Abstract
Atmospheric deposition of reactive nitrogen (Nr) has enriched oligotrophic lakes with nitrogen (N) in many regions of the world and elicited dramatic changes in diatom community structure. The lakewater concentrations of nitrate that cause these community changes remain unclear, raising interest in the development of diatom-based transfer functions to infer nitrate. We developed a diatom calibration set using surface sediment samples from 46 high-elevation lakes across the Rocky Mountains of the western US, a region spanning an N deposition gradient from very low to moderate levels (<1 to 3.2 kg Nr ha−1 year−1 in wet deposition). Out of the fourteen measured environmental variables for these 46 lakes, ordination analysis identified that nitrate, specific conductance, total phosphorus, and hypolimnetic water temperature were related to diatom distributions. A transfer function was developed for nitrate and applied to a sedimentary diatom profile from Heart Lake in the central Rockies. The model coefficient of determination (bootstrapping validation) of 0.61 suggested potential for diatom-inferred reconstructions of lakewater nitrate concentrations over time, but a comparison of observed versus diatom-inferred nitrate values revealed the poor performance of this model at low nitrate concentrations. Resource physiology experiments revealed that nitrogen requirements of two key taxa were opposite to nitrate optima defined in the transfer function. Our data set reveals two underlying ecological constraints that impede the development of nitrate transfer functions in oligotrophic lakes: (1) even in lakes with nitrate concentrations below quantification (<1 μg L−1), diatom assemblages were already dominated by species indicative of moderate N enrichment; (2) N-limited oligotrophic lakes switch to P limitation after receiving only modest inputs of reactive N, shifting the controls on diatom species changes along the length of the nitrate gradient. These constraints suggest that quantitative inferences of nitrate from diatom assemblages will likely require experimental approaches.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enhanced reactive nitrogen (Nr) deposition has widely affected lake ecosystems across the Northern Hemisphere over the last century (Wolfe et al. 2001; Bergström and Jansson 2006; Elser et al. 2009). Nr enrichment alters nutrient limitation patterns (Bergström et al. 2008; Gardner et al. 2008) and algal community structure (Wolfe et al. 2001; Saros et al. 2005a), and may affect food quality for zooplankton (Elser et al. 2010). Given the widespread effects of Nr deposition, there is interest in quantifying surface-water concentrations of nitrate that elicit ecological change in order to implement management strategies to reduce deposition levels below ecological thresholds.
Alpine lakes in low to moderate Nr deposition regions, such as the Rocky Mountains of the western US, may provide an ideal setting to quantify the effects of Nr deposition on aquatic biota. These lakes are particularly sensitive to enhanced Nr deposition because many of them are limited by nitrogen (N) (Morris and Lewis 1988; Saros et al. 2005a). Gradients along the low end of Nr deposition exist across the Rocky Mountains, with some areas of Colorado currently receiving more than 3.0 kg Nr ha−1 year−1 wet deposition (NADP 2007) whereas many areas further north along the Rockies, particularly in Montana, receive lower rates of Nr deposition (below 1.0 kg Nr ha−1 year−1 (NADP 2007)). Analyses over the last few decades in the Colorado Front Range have revealed increasing nitrate concentrations in some alpine lakes (Williams and Tonnessen 2000; Wolfe et al. 2003), while many lakes in the central Rockies have nitrate concentrations below quantification levels of 1 μg L−1 (Saros et al. 2010).
Changes in algal species are early indicators of increased Nr deposition (Baron et al. 2000; Wolfe et al. 2003, Saros et al. 2005a). Saros et al. (2003; 2005a) demonstrated that Asterionella formosa (Hassall) and Fragilaria crotonensis (Kitton) are early indicators of moderate Nr enrichment. Increases in these two taxa occurred around the 1950s–1960s in the Colorado Rockies (Wolfe et al. 2003) and in the 1980s in the central Rocky Mountains of southern Montana (Saros et al. 2005b). Although several paleolimnological studies have demonstrated that Nr deposition has driven changes in diatom community structure in western alpine lakes, the surface-water concentrations of nitrate that elicit these changes remains unclear. Establishing critical loads of Nr deposition that cause ecological change is a key step in developing remediation strategies and preventing further ecological change in sensitive lake ecosystems.
