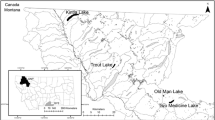
Regardless of the N source, the type and mass balance of a glacier will influence the quantity and composition of its runoff. Given that the mass balance of alpine glaciers has varied through the late Holocene (Carrara 1989), a key question for paleolimnological interpretation is how these various effects from glaciers are integrated to influence past lake communities and affect the accuracy of paleolimnological inferences. In this study, we examined how algal communities responded to glacial meltwater through the late Holocene (~present to 3000 years BP; years before present) in the central Rocky Mountains of the US by comparing records from a GSF alpine lake, Jasper Lake, to those of a neighboring SF lake, Albino Lake (Fig. 1). We measured fossil algal pigments and diatom assemblage structure and turnover, as well as sediment geochemistry in these paired lakes.
Study site
The Beartooth Mountains, part of the central Rocky Mountains, are located along the Montana and Wyoming border. This region contains in excess of 600 lakes, many of which are above 2,700 m and above treeline (Fig. 1). The geology of the region is composed primarily of 2.8–3.2 billion year old Precambrian granite and crystalline metamorphic rock. Slow bedrock weathering rates lead to low conductivity (average of 11 µS cm−1, Arnett et al. 2012) and phosphorus concentrations in lakes in this area (Saros et al. 2005b). Since the beginning of the twentieth century, the area covered by glaciers in this region has declined by ~42 % (Fountain et al. 2007), with only 25 small glaciers and remnant ice patches remaining today. Low N deposition rates (<200 kg N km−2 year−1; NADP 2011) in the region result in very low N concentrations (<10 µg NO3 −-N L−1; Saros et al. 2010) in SF lakes, with many of the lakes in this region N limited (Saros et al. 2005a). Fossil diatom assemblages suggest that enhanced N deposition during the twentieth century elicited changes in phytoplankton assemblages after 1980 in SF lakes in this area (Saros et al. 2005a).
Glacier advance and retreat in the central Rocky Mountains through the late Holocene (~3000 years BP) have exhibited temporal and spatial variation (Whitlock and Bartlein 1993) and are largely controlled by the interplay between the Pacific Decadal Oscillation (McCabe and Dettinger 2002) and El Niño events (Barron and Anderson 2011). In particular, during the late Holocene, major glacial fluctuations included advances during the Little Ice Age (100–500 years BP), and the Neoglacial period (2500–3400 years BP), and recession during the Medieval Climate Anomaly (750–1150 years BP; Carrara 1989). Evidence suggests that glaciers in this region did not grow to their maximum extent until the end of the Little Ice Age (~AD 1850). Although determining the age of the glaciers is difficult, evidence suggests that they date to approximately 7000 years BP (Carrara 1989). Over the last 150 years, glaciers have rapidly receded due to a sizable decrease in winter snowpack in conjunction with hot, dry summers, resulting in recession rates up to 100 m year−1 during the period of AD 1917–1941 (Pederson et al. 2004).
We selected two lakes in this region, Jasper Lake, fed by a remnant glacier (area <0.1 km2), and Albino Lake, fed by snowmelt alone (Fig. 1), to examine patterns of change in fossil algal records. Given that glaciers did not grow to their maximum extent until the end of the Little Ice Age, the glacial history of Albino Lake prior to that time is unknown. However, Albino Lake did not receive glacial meltwater during the Little Ice Age. While separated hydrologically, these two lakes are in close proximity and share similarities in many physical and chemical characteristics (Table 1; Saros et al. 2010; Slemmons and Saros 2012). The two lakes are similar in area and maximum depth. The study lakes have similar water transparencies, with 1 % attenuation depths for photosynthetically active radiation (PAR), as determined from diffuse attenuation coefficient (Kd) values (Z1 % = 4.6/Kd PAR), of 15 m for Jasper and 14 m for Albino. Total phosphorus concentrations were also similar (Jasper = 9 µg L−1, Albino = 10 µg L−1). Silica concentrations are higher in Albino Lake (2,104 µg L−1) compared to Jasper (1,321 µg L−1). While silica requirements vary among diatom species (~average 55 µg L−1; Tilman et al. 1982), the concentrations in these lakes are more than adequate for phytoplankton growth. A key difference at present between the two lakes is their nitrate concentrations, with 125 µg NO —3 N L−1 in Jasper compared to 2 µg NO —3 N L−1 in Albino. Subsequently, average water column chlorophyll concentrations are three times higher in Jasper Lake (4.2 µg L−1) than in Albino Lake (1.4 µg L−1). Sedimentation rates differ dramatically between Jasper and Albino Lakes largely given the glacial meltwater input into Jasper Lake.
Materials and methods
Core collection, dating and characterization
A 71.5-cm core was extracted from Jasper Lake and a 64.5-cm core was extracted from Albino using a rod-operated piston corer in July of 2010. Cores were dated using 210Pb activity counted by using a high purity germanium well detector with a low background graded lead shield for thirteen (Jasper) and nine (Albino) samples (Appleby and Oldfield 1978). Counts were conducted over a 24 h period for most samples. Position of the Cesium peak was used to verify 210Pb dates (Eakins and Morrison 1978). Chronology was based on the constant rate of supply model (Appleby and Oldfield 1978). Radiocarbon dating was conducted on concentrated pollen isolations from depths of 27, 40, and 62.5 cm in Jasper Lake and 11.5, 17.5, 20, and 40 cm in Albino Lake. Pollen was isolated by screening with a 6-µm Nitex nylon mesh to remove clay particles <6 µm, processed with hydrofluoric acid to remove excessive biogenic silica (Bsi) in a few cases, and separated by centrifugation using POLY-GEE sodium polytungstate (density 2.8) powder (GEOLiquids Inc.,). Samples were dated at the Lawrence Livermore Center for Accelerator Mass Spectrometry (CAMS). The age model and error estimates was created using R package, BChron (version 2.12.2) which determined the most likely age-depth estimate using Bayesian probability techniques. All radiocarbon dates were calibrated to calendar years using CALIB 5.0 (M. Stuiver, PJ Reimer and R. Reimer) and will be referred to herein as calibrated ages in years BP.