The goal of this study was to develop a transfer function for lakewater nitrate concentrations, and apply it to sedimentary diatom profiles from the US Rocky Mountains to assess whether nitrate concentrations could be reconstructed over the last century. A robust transfer function could be used to infer nitrate concentrations at which key diatom community changes occurred, and inform the establishment of critical loads based on surface-water nitrate concentrations.
Site description
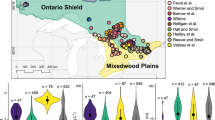
The Rocky Mountains of the western US span from northern New Mexico to northern Montana. We selected 46 high-elevation lakes across Colorado, Wyoming, and Montana based on their distribution along an Nr deposition gradient (Fig. 1). Of these 46 lakes, 26 were located in Rocky Mountain National Park and Great Sand Dunes National Park in Colorado (hereafter referred to as the southern Rockies), 11 were in the Beartooth Mountains in Wyoming and Montana (the central Rockies), and 9 were in Glacier National Park in Montana (the northern Rockies). More lakes were selected in the southern Rockies because of the large gradient in Nr deposition in this region (Fig. 1).
Materials and methods
In this study, we collected and analyzed sedimentary diatoms for a calibration set, developed a set of transfer functions, applied the nitrate transfer function to a previously published sedimentary diatom profile from the region, and conducted a set of resource physiology experiments to test whether diatom distribution patterns were consistent with their N requirements.
Diatom calibration set
Environmental parameters
Fourteen physical, chemical, and biological parameters were measured and used to construct the calibration set (Table 1). Lakes were sampled in July or August of 2007 or 2008. Water column temperature, specific conductance, and pH were measured with a Hydrolab® DataSonde 5a (Hach Environmental, Loveland, Colorado). The temperature profiles were used to determine average temperature of the photic zone as well as the mixing depths. Water samples were collected from the epilimnion and hypolinmion for determination of nutrients, chlorophyll a, and dissolved organic carbon (DOC) using standard methods (American Public Health Association 2000). Samples for dissolved nutrient analysis were filtered through 0.4-μm polycarbonate filters. Dissolved nitrate + nitrite was analyzed using the cadmium reduction method. Soluble reactive phosphorus (SRP) was measured with the ascorbic acid method and dissolved silica with the heteropoly blue method (American Public Health Association 2000). All three dissolved nutrient methods had a limit of quantification of 1 μg L−1. Particulate phosphorus was collected onto 0.4-μm polycarbonate filters and measured using persulfate digestion followed by measurement of SRP. Total phosphorus was estimated by adding the dissolved and particulate fractions. This provides a conservative, low-end estimate of total nutrient pools because of the fact that dissolved organic fractions are not included but are generally low in these alpine lakes (Saros et al. 2005a). The ratio of dissolved inorganic N (measured as nitrate) to total P (expressed as DIN:TP) was calculated based on concentrations in μg L−1. Bergström (2010) demonstrated that the DIN:TP ratio is a better indicator of nutrient limitation patterns in oligotrophic lakes than the TN:TP ratio typically used, with a DIN:TP below 1.5 suggesting N limitation.
Chlorophyll a samples were filtered through Whatman GF/F filters, which were stored in petri dishes wrapped in aluminum foil and frozen until analysis. Analyses were performed within 3 weeks of filtration according to standard methods (American Public Health Association 2000). Chlorophyll was extracted in 90% acetone solution and the extract was clarified via centrifugation. Final chlorophyll a concentrations were determined with a spectrophotometer (Varian Cary-50 UV–VIS).
Water samples for DOC were filtered through pre-combusted Whatman GF/F filters and stored in the cold and dark until analysis. DOC concentrations were measured using a Shimadzu TOC-VCPH analyzer run in regular sensitivity mode.