The fraction of organic sediments was calculated by weight of loss on ignition at 550 °C for 2 h. The percent inorganic material in sediment was measured following combustion at 950 °C (Heiri et al. 2001). Minerogenic flux, used as an alternative measure of glaciofluvial output, was calculated using Pb210 dates and loss on ignition values. BSi, a measure of the amorphous silica content of sediment that is used to infer overall diatom productivity over time (Conley 1998; Conley and Schelske 2001), was quantified based on the procedures outlined in Conley (1998). Freeze-dried sediment (30 mg) was digested by adding 40 mL of 1 % Na2CO3 and placing in a shaker bath at 85 °C and 100 rpm. At 2, 3, 4 and 5 h of digestion time, samples were allowed to cool to room temperature and 1 mL aliquots were extracted and placed into 10 mL sample vials containing 9 mL of 0.021 N HCl. These diluted extracts were analyzed for dissolved reactive silica via the heteropoly blue method (APHA 2000) with a Varian Cary-50 UV–VIS Spectrophotometer. Given the high weight percentage of silica in these sediments, digested sediment was examined to verify complete dissolution. These sediments were compared to samples that were digested in a 5 % Na2CO3 solution. Least squares regression analysis was used to determine the intercept from the change in dissolved silica concentration versus time. Taking into account sample weight and dilutions the intercept of this equation is typically the BSi concentration of the sediment (Conley 1998). However, in cases of high Bsi concentrations such as in these samples, concentrations do not increase with digestion time and therefore determining silica concentrations based on an increasing slope cannot be calculated. As a result, the mean concentration from the sub-samplings was used to estimate the Bsi concentration (Conley 1998; Conley and Schelske 2001).
Pigment analysis
Pigments derived from photosynthetic organisms can be used to estimate past lake primary production and, since pigments have some taxonomic specificity, can provide information regarding past algal assemblage structure (McGowan 2013). Sedimentary pigments were quantified using standard high performance liquid chromatographic separations of chlorophylls (chls), carotenoids and associated derivatives (Leavitt and Findlay 1994). Pigments were extracted, filtered, and dried under N2 gas. Extracts were separated in an Agilent 1200 series separation module with quaternary pump following methods developed by Chen et al. (2001). Pigments were identified by spectral characteristics and chromatographic mobility and compared to unialgal cultures, which were also used to calibrate pigment concentrations (Chen et al. 2001). Analysis included pigments from all algae and plant chlorophylls (chlorophyll a and derivatives, β-carotene), chlorophytes (chlorophyll b and derivatives, lutein), total cyanobacteria (zeaxanthin), colonial cyanobacteria and zooplankton (canthaxanthin), diatoms (diatoxanthin, fucoxanthin), cryptophytes (alloxanthin), chrysophtes (fucoxanthin, diatoxanthin), and dinoflagellates (diatoxanthin). Total algal biomass was based on the sum of β-carotene and pheophytin a, two chemically stable indicators found in all algal groups (Leavitt and Carpenter 1990). Pigment concentrations were normalized to sediment organic matter and were measured at ~2.0 cm resolution.
Diatom enumeration
Diatom assemblages were determined in every 0.5 cm increment throughout each core. Samples for diatom analysis were treated with 10 % HCl to remove carbonate material, followed by 30 % H2O2 to remove organic matter. Diatom slides were prepared according to standard procedures (Battarbee 1986) and examined using an Olympus BX51 microscope with differential interference contrast. A minimum of 300 diatom valves were identified and enumerated from random transects from each slide under oil immersion at 600× magnification. Diatoms were identified using Krammer and Lange-Bertalot (1986–1991) and Camburn and Charles (2000). The ratio of planktic to benthic (P:B) diatoms was calculated for each depth in the cores based on ecology of the species (Krammer and Lange-Bertalot 1986; Spaulding et al. 2010). Tychoplanktic and meroplanktic species were included in the planktic component of the assemblage. Comparisons were made excluding these species from the analysis to determine if P:B yielded similar patterns.
Statistical analyses
Principal components analysis was conducted on pigment concentrations from each lake to express dominant trends or patterns of variation over time using R (version 2.12.2; Legendre and Birks 2001). Detrended correspondence analysis (DCA) in R (version 2.12.2) was used to summarize the dominant trend in diatom assemblage structure and the amount of compositional change or turnover (Hill and Gauch 1980; Legendre and Birks 2001). Analysis included the percent relative abundance of all diatom species, square root transformed, with down-weighting of rare taxa. To identify the change in species richness through time, average diatom species richness was calculated with a consistent sample size of 300 individuals using rarefaction analysis (Analytic Rarefaction 1.3, Steven M. Holland). Relative species richness was normalized to the average deviation of the mean and will be referred to hereafter as species richness unless otherwise indicated.
Results
Core chronology and characterization
Ten 210Pb dates and three 14C dates were calculated in the Jasper Lake (GSF). For Jasper Lake, the age-depth relationship was highly collinear and the age model produced an inferred age at the bottom of the 71.5 cm core as ~3000 years BP. Eight 210Pb dates and three 14C dates were calculated in Albino Lake (SF) and the age-model was calculated in the same manner as Jasper Lake. For Albino Lake, there was some disagreement between the 210Pb dates and the 14C dates. Results from the 210Pb and 14C dating models for Jasper (Fig. 2a) and Albino (Fig. 2b) reveal that the sedimentation rate in Jasper is greater than that in Albino, with 71 cm representing ~3000 years BP in Jasper and ~18 cm representing the same time frame in Albino. As a result, only the first 18 cm of Albino were used in further analyses.