Diatom collection and enumeration
Sediment cores were taken from the deepest part of the lake using a gravity corer deployed off the side of an inflatable raft. Cores were sectioned in 0.5-cm intervals and the top interval (0–0.5 cm), containing the most recently deposited sediment, was analyzed for diatoms. Out of each homogenized top interval, 0.3–0.5 g of wet sediment or 0.1 g of dry sediment was weighed into 20 ml borosilicate scintillation vials. After treatment with 10% HCl to remove carbonates, samples were treated with 30% hydrogen peroxide to remove organic material. The processed material was settled onto coverslips and mounted on slides with Naphrax®. Diatom frustules were counted when greater than 50% of the valve was present; a minimum of 500 diatom valves was counted for each sample under oil immersion at a magnification of 600× or 1000× using an Olympus BX51 microscope with differential interference contrast. Diatom taxonomy was based on Krammer and Lange-Bertalot (1986–1991) and Camburn and Charles (2000).
Statistical analysis
Multivariate statistical approaches were used to relate diatom species distributions to the measured environmental variables of the 46 lakes. Detrended correspondence analysis (DCA) and constrained correspondence analysis (CCA) were used to determine the dominant gradients and environmental parameters controlling diatom distributions across the lakes. With both the DCA and CCA, rare species were downweighted and taxa that appeared in fewer than two lakes or never represented more than 2% of the total assemblage in a given sample were not included. Statistics were performed in the programming environment R (Version 2.9.0 R Development Core Team 2006). With R, the programming libraries vegan (Jari Oksanen Version 1.17-2), car (John Fox Version 1.2-16), MASS (Brian Ripley Version 7.3-5), leaps (Thomas Lumley Version 2.5), and akima (Albrecht Gebhard, Version 0.5-4) were used. Source code files RFunctions and big.cor were also included (Steve Juggins 2009). Water chemistry data from the hypolimnion were used in these analyses because many of these lakes have deep chlorophyll maxima, hence most diatoms grow at that depth (Saros et al. 2005b). Species and environmental data were examined for outliers and normality. Normality testing included histograms and normal plots to identify skewing. Environmental data that were not normally distributed were transformed as indicated in Table 1 and used in the DCA and CCA models. Models were run with species abundances untransformed or square-root transformed to find the best performance, and resulted in the use of untransformed percent relative abundances.
DCA analysis was used to determine which variables were most strongly correlated with the species axes. Using manual backward selection, the variable with the highest P value was dropped in each run until all remaining variables were significant (P < 0.05). When two parameters co-varied, correlation analysis was used to remove one of the co-variates before running the model, retaining the most ecologically relevant or most directly measured variable.
Transfer functions and nitrate reconstruction
Transfer functions were developed for the environmental variables identified as significant in the CCA model. The program C2 (Steve Juggins, Version 1.5.1) was used to construct and validate these equations using weighted-averaging with bootstrap error estimation. Weighted averaging is robust for small (< 50 sites), noisy datasets. Diagnostics were run with both types of deshrinking (classic and inverse), as well as two types of weighted averaging (basic and tolerance down-weighted). The power of the model was determined using the coefficient of determination (\( {\text{r}}_{\text{boot}}^{2} \) value) and the root mean square error of prediction (RMSEPboot) based on bootstrapping errors of 100 cycles.
The nitrate transfer function was applied to a previously published sediment core from Heart Lake in the central Rockies (Saros et al. 2011). Heart Lake is located in a region receiving low Nr deposition (<2 kg N ha−1 year−1 wet deposition; NADP 2007), and changes in diatom community structure across lakes in this area suggest that enhanced Nr deposition began affecting these lakes around 1980 (Saros et al. 2005a; Saros et al. 2011). The sedimentary diatom profile from Heart is characteristic of many in this region, with increases in the relative abundance of A. formosa starting in 1980, before which time small Fragilaria sp., small Achnanthes sp. and Discostella stelligera (Cleve and Grunow) Houk and Klee were common. In addition to applying the nitrate transfer function to the Heart core, fossil diatom assemblages along the length of the core were plotted passively onto the CCA biplot to track the direction and magnitude of change with respect to environmental variables.
Resource physiology experiments
To assess the N requirements of select diatom taxa, we determined the resource physiology for N for two species, A. formosa and D. stelligera, for which analysis of the calibration set suggested that they have nitrate optima at opposite ends of the measured nitrate gradient. To determine N requirements, batch culture experiments along a nitrate gradient were conducted using whole lake water from Fossil Lake in the Beartooth Mountains of the central Rockies. This lake had both species present, and also had very low initial concentrations of nitrate (<1 μg N L−1) in the epilimnion. Fossil Lake has a conductivity of 2 μS cm −1 and the pH of the epilimnion varies from 6.5 to 7.1 (Saros et al. 2005b).