Age-depth model from central basin of a Jasper Lake and b Albino Lake. The age-depth model from the central basin of Jasper Lake, based on 11 210Pb dates (circles) and 3 AMS 14C dates (squares) and for Albino Lake, based on 8 210Pb dates (circles) and 4 AMS 14C dates (squares). The gray shaded areas represent the error envelope
Both lakes had sediments that were largely composed of inorganic material (Jasper 86–92 %; Albino 85–91 %, Fig. 3). Minerogenic flux was greater in Jasper Lake (GSF) relative to Albino (SF). Minerogenic flux ranged from 0.022 to 0.041 g cm−2 year−1 in Jasper, and 0.004–0.009 g cm−2 year−1 in Albino. The percent organic content of the Jasper core (GSF; Fig. 3a) showed little directional change (~10–10.5 %) throughout the record until approximately the middle of the LIA when it decreased, possibly owing to greater influx of inorganic material from glacier meltwater. Albino Lake (SF) also had a similar organic content (10–10.5 %) throughout the record until the end of the LIA, and then increased throughout the twentieth century (Fig. 3b). Jasper Lake only exhibited this increase at the top of the core. BSi concentrations were high in both lakes (Jasper average = 29.7 % weight SiO2, Fig. 3a; Albino = 21.8 % weight SiO2, Fig. 3b). The BSi profile in Jasper Lake showed a marked decline at the start of the MCA; concentrations remained low throughout the LIA, and then increased since 1850 AD. Bsi in Albino Lake was highly variable but with no directional change. Bsi accumulation rates were higher in Jasper Lake (0.007–0.014 g cm−2 year−1) compared to Albino Lake (0.0003–0.005 g cm−2 year−1).
Biogeochemical stratigraphy of a Jasper Lake and b Albino Lake. Biogenic silica based on percent weight of SiO2. Percent organic based on loss-on-ignition analysis. Algal pigment analysis included: alloxanthin, diatoxanthin, lutein–zeaxanthin (lutein–zea), canthaxanthin, total algal biomass (sum of β-carotene and pheophytin a), and total chlorophyll pigments (Tchl; a sum of chlorophyll a, pheophytin a and b, and pheophorbide a). Principal components analysis (PCA) based on entire pigment analysis. Light gray bars capture the Little Ice Age (100–500 years BP), Medieval Climate Anomaly (750–1150 years BP), and the Neoglacial period (2500–3400 years BP)
Pigment analysis
PCA scores were strong estimators of the major patterns in algal change in Jasper Lake (GSF; axis 1 explained 44.5 % of the variance; Fig. 3a) and to a lesser degree in Albino Lake (SF; axis 1 explained 32.5 %; Fig. 3b). In Jasper Lake (GSF) a substantial increase in the total algal biomass (β-carotene and pheophytin a), total chlorophyll pigments (chlorophyll a, pheophytin a and b, and phaeophorbide a), lutein–zeaxanthin (chlorophytes and total cyanobacteria), canthaxanthin (colonial cyanobacteria) and alloxanthin (cryptophytes) occurred during the start of the MCA (circa 1150 years BP) and peaked at the middle of the LIA (AD 1700). Prior to this, these pigments had low concentrations and showed minimal directional change. In contrast, diatoxanthin (diatoms, dinoflagellates, chrysophytes) concentrations declined during the MCA and remained lower during the LIA (average 4.5 nmol g−1 organic carbon). Concentrations of this pigment gradually increased at the end of the LIA and throughout the modern period (present to AD 1950; average = 7.1 nmol g−1). Trends in average diatoxanthin concentrations over a given period reflect those of the average Bsi concentrations (pre-MCA average = 28.7 % weight SiO2; MCA average = 21.3 % weight SiO2; modern average = 34.3 % weight SiO2). In Albino Lake (SF), total algal biomass was fairly constant throughout the entire record with highs occurring in the mid-Neoglacial (2750 years BP), the end of the MCA (~750 years BP), and recent times (AD 1997–present) and lows occurring mid-Neoglacial (2230 and 2660 years BP), and recent times (AD 1971). Diatoxanthin and lutein–zeaxanthin increased towards the end of the Neoglacial (~2600 years BP) and remained high until the start of the MCA. In contrast to Jasper pigments, alloxanthin, lutein–zeaxanthin and canthaxanthin declined throughout the MCA and most of the LIA, and rebounded during the twentieth century. Similarly to Jasper pigments, diatoxanthin declined during the MCA and LIA, and then rebounded during the twentieth century.
Diatom stratigraphy
A total of 110 diatom taxa were identified in the sediment core from Jasper Lake (GSF; Fig. 4a). Jasper showed significant shifts in the dominant species throughout the core. Throughout the early portion of the record, Achnanthes and Fragilaria (sensu lato) species dominated. Taxa such as Pinnularia biceps W. Gregory and Sellaphora pupula (Kützing) Mereschkovsky each comprised about 10 % of assemblages over this time, while Aulacoseira alpigena/distans (Grunow) Krammer and Aulacoseira lirata (Ehrenberg) R. Ross varied from about 0 to 10 % of assemblages. Shortly before the MCA, the relative abundances of the planktic species Discostella stelligera (Cleve & Grunow) Houk & Klee and Asterionella formosa Hassall, began to increase. During the LIA, they sharply increased; Fragilaria crotonensis Kitton also increased and Tetracyclus glans (Ehrenberg) Mills showed a maximum peak, while Aulacoseira species declined notably. During the twentieth century, A. formosa, F. crotonensis, and D. stelligera dominated diatom assemblages. During this same time, there was a decline in small Fragilaria and Achnanthes (sensu lato) species.
Diatom stratigraphy of a Jasper Lake, a glacially-fed lake and b Albino Lake ordered by appearance in the record and showing the relative frequencies of species composing at least 10 % of the assemblage at a given depth. Small colonial Fragilariales included: Staurosira construen Ehrenberg, S. construens var.venter (Ehrenberg) Hamilton, S. pinnata and Fragilaria brevistriata Grunow. Planktic to benthic ratio, with planktic including meroplanktic species such as Aulacoseira sp. Detrended correspondence analysis (DCA) axis 1 scores of the entire diatom assemblage. Species richness values are the deviation from the average richness. Light gray bars capture the Little Ice Age (100–600 years BP), Medieval Climate Anomaly (700–1000 years BP), and the Neoglacial period (2500–3400 years BP)
A total of 112 diatom taxa were found in the sediment core from Albino Lake (SF; Fig. 4b) with the dominant diatom species, D. stelligera and small Fragilaria species, remaining relatively consistent over the ~3,000 year period. There was a slight increase in A. formosa during the twentieth century.