Lake water was collected from the eplimnion at a depth of 3 m using a van Dorn bottle, and pre-screened through 100-μm mesh to remove zooplankton grazers. Three 50-ml aliquots of this initial water were preserved with Lugol’s iodine solution and used to determine initial cell densities. Six 500-mL aliquots of this water were added to acid washed (20% HCl) 500-mL bottles. All six bottles received phosphorus (1 μM in the form of NaH2PO4) and silica (100 μM in the form of Na2SiO3) amendments so that the response to N additions could be isolated. The bottles then received the following amounts of N in the form of NaNO3: 0, 0.005, 0.01, 0.05, 0.1, and 0.5 μM. Nitrate concentrations are focused at the low end of the gradient to capture growth kinetics. After homogenizing each bottle, the amended water from each bottle was distributed into three flat, polystyrene culture flasks. The 18 flasks were placed between two pieces of 3.2-mm Lucite plastic sheeting held together with 2.5-cm PVC pipe, as in Michel et al. (2006), and incubated in the lake at a depth of 3 m for 6 days. Lakewater transparency data for this lake are provided in Rose et al. (2009), and reveal that only 1% of the surface ultraviolet B radiation remained at the incubation depth, while the 1% attenuation depth for photosynthetically active radiation was 15.6 m.
At the end of the experiment, Lugol’s iodine solution was added to each flask to preserve cells. The densities of A. formosa and D. stelligera were determined in each flask and the three initial samples by settling in an Utermöhl-style chamber and examining through a Nikon TS-100 inverted microscope at 400× magnification. Cell densities were used to calculate growth rates and Monod growth kinetics were calculated using the program JMP (SAS Institute Inc., Cary, NC, USA), as in Michel et al. (2006). The half-saturation constant for growth (K s) was used to compare the N requirements of these two species. K s is the nutrient concentration at which a species reaches half of its maximum growth rate; species with a lower K s have a lower requirement for that nutrient.
Results
The range in values for each environmental parameter across the 46 lakes is indicated in Table 1. Comparing parameters across the three regions, lakes in the southern Rockies are situated at the highest elevations, have the deepest mixing depths, and span the largest nitrate gradient. Those in the northern Rockies are at the lowest elevations, and generally have the lowest chlorophyll a concentrations and higher specific conductance. Lakes in the central Rockies had lower hypolimnetic temperatures and lower specific conductance. These 46 lakes spanned a strong gradient in nitrate concentrations, ranging from extremely low (below quantification of <1 μg L−1) to moderate concentrations (maximum of 484 μg L−1).
Ninety-nine diatom taxa were identified in the surface sediments across the 46 lakes (“Appendix 1”). Across all regions of the Rockies, lakes with low to moderate nitrate concentrations (<30 μg L−1) were dominated by either A. formosa or F. crotonensis. Aulacoseira lirata (Ehrenberg) Ross was also present in these lakes. Staurosirella pinnata (Ehrenberg) Williams and Round was common in lakes with moderate to high nitrate. Lakes with the highest nitrate concentrations (>100 μg L−1), all located in the southern Rockies, were dominated by Achnanthidium minutissimum (Kützing) Czarnecki as well as D. stelligera. The distributions of these six species along the nitrate gradient are indicated in Fig. 2. In some cases, when A. formosa or D. stelligera dominated, they comprised more than 80% of the total diatom assemblages. These two species, however, did not co-dominate: when the relative abundance of A. formosa increased, that of D. stelligera declined. In contrast, D. stelligera often co-dominated with A. minutissimum. We note that some of the highest relative abundances of A. formosa occurred in lakes in which nitrate concentrations were still below quantification (Fig. 2a).