Diatom species richness in Jasper Lake (raw richness values range = 23–47; mean = 36) was relatively constant up until the LIA, at which point it shifted downward, followed by an additional downward shift during the twentieth century (Fig. 4a). Likewise, planktic:benthic ratios indicate that diatom communities were dominated by benthic taxa until the start of the LIA, at which point these ratios progressively shifted towards planktic domination (Fig. 4a). Diatom species richness in Albino Lake (raw richness values range = 28–47; average = 37) was less variable than that in Jasper Lake (Fig. 4b) and did not show a directional decline starting with the LIA as in Jasper. Planktic:benthic ratio varied throughout the Albino core but with no directional trends (Fig. 4b).
Assemblage change
The DCA axis 1 gradient length for Jasper was 1.9 with the axis 1 scores explaining 16.5 % of the variation in diatom communities (Fig. 4a). The first axis 1 gradient length for Albino was 0.96 with the axis 1 scores explaining 6.5 % of the variation in Albino communities (SF; Fig. 4b). Assemblage turnover (DCA axis 1 scores) in Albino was highly variable throughout the core but demonstrated no directional change, whereas in Jasper, assemblage turnover was relatively stable until near the middle of the MCA (~800 years BP), at which point turnover increased.
Discussion
The fossil algal records in glacier-fed Jasper Lake showed greater change through the late Holocene, particularly since the MCA, when compared to those of nearby snow-fed Albino Lake, which showed relatively little change over this time frame (Fig. 5). This underscores the importance of considering the presence of glaciers on the watershed when interpreting environmental change from algal records in alpine lakes, as it can create disparate records across lakes in close proximity to one another. We attribute the differences in the GSF and SF lakes in our study primarily to N enrichment from glacier meltwater starting just prior to the MCA when glacial meltwater influx to Jasper Lake would have increased with warming climate. In addition, to a lesser degree, these differences may be due to possible lake-level rise with increased glacial meltwater flow following the LIA.
Comparison of select data for the two lakes, including, minerogenic flux in grams of mineral matter per cm2 per year, biogenic silica accumulation rates, detrended correspondence analysis (DCA) axis 1 scores of the entire diatom assemblages, total chlorophyll pigments (Tchl; a sum of chlorophyll a, pheophytin a and b, and pheophorbide a), and tree-ring inferred air temperature for the northern Rocky Mountains (at annual resolution smoothed with a 20-year spline; Luckman and Wilson 2005). Solid circles represent Jasper Lake (GSF) and hollow triangles represent Albino Lake (SF). Light gray bars capture the Little Ice Age (100–600 years BP), Medieval Climate Anomaly (700–1000 years BP), and the Neoglacial period (2500–3400 years BP)
Several features of the diatom record in Jasper Lake (GSF) provide evidence for mild N enrichment starting approximately 1000 years BP. A. formosa increased in relative abundance at the onset of the MCA and F. crotonensis increased during the LIA. These two species are indicators of N enrichment in alpine lakes of the Rocky Mountains, and have increased in SF lakes in this region during the twentieth century owing to enhanced atmospheric N deposition (Saros et al. 2005a). This same pattern of twentieth century change is apparent in the Albino Lake (SF) diatom record, with an increase in the relative abundance of A. formosa only over the last century. However, the increase in relative abundances of A. formosa and F. crotonensis much earlier in the diatom record (MCA) in Jasper suggests that glacial meltwater had become a source of N enrichment to the lake. The continuing increases in relative abundances of these two species during the LIA and into the twentieth century indicate that N enrichment grew stronger over time. The half-saturation constants (KS) for N that are available for some of the diatom taxa in the Jasper record further support that N enrichment began over 1000 years BP and has increased over time. Lower KS values indicate lower requirements for a given nutrient and better competitive ability for that nutrient. Beginning at the start of the MCA, the sequential shift in the relative abundances of Staurosirella pinnata (Ehrenberg) D. M. Williams & Round; KS = 0.003 µM), T. glans (KS = 0.012 µM), F. crotonensis (0.028 µM), and A. formosa (K S = 0.041 µM) (Michel et al. 2006) reveal a directional change through time to a greater relative abundance of taxa with higher N requirements.
The Jasper Lake algal pigment record also suggests N enrichment in this lake starting with the MCA, as concentrations of various algal pigments (alloxanthin, total chlorophyll, canthaxanthin, and total algal abundance) increased markedly during this time. With the exception of diatoxanthin, these pigments showed low concentrations prior to the MCA and no directional change. The increase in algal pigments, often an indication of a change in lake production, may have occurred due to an increase in N-enriched runoff as a result of increased glacier volume during cold times (LIA) and an increase in runoff with glacial melting during warmer periods (MCA and modern times). In Jasper Lake, the algal assemblage was dominated intermittently by diatoms (as indicated by an increase in BSi concentrations) from the beginning of the record (~3000 years BP) to the middle of the MCA (~850 years BP). This decline preceded an increase in cryptophytes (alloxanthin) and chlorophytes (lutein) during the latter part of the MCA and the LIA, possibility indicating an escalation in plankton production of these other algal groups. This increase in non-diatom plankton productivity as indicated through the pigment analysis is synchronous with a decline in some diatom taxa, particularly small Fragilaria species and Aulacoseira species, but the emergence of others (A. formosa, F. crotonensis and D. stelligera) at the start of the MCA. While these species increase only slightly during the MCA, they are key indicator taxa of mild N increase, indicating that N enrichment is a more probable explanation than lake-level change. While these changes in algal communities indicate N enrichment in Jasper, they are not apparent in the snow-fed Albino Lake, where algal pigments of various groups decline during this same time. Consequently, these differences in the responses of algal communities between these two lakes in close proximity to each other suggest that changes in the duration of ice cover are not likely primary drivers of these patterns in the algal records.
The decline in diatom species richness in Jasper Lake at the start of the LIA is also consistent with N enrichment. Historically, many SF lakes in this region of the Rocky Mountains were N limited (Morris and Lewis 1988) and today show N and phosphorus co-limitation. In contrast, GSF lakes in the central Rocky Mountains, including Jasper Lake, are phosphorus-limited (Slemmons and Saros 2012) owing to high nitrate concentrations that are delivered via glacier meltwater. Today, GSF lakes in the central Rocky Mountains, including Jasper Lake, have lower species richness than SF lakes in the area (Slemmons and Saros 2012). Species richness decline has been linked to N enrichment and subsequent decline in the number of nutrients limiting algal growth (Interlandi and Kilham 2001).
Discostella stelligera was a relatively abundant taxon in both lake records. Interpreting environmental change from this species alone can be difficult because its environmental requirements can be somewhat diverse and may vary regionally (Köster and Pienitz 2006; Arnett et al. 2012; Saros and Anderson 2014), underscoring the importance of incorporating lake context and a multi-species approach for accurate interpretation. Increases in the relative abundance of this particular species have been attributed to changes in the length of the ice-free season (Rühland et al. 2003), mixing depth (Winder et al. 2009), nitrate (Köster and Pienitz 2006) or to a combination of factors such as mixing depth and nitrate (Saros et al. 2012). While this species has very low N and phosphorus requirements (Tibby 2004; Arnett et al. 2012), it is also opportunistic and can dominate when nutrients increase (Köster and Pienitz 2006). Consequently, the use of this species for paleolimnological interpretations must be done cautiously and in tandem with other indicator species. However, the increases in A. formosa and F. crotonensis, two reliable N indicators, in conjunction with the increase in D. stelligera in Jasper Lake suggests that D. stelligera may also have responded to N enrichment, with this enrichment enhanced in the twentieth century with accelerated glacial melting. In comparison, the consistent presence of D. stelligera throughout the Albino record exclusive of other N indicating species suggests that D. stelligera responded to factors other than nutrients. It may be consistently, relatively abundant in this dilute alpine lake because few other taxa can flourish there.
Lake-level rise or longer ice free periods in Jasper Lake following the LIA are also possible secondary mechanisms to explain some of the changes in fossil algal records, because a substantial increase in the proportion of planktic species occurs over the last 50 years. We recognize that without the bathymetry of these lakes, it is difficult to determine how the available planktic and benthic habitat areas would change with lake level. While a variety of other conditions may influence the planktic to benthic ratio of diatoms such as changes in PAR, UV, lake morphology, and food web dynamics (Stone and Fritz 2004), an increase in the planktic to benthic ratio in simple basins is often attributed to an increase in lake level (Hoagland and Peterson 1990) or an increase in overall algal productivity as a result of nutrient enrichment (Anderson 1989). Increases in algal pigments and a relatively stable P:B suggest nutrient enrichment during the LIA. Following the LIA, during accelerated glacial melting, the increase in P:B and diatoxanthin concentrations denote a possible lake-level increase as melting glaciers have been linked to increased lake level in other regions such as the Tibetan Plateau (Zhang et al. 2011). In terms of the length of ice-free period, changes in ice-off generally occur coherently within a region (Magnuson et al. 2000) and would consequently be apparent in both Jasper and Albino if this were the mechanistic link stimulating the shift in P:B communities. Our understanding of how glacier meltwater may alter the timing of lake ice-off is limited and merits more attention.
Although the possibility of pigment degradation exists, Bsi concentrations generally reflect similar trends to those of the diatoxanthin concentrations, suggesting that the diatoxanthin profile reflects patterns in diatom abundance. While fossil pigment preservation can vary independent of diatom productivity (Leavitt 1993), and pigments can degrade rapidly and selectively (Furlong and Carpenter 1988; Hurley and Armstrong 1990) or be lost during deposition, fossil pigments and algal abundance remain correlated through time barring changes in morphometry, light attenuation, stratification or hypolimnion oxygen content (Leavitt 1993). It is possible that light attenuation conditions may have changed in Jasper Lake over time if glacial flour loading varied. Increased turbidity from this material can negatively affect the preservation of fossil algal records (Karabanov et al. 2004). Currently, Jasper is not turbid, and has essentially equal water transparency to Albino Lake (Table 1), but this may not have always been the case. However, the period of highest percent inorganic material in Jasper sediments occurred from the end of the LIA to near present (AD 2007), with other periods of high percent organic also observed during 1350–1450 and 1700–2000 years BP. During all of these periods, glacial flour may have increased turbidity and caused a shift to taxa tolerant of low light conditions.
While these results do indicate that N rich glacial meltwater is not only a recent (twentieth century) phenomenon as is evident by increasing relative abundances of N-indicating diatom species in lake sediments over the last 1,000 years, it raises the question of why N does not appear to be a driver during and following other periods of glacial advance and retreat such as in the Neoglacial period. Evidence suggests that glaciers in this region did not grow to their maximum extent until the end of the Little Ice Age, estimated to be AD 1850 for the Rocky Mountain region, and therefore, determining the age, growth and retreat of the glaciers early on in the record is difficult (Carrara 1989). The magnitude of glacier meltwater input during the Neoglacial therefore remains unclear, but the fossil algal records from Jasper suggest that if the lake was receiving meltwater it was not sufficiently enriched in N to elicit an ecological change.
An additional consideration is the role of permafrost in determining N concentrations in surface waters in this region. Thawing of discontinuous permafrost has been linked to higher total N concentrations in Arctic and subarctic regions (Frey et al. 2007; Walvoord and Striegl 2007). However, little is known about the extent, distribution and composition of permafrost in alpine areas (Ives and Fahey 1971) and has only recently been explored (Janke 2005) with one of the possible sources for elevated nitrogen being linked to the thawing of glacial and permafrost features (Barnes et al. 2013). Furthermore, thawing would potentially deliver additional dissolved organic carbon to the GSF lake, which could favor facultative heterotrophs such as cryptophytes and also potentially alter lake thermal structure (Fee et al. 1996). This potential source of N and dissolved organic carbon along with the role of chemical weathering following deglaciation on the nutrient budgets of oligotrophic lakes merits more attention (Fritz and Anderson 2013) and is an important consideration for paleolimnological interpretation.