The CCA of all environmental variables for the 46 lakes revealed four significant gradients: specific conductance (P = 0.005), nitrate (P = 0.005), TP (P = 0.005), and hypolimnetic temperature (P = 0.02) (Fig. 3). Together, these four variables explained 17.4% of the total variance in diatom distributions (37.2% of the variance was explained by the complete set of 14 variables). Of the 37.2% of the total explained variance, axis one accounted for 35.2%, axis two for 27.2%, axis three for 20.6%, and axis four explained the last 17.0%. CCA biplots and supplemental DCA axis scores revealed that specific conductance was correlated most strongly with axis one, nitrate with axis two, TP with axis three, and hypolimnetic temperature with axis four.
With nitrate concentrations as one of the significant variables in the CCA, we developed a transfer function for this variable as well as the other three (specific conductance, TP, and hypolimnetic temperature) that were significant for comparison. Tolerance downweighted weighted averaging equations were developed for nitrate and TP, while weighted averaging was used for specific conductance and temperature, based on the highest level of performance in C2. All transfer functions employed inverse deshrinking to correct for the mathematical contraction of the species distribution that overestimates low values and underestimates high values.
Bootstrap cross-validation revealed that the predictive abilities of the transfer functions varied (Fig. 4). The nitrate model had a fair \( {\text{r}}_{\text{boot}}^{2} \) (0.610) but the RMSEPboot was high, with a value of 0.548 log (nitrate + 1) units. A comparison of observed versus diatom-inferred nitrate values reveals the very poor performance of this model at low nitrate concentrations (<10 μg L−1; Fig. 4a). Specific conductance had the highest \( {\text{r}}_{\text{boot}}^{2} \) value (0.627) and a moderate RMSEPboot. The model performed well with moderate specific conductance values but had high error at either end of the gradient (Fig. 4b). The TP model had the lowest \( {\text{r}}_{\text{boot}}^{2} \) of 0.485 but also the lowest RMSEPboot value of 0.212 log (TP + 1) units. The TP model performed well with moderate values but, like the nitrate model, did poorly at low concentrations (Fig. 4c). Finally, the hypolimnetic temperature model had an \( {\text{r}}_{\text{boot}}^{2} \) of 0.554 and a moderate RMSEPboot of 0.259 square root temperature units, but prediction errors increased with increasing temperature values (Fig. 4d).
Based on the distributions of species across the lakes, the most abundant species in the calibration set lakes have nitrate optima that span from about 10 to 120 μg L−1 based on the weighted averaging model. Aulacoseira sp., A. formosa, F. crotonensis, and Tabellaria sp. (flocculosa v. linearis and flocculosa Strain IV) have optima at the low to moderate end of the range of nitrate concentrations (ranging from 10 to 35 μg L−1). Staurosirella pinnata, A. minutissimum, and D. stelligera had higher optima, ranging from about 70 to 120 μg L−1.
However, results of the resource physiology experiments provide a different perspective on nitrate requirements (Fig. 5). The nitrogen Ks for D. stelligera was 0.04 μg L−1 (0.003 ± 0.003 μM N) while it was 2.5 μg L−1 (0.18 ± 0.06 μM N) for A. formosa. Although the standard error for D. stelligera is large relative to the Ks value, and this is a common problem encountered with Ks estimates (Grover 1989), the shape of the growth curves reveal that D. stelligera has much lower N requirements than A. formosa.
Application of the nitrate transfer function to the Heart Lake diatom profile further demonstrates the problems with this approach for inferring lakewater nitrate. Contrary to expectations, diatom-inferred nitrate values dropped substantially when the shift from small generalist taxa (such as small Fragilaria sp., small Achnanthes sp., and D. stelligera) to A. formosa occurred (average of 157 μg L−1 nitrate before 1980 and average of 54 μg L−1 after 1980; data not shown). The passive plot of Heart fossil diatom assemblages onto the CCA biplot (Fig. 6) reveals that assemblages plot along the nitrate trajectory, but track from higher to lower concentrations over time.