Our results indicate that this GSF lake has experienced N enrichment for over 1,000 years as the mass balance of glaciers has varied. This underscores the importance of considering the effects of glaciers over time as they may skew the interpretations gleaned from paleolimnological indicators, particularly when evidence of alpine glaciers is gone, further reinforcing the need for documentation of glacier size, presence and recession in a watershed. With the recent focus on N deposition issues in the Rocky Mountains (Baron et al. 2009; Saros et al. 2011), the confounding effects of N rich meltwater on lakes, in addition to atmospheric deposition serving as an additional source of N delivery to GSF lakes, may alter the response observed in the fossil record, warranting that GSF lakes remain outside of these types of studies focused on deposition changes over the twentieth century.
Overall we found that, in the central Rocky Mountains, glacial meltwater is a powerful driver of algal community change and that nitrogen delivery is the most likely driver of that change. This N enrichment has altered the fossil algal record, resulting in differences in algal communities relative to the SF lake in this study. The GSF Jasper Lake showed a shift to diatom species with higher N requirements over the last 1,000 years. During the twentieth century, Jasper Lake exhibited a substantial decline in species richness and increase in P:B. In contrast, Albino Lake showed minimal directional change over the 3,000-year period. This study highlights the importance of glaciers as a landscape feature that affects algal communities in lakes. It also sheds light on the importance of integrating glaciers as a driver of ecosystem processes for accurate paleolimnological interpretations particularly when alpine glaciers have disappeared. While this study indicates that glaciers are substantial drivers of algal community change, the sample size is limited. However, with both Jasper (GSF) and Albino (SF) receiving equivalent N deposition during the twentieth century, the augmented N supplied by a rapidly melting proximal glacier to Jasper Lake elicited a divergent pattern of diatom community change relative to snow-fed Albino Lake. Determining whether this pattern in algal community change occurs among other lakes receiving glacial meltwater merits more attention.
References
Anderson NJ (1989) A whole-basin diatom accumulation rate for a small eutrophic lake in Northern Ireland and its palaeoecological implications. J Ecol 77:926–946
Appleby PG, Oldfield F (1978) The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. Catena 5:1–8
Arnett H, Saros JE, Mast MA (2012) A caveat regarding diatom-inferred nitrogen concentration in oligotrophic lakes. J Paleolimnol 47:277–291
Barnes RT, Williams MW, Parman JN, Hill K, Caine N (2013) Thawing glacial and permafrost features contribute to nitrogen export from Green Lakes Valley, Colorado Front Range, USA. Biogeochemistry. doi:10.1007/s10533-013-9886-5
Baron J, Schmidt T, Hartman M (2009) Climate-induced changes in high elevation stream nitrate. Glob Chang Biol 15:1777–1789
Barron JA, Anderson L (2011) Enhanced late Holocene ENSO/PDO expression along the margins of the eastern North Pacific. Quat Int 235:3–12
Battarbee RW (1986) Diatom analysis. In: Berglund BE (ed) Handbook of Holocene palaeoecology and palaeohydrology. Wiley, Hoboken, pp 527–570
Boyd ES, Lange RK, Mitchell AC, Havig JR, Hamilton TL, Lafreniére MJ, Shock EL, Peters JW, Skidmore M (2011) Diversity, abundance, and potential activity of a nitrifying and nitrate-reducing microbial assemblages in a subglacial ecosystem. Appl Environ Microbiol 77:4778–4787
Camburn KE, Charles DF (2000) Diatoms of low-alkalinity lakes in the Northeastern United States. Special Publication 18, Academy of Natural Sciences of Philadelphia. Scientific Publication, Philadelphia, Pennsylvania, p 152
Carrara PE (1989) Late quaternary glacial and vegetative history of the Glacier National Park Region, Montana. United States Government Printing Office, Denver
Chen N, Bianchi TS, McKee BA, Bland JM (2001) Historical trends of hypoxia on the Louisiana shelf: applications of pigments as biomarkers. Org Geochem 32:543–561
Conley DJ (1998) An interlaboratory comparison for the measurement of biogenic silica in sediments. Mar Chem 63:39–48
Conley DJ, Schelske CL (2001) Biogenic silica. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments. Terrestrial, algal and siliceous indicators, vol 3. Kluwer, Dordrecht
Daly GL, Wani F (2005) Organic contaminants in Mountains. Environ Sci Technol 39:385–398
Eakins JD, Morrison RT (1978) A new procedure for the determination of lead-210 in lake and marine sediments. Int J Appl Radiat Isotopes 29:531–536
Fee EJ, Hecky RE, Kasian SEM, Cruikshank DR (1996) Effects of lake size, water clarity, and climatic variability on mixing depths in Canadian Shield lakes. Limnol Oceanogr 5:912–920
Fountain AG, Hoffman MJ, Jackson KM, Basagic HJ, Nylen T, Percy D (2007) Digital outlines and topography of the glaciers of the American West. US geological survey open-file report 2006-1340, 23 p
Frey KE, McClelland JW, Holmes RM, Smith LC (2007) Impacts of climate warming and permafrost thaw on the riverine transport of nitrogen and phosphorus to the Kara Sea. J Geophys Res 112:1–10
Fritz SC, Anderson NJ (2013) The relative influences of climate and catchment processes on Holocene lake development in glaciated regions. J Paleolimnol 49:349–362
Furlong ET, Carpenter R (1988) Pigment preservation and remineralization in oxic coastal marine sediments. Geochim Cosmochim Acta 52:87–99