Discussion
Although nitrate concentrations affected the distribution of diatom taxa across these lakes, and this suite of lakes included those with some of the lowest nitrate concentrations possible, we were unable to develop a transfer function capable of accurately reconstructing lakewater nitrate concentrations. The problem was more than a matter of overestimation of nitrate concentrations: the direction of reconstructed change was opposite to that previously inferred (Wolfe et al. 2001; Saros et al. 2011). We believe this is due to two major issues, both of which could apply beyond this study region and hinder this approach in other areas as well. The primary issue was that changes in diatom assemblages occurred before nitrate concentrations were quantifiable (below 1 μg L−1 in this study). Species such as A. formosa and F. crotonensis that respond rapidly to moderate increases in N (Saros et al. 2005a; Michel et al. 2006) dominated at the low end of the N gradient spanned by these lakes. This suggests that focusing our analysis on the low end of the gradient would not improve the model. A second issue was that, as nitrate concentrations increased in these oligotrophic lakes, these historically N-limited lakes underwent a transition to P limitation (Nydick et al. 2003; Gardner et al. 2008), changing the importance of nitrate availability for diatoms. Of the 46 lakes in our calibration set, only the 11 lakes with the lowest nitrate concentrations had DIN:TP ratios below 1.5, indicative of N-limitation. All remaining lakes had higher nitrate concentrations and therefore, DIN:TP ratios indicative of P limitation. One could address this problem by focusing on the low end of the nitrate gradient, if the primary problem identified above was not an issue.
Our resource physiology experiments confirmed that A. formosa has higher N requirements than D. stelligera, a species that was abundant at the high end of the measured nitrate gradient. We suggest that small, generalist diatoms such as D. stelligera and A. minutissimum dominated at higher nitrate concentrations because of their low P requirements, not high N requirements. DIN:TP ratios suggest that these high N lakes were P-limited, and these species would have low enough P requirements to tolerate these high DIN:TP supply ratios (Fairchild et al. 1985; Stelzer and Lamberti 2001; Soininen 2002). Because these generalist species dominated at high nitrate concentrations across the lakes, they had high nitrate optima in the transfer function. As a result, the dominance of generalist species in fossil diatom assemblages throughout the bottom of the Heart Lake core led to high diatom-inferred nitrate concentrations prior to the period in which atmospheric Nr deposition is thought to have increased.
When including N in calibration sets, total nitrogen (TN) is often the metric employed (Siver 1999; Lim et al. 2001; Bloom et al. 2003), presumably to more completely capture N dynamics. However, even in oligotrophic lakes, refractory dissolved organic N can comprise a significant portion of TN (Lewis and Wurtsbaugh 2008), raising questions about how relevant this metric is for understanding the effects of N on diatom distributions. Bergström (2010) demonstrated that DIN:TP ratios are better predictors than TN:TP ratios of the nutrient limitation status of oligotrophic lakes. This suggests that biologically-available forms of N, such as nitrate, would be more suitable metrics to use in diatom-based calibration sets; however, our study as well as others have encountered problems employing this metric. In a study of European alpine lakes, Curtis et al. (2009) found that nitrate was a significant variable controlling diatom distributions, but found that it co-varied with DOC, complicating the potential for nitrate inferences.
Although there are problems with diatom-based nitrate transfer functions, other diatom-based approaches to quantifying critical loads remain some of the best available methods for assessing the effects of Nr on aquatic ecosystems. Using fossil diatom profiles from lakes in the Colorado Front Range, Baron (2006) used hindcasting methods to establish a critical Nr deposition load for that area of 1.5 kg Nr ha−1 year−1, which is well below current deposition rates in this area (NADP 2007). In this method, an exponential equation was fit to a 19-year record of Nr deposition rates to hindcast values back to the period of key diatom changes (i.e. increases in the relative abundances of A. formosa and F. crotonensis) in the sediment record. The same approach was employed by Saros et al. (2011) to define critical loads for the central Rocky Mountains and Sierra Nevada of California. This approach focuses on indicator species known to respond to N (Saros et al. 2005a). Bradshaw et al. (2002) also found that if only certain species known to have ecological relevance to the study were included, environmental inferences tended to improve due to reduction of species that have little ecological relevance to the problem. To quantify and infer surface water nitrate concentrations that elicit ecological change, experimental approaches hold promise, if the experimental design and conditions allow for low enough Nr concentrations to be tested. In our experiments here, we found that A. formosa, a consistently strong indicator of Nr enrichment in oligotrophic, alpine lakes, would already achieve maximum growth when supplied with only 5 μg N L−1.