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110
Hill MO, Gauch HG Jr (1980) Detrended correspondence analysis—an improved ordination technique. Vegetatio 42:47–58. doi:10.1007/BF00048870
Hoagland KD, Peterson CG (1990) Effects of light and wave distribution on vertical zonation of attached microalgae in a large reservoir. J Phycol 26:450–457
Hobbs WO, Telford RJ, Birks HJB, Saros JE, Hazewinkel RRO, Perren BB, Saulnier-Talbot E, Wolfe AP (2010) Quantifying recent ecological changes in remote lakes of North America and Greenland using sediment diatom assemblages. PLoS One 5:1–12. doi:10.1371/journal.pone.001002
Hobbs WO, Vinebrooke RD, Wolfe AP (2011) Biogeochemical responses of two alpine lakes to climate change and atmospheric deposition, Jasper and Banff National parks, Canadian Rocky Mountains. Can J Fish Aquat Sci 68:1480–1494
Holtgrieve GW, Schindler DE, Hobbs WO, Leavitt PR, Ward EJ, Bunting L, Chen G, Finney BP, Gregory-Eaves I, Holmgren S, Lisac MJ, Lisi PJ, Nydick K, Rogers LA, Saros JE, Selbie DT, Shapley MD, Walsh PB, Wolfe AP (2011) A coherent signature of anthropogenic nitrogen deposition to remote watersheds of the northern hemisphere. Science 334:1545–1548
Hurley JP, Armstrong DE (1990) Fluxes and transformations of aquatic pigments in Lake Medota, Wisconsin. Limnol Oceanogr 35:384–398
Hylander S, Jephson T, Lebret K, von Einem J, Fagerberg T, Balseiro E, Modenutti B, Souza MS, Laspoumaderes C, Jönsson M, Ljungberg P, Nicolle A, Nilsson PA, Ranaker L, Hansson L (2011) Climate-induced input of turbid glacial meltwater affects vertical distribution and community composition of phyto- and zoo-plankton. J Plankton Res 33:1239–1248
Interlandi S, Kilham S (2001) Limiting resources and the regulation of diversity in phytoplankton communities. Ecology 82:1270–1282
Irwin J (1974) Water clarity records from twenty-two New Zealand Lakes. N Z J Mar Fresh 8:223–227
Ives JD, Fahey BD (1971) Permafrost occurrence in the Front Range, Colorado Rocky Mountains. J Glaciol 10:105–111
Janke JR (2005) The occurrence of alpine permafrost in the Front Range of Colorado. Geomorphology 67:375–389
Jansson P, Hock R, Schneider T (2003) The concept of glacier storage: a review. J Hydrol 282:116–129
Karabanov E, Williams D, Kuzmin M, Sideleva V, Khursevich G, Prokopenko A, Solotchina E, Tkachenko L, Fedenya S, Kerber E, Gvozdkov A, Khlustov O, Bezrukova E, Lutnova P, Krapivina S (2004) Ecological collapse of Lake Baikal and Lake Hovsgol ecosystesms during the Last Glacial and consequences for aquatic species diversity. Palaeogeogr Palaeoclimatol Palaeoecol 209:227–243
Koenings JP, Burkett RD, Edmundson JM (1990) The exclusion of limnetic cladocera from turbid glacier-meltwater lakes. Ecology 71:57–67
Köster D, Pienitz R (2006) Seasonal diatom variability and paleolimnological inferences—a case study. J Paleolimnol 35:395–416
Krammer K, Lange-Bertalot H (1986–1991) Bacillariophyceae. In: Ettl H, Gartner G, Gerloff J, Heynig H, Mollenhauer D (eds) Sußwasserflora von Mitteleuropa (Freshwater flora of central Europe), vol 2. Gustav Fischer Verlag, Stuttgart, pp 1–4
Leavitt PR (1993) A review of factors that regulate carotenoid and chlorophyll deposition and fossil pigment abundance. J Paleolimnol 9:109–127
Leavitt PR, Carpenter SR (1990) Regulation of pigment sedimentation by photo-oxidation and herbivore grazing. Can J Fish Aquat Sci 47:1166–1176
Leavitt PR, Findlay DL (1994) Comparison of fossil pigments with 20 years of phytoplankton data from eutrophic Lake 227, Experimental Lakes Area, Ontario. Can J Fish Aquat Sci 51:2286–2299
Legendre P, Birks HJB (2001) Statistical learning in Palaeolimnology. In: Birks HJB, Lotter AF, Juggins S, Smol JP (eds) Tracking environmental change using lake sediments. Data handling and numerical techniques, vol 5. Kluwer, Dordrecht
Luckman BH, Wilson RJS (2005) Summer temperatures in the Canadian Rockies during the last millennium: a revised record. Clim Dyn 24:131–144
Magnuson JJ, Robertson DM, Benson BJ, Wynne RH, Livingstone DM, Arai T, Assel RA, Barry RG, Card V, Kuusisto E, Granin NG, Prowse TD, Stewart KM, Vuglinski VS (2000) Historical trends in lake and river ice cover in the Northern Hemisphere. Science 289:1743–1746
McCabe GJ, Dettinger MD (2002) Primary modes and predictability of year-to-year snowpack variations in the Western United States from teleconnections with Pacific Ocean climate. J Hydrometerol 3:13–25
McGowan S (2013) Pigment Studies. In: Elias S et al (eds) Encyclopedia of quaternary sciences, 2nd edn. Elsevier, Amsterdam
Michel TJ, Saros JE, Interlandi SJ, Wolfe AP (2006) Resource requirements of four freshwater diatom taxa determined by in situ growth bioassays using natural populations from alpine lakes. Hydrobiologia 568:235–243
Milner AM, Brown LE, Hannah DM (2009) Hydroecological response of river systems to shrinking glaciers. Hydrol Process 23:62–77
Morris DP, Lewis WM (1988) Phytoplankton nutrient limitation in Colorado mountain lakes. Freshw Biol 20:315–327
Naftz DL, Schuster PF, Johnson CA (2011) A 50-year record of NOx and SO2 sources in precipitation in the Northern Rocky Mountains, USA. Geochem Trans 12:2–10
National Atmospheric Deposition Program (NADP) (2011) Nitrate wet ion deposition. http://nadp.sws.uiuc.edu. (Accessed 5 Jan 2013)
Pederson, GT, Fagre DB, Gray ST, Graumlich LJ (2004) Decadal-scale climate drivers for glacial dynamics in Glacier National Park, Montana, USA. Geophys Res Lett 31. doi:10.1029/2004GL0197770
Robinson C, Kawecka B (2005) Benthic diatoms of an Alpine stream/lake network in Switzerland. Aquat Sci 67:492–506