Conclusions
Oligotrophic alpine lakes can serve as ideal systems to understand the effects of enhanced Nr deposition on lake ecosystems. Excess nutrient loading can quickly alter the chemistry of these dilute lake systems, and elicit community- and ecosystem-level changes. Understanding the threshold concentration that causes changes in the diatom communities in oligotrophic alpine lakes is an important step towards improving protection of remote, oligotrophic lakes in the Northern Hemisphere. We attempted to quantify the surface-water nitrate concentrations that elicit diatom community changes in these lakes through the development of a diatom-based transfer function, but found that the abundances of key indicator species of N enrichment were already high before changes in nitrate concentrations could be detected. Despite this problem, we suggest that other diatom-based approaches to quantifying critical loads, such as hindcasting and experimental approaches, still provide some of the best available methods for assessing the effects of Nr on aquatic ecosystems.
References
American Public Health Association (2000) Standard methods for the examination of water and wastewater, 20th edition. American Public Health Association, Washington, DC
Baron JS (2006) Hindcasting nitrogen deposition to determine an ecological critical load. Ecol Appl 16:433–439
Baron JS, Rueth HM, Wolfe AM, Nydick KR, Allstott EJ, Minear JR, Moraska B (2000) Ecosystem responses to nitrogen deposition in the Colorado Front Range. Ecosystems 3:352–368
Bergström AK (2010) The use of TN:TP and DIN:TP ratios as indicators for phytoplankton nutrient limitation in oligotrophic lakes affected by N deposition. Aquat Sci. doi:10.1007/s00027-010-0132-0
Bergström AK, Jansson M (2006) Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Glob Change Biol 12:635–643
Bergström AK, Johnsson A, Jansson M (2008) Phytoplankton responses to nitrogen and phosphorus enrichment in unproductive Swedish lakes along a gradient of atmospheric nitrogen deposition. Aquat Biol 4:55–64
Bloom AM, Moser KA, Porinchu DF, MacDonald GM (2003) Diatom-inference models for surface-water temperature and salinity developed from a 57-lake calibration set from the Sierra Nevada, California, USA. J Paleolimnol 29:235–255
Bradshaw EG, Anderson NJ, Jensen JP, Jeppesen E (2002) Phosphorus dynamis in Danish lakes and the implications for the diatom ecology and palaeoecology. Freshw Biol 47:1963–1975
Camburn KE, Charles DF (2000) Diatoms of low-alkalinity lakes in the Northeastern United States. Publication, Proc Acad Natl Sci Phila Special 18
Curtis CJ, Juggins S, Clarke G, Battarbee RW, Kernan M, Catalan J, Thompson R, Posch M (2009) Regional influence of acid deposition and climate change in European mountain lakes assessed using diatom transfer functions. Freshw Biol 54:2555–2572
Elser JJ, Andersen T, Baron JS, Bergström AK, Jansson M, Kyle M, Nydick KR, Steger L, Hessen DO (2009) Shifts in Lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326:835–837
Elser JJ, Peace AL, Kyle M, Wojewodzic M, McCrackin ML, Andersen T, Hessen DO (2010) Atmospheric nitrogen deposition is associated with elevated phosphorus limitation of lake zooplankton. Ecol Lett 13:1256–1261
Fairchild GW, Lowe RL, Richardson WB (1985) Algal periphyton growth on nutrient-diffusing substrates: an insitu bioassay. Ecology 66:465–472
Gardner EM, McKnight DM, Lewis WM Jr, Miller MP (2008) Effects of nutrient enrichments on phytoplankton in an alpine lake, Colorado, USA. Arct Antarct Alp Res 40:55–64
Grover JP (1989) Phosphorus-dependent growth kinetics of 11 species of freshwater algae. Limnol Oceanogr 34:341–348
Krammer K, Lange-Bertalot H (1986–1991) Bacillariophyceae. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, vol 2(1–4)
Lewis WM, Wurtsbaugh WW (2008) Control of lacustrine phytoplankton by nutrients: erosion of the phosphorus paradigm. Int Rev Hydrobiol 93:446–465