Rühland K, Priesnitz A, Smol JP (2003) Paleolimnological evidence from diatoms for recent environmental changes in 50 lakes across Canadian arctic treeline. Arct Antarct Alp Res 35:110–123
Saros JE, Anderson NJ (2014) The ecology of the planktonic diatom Cyclotella and its implications for global environmental change studies. Biol Rev. doi:10.1111/brv.12120
Saros JE, Interlandi SJ, Doyle S, Michel TJ, Williamson CE (2005a) Are the deep chlorophyll maxima in alpine lakes primarily induced by nutrient availability, not UV avoidance? Arct Antarct Alp Res 37:557–563
Saros JE, Michel TJ, Interlandi SJ, Wolfe AP (2005b) Resource requirements of Asterionella formosa and Fragilaria crotonensis in oligotrophic alpine lakes. Can J Fish Aquat Sci 62:1681–1689
Saros JE, Rose KC, Clow DW, Stephens VC, Nurse AB, Arnett HA, Stone JR, Williamson CE, Wolfe AP (2010) Melting alpine glaciers enrich high elevation lakes with reactive nitrogen. Environ Sci Technol 44:4891–4896
Saros JE, Clow DW, Blett T, Wolfe AP (2011) Critical nitrogen deposition loads in high-elevation lakes of the Western US inferred from paleolimnological records. Water Air Soil Pollut 216:193–202
Saros JE, Stone JR, Pederson GT, Slemmons KEH, Spanbauer T, Schliep A, Cahl D, Williamson CE, Engstrom DR (2012) Climate-induced changes in lake ecosystem structure inferred from coupled neo- and plaeoeco-logical approaches. Ecology 93:2154–2155
Schindler DW, Donahue WF (2006) An impending water crisis in Canada’s western prairie provinces. Proc Nat Acad Sci Biol 103:7210–7216
Schuster P, White D, Naftz D, Cecil L (2000) Chronological refinement of an ice core record at Upper Fremont Glacier, south central North America. J Geophys Res 105:4657–4666
Skidmore ML, Foght JM, Sharp MJ (2000) Microbial life beneath a High Arctic glacier. Appl Environ Microbiol 66:3214–3220
Slemmons KEH, Saros JE (2012) Implications of nitrogen-rich glacial meltwater for phytoplankton diversity and productivity in alpine lakes. Limnol Oceanogr 57:1651–1663
Spaulding SA, Lubinski DJ, Potapova M (2010) Diatoms of the United States. http://westerndiatoms.colorado.edu. (Accessed 09 May 2012)
Stevens LR, Stone JR, Campbell J, Fritz SC (2006) A 2200-yr record of hydrological variability from Foy Lake, Montana, USA inferred from diatom and geochemical data. Quat Res 65:264–274
Stone JR, Fritz SC (2004) Three-dimensional modeling of lacustrine diatom habitat areas: improving paleolimnological interpretation of planktic:benthic ratios. Limnol Oceanogr 49:1540–1548
Stone JR, Fritz SC (2006) Multidecadal drought and Holocene climate instability in the Rocky Mountains. Geology 34:409–412
Thies H, Nickus U, Mair V, Tessadri R, Tait D, Thaler B, Psenner R (2007) Unexpected response of high alpine lake waters to climate warming. Environ Sci Technol 41:7424–7429
Tibby J (2004) Development of a diatom-based model for inferring total phosphorus in southeastern Australian water storages. J Paleolimnol 31:23–36
Tilman D, Kilham SS, Kilham P (1982) Phytoplankton community ecology: the role of limiting nutrients. Annu Rev Ecol Syst 13:349–372
Vinebrooke RD, Thompson PL, Hobbs WO, Luckman BH, Graham MD, Wolfe AP (2010) Glacially mediated impacts of climate warming on alpine lakes of the Canadian Rocky Mountains. Verhandlungen des Internationalen Verein Limnologie 30:1449–1452
Whitlock C, Bartlein PJ (1993) Spatial variations of Holocene climatic change in the Yellowstone region. Quat Res 39:231–238
Walvoord MA, Striegl RG (2007) Increased groundwater to stream discharge from permafrost thawing in the Yukon River basin: potential impacts on lateral export of carbon and nitrogen. Geophys Res Lett 34. doi:10.1029/2007GL030216
Williams MW, Knauf M, Caine N, Liu F, Verplanck PL (2006) Geochemistry and Source Waters of Rock Glacier Outflow, Colorado Front Range. Permafr Periglac 17:13–33
Winder M, Reuter JE, Schladow SG (2009) Lake warming favours small-sized planktonic diatom species. Proc R Soc B 276:427–435
Wynn PM, Hodson AJ, Heaton THE, Chenery SR (2007) Nitrate production beneath a high arctic glacier, Svalbard. Chem Geol 244:88–102
Yang Q, Mayewski PA, Linder E, Whitlow S, Twickler M (1996) Chemical species spatial distribution and relationship to elevations and snow accumulation rate over the Greenland Ice Sheet. J Geophys Res 101:18629–18637
Zhang G, Xie H, Kang S, Yi D, Ackley SF (2011) Monitoring lake level changes on the Tibetan Plateau using ICE Sataltimetry data (2003–2009). Remote Sens Environ 115:1733–1742
Acknowledgments
We thank Dennis Anderson, Andrea Nurse, and Clive Devoy for assistance with chemical analyses and pollen preparation at the University of Maine, and Teresa Needham and Graham Morris for assistance with pigment analyses at the University of Nottingham. Erin Overholt assisted with analysis of light data from Jasper and Albino. We are grateful for field and laboratory assistance provided by Carl Tugend, Carmen Daggett, Caleb Slemmons, Courtney Wigdahl, and Dom Winski. We thank Will Hobbs and Joy Ramstack Hobbs for assistance with the diatom statistical analysis. This project was funded by the US National Science Foundation (Division of Environmental Biology-0734277), as well as the Dan and Betty Churchill Fund and a University of Maine Correll Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slemmons, K.E.H., Saros, J.E., Stone, J.R. et al. Effects of glacier meltwater on the algal sedimentary record of an alpine lake in the central US Rocky Mountains throughout the late Holocene. J Paleolimnol 53, 385–399 (2015). https://doi.org/10.1007/s10933-015-9829-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-015-9829-3