Lim DSS, Douglas MSV, Smol JP (2001) Diatoms and their relationship to environmental variables from lakes and ponds on Bathurst Island, Nunavut, Canadian High Arctic. Hydrobiologia 450:215–230
Michel TJ, Saros JE, Interlandi SJ, Wolfe AP (2006) Resource requirements of four freshwater diatom taxa determined by in situ growth bioassays using natural populations from alpine lakes. Hydrobiologia 568:235–243
Morris DP, Lewis WM Jr (1988) Phytoplankton nutrient limitation in Colorado mountain lakes. Freshw Biol 20:315–327
National Atmospheric Deposition Program (2007) Nitrate we ion deposition. Accessed 16 April 2009 <http://nadpiswsillinoisedu/isopleths/maps2007/no3depgif>
Nydick KR, Lafroncois BM, Baron JS, Johnson BM (2003) Lake-specific responses to elevated atmospheric nitrogen deposition in the Colorado Rocky Mountains, U.S.A. Hydrobiologia 510:103–114
Rose KC, Williamson CE, Saros JE, Sommaruga R, Fischer JM (2009) Differences in UV transparency and thermal structure between alpine and subalpine lakes: implications for organisms. Photochem Photobiol Sci 8:1244–1256
Saros JE, Interlandi SJ, Wolfe AP, Engstrom DR (2003) Recent changes in the diatom community structure of lakes in the Beartooth Mountain Range, USA. Arct Antarct Alp Res 35:18–23
Saros JE, Michel TJ, Interlandi SJ, Wolfe AP (2005a) Resource requirements of Asterionella formosa and Fragilaria crotonensis in oligotrophic alpine lakes: implications for recent phytoplankton community reorganizations. Can J Fish Aquat Sci 62:1681–1689
Saros JE, Interlandi SJ, Doyle S, Michel TJ, Williamson CE (2005b) Are the deep chlorophyll maxima in alpine lakes primarily induces by nutrient availability, not UV avoidance? Arct Antarct Alp Res 37:557–563
Saros JE, Rose KC, Clow DW, Stephens VC, Nurse AB, Arnett HA, Stone JE, Williamson CE, Wolfe AP (2010) Melting alpine glaciers enrich high-elevation lakes with reactive nitrogen. Environ Sci Technol 44:4891–4896
Saros JE, Clow DW, Blett T, Wolfe AP (2011) Critical nitrogen deposition loads in high-elevation lakes of the western U.S. inferred from paleolimnological records. Water Air Soil Pollut 216:193–202
Siver PA (1999) Development of paleolimnological inference models for pH, total nitrogen and specific conductivity based on planktonic diatoms. J Paleolimnol 21:45–59
Soininen J (2002) Responses of epilithic diatom communities to environmental gradients in some Finnish rivers. Int Rev Hydrobiol 87:11–24
Stelzer RS, Lamberti GA (2001) Effects of N:P ratio and total nutrient concentration on stream periphyton community structure, biomass and elemental composition. Limnol Oceanogr 46:356–367
Williams MW, Tonnessen KA (2000) Critical loads for inorganic nitrogen deposition in the Colorado Front Range, USA. Ecol Appl 10:1648–1665
Wolfe AP, Baron JS, Cornett RJ (2001) Anthropogenic nitrogen deposition induces rapid ecological changes in alpine lakes of the Colorado Front Range (USA). J Paleolimnol 25:1–7
Wolfe AP, Van Gorp AC, Baron JS (2003) Recent ecological and biogeochemical changes in alpine lakes of Rocky Mountain National Park (Colorado, USA): a response to anthropogenic nitrogen deposition. Geobiology 1:153–168
Acknowledgments
We thank Kevin Rose, William Grey, Chelsea Lucas, Erin Wilcox, and Neil Winn for valuable field assistance. Steve Juggins assisted with statistical analyses. Two anonymous reviewers provided comments and feedback that substantially improved this manuscript. This project was funded by the National Park Service and the U.S. National Science Foundation (DEB-0089600 and DEB-0734277).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Arnett, H.A., Saros, J.E. & Alisa Mast, M. A caveat regarding diatom-inferred nitrogen concentrations in oligotrophic lakes. J Paleolimnol 47, 277–291 (2012). https://doi.org/10.1007/s10933-011-9576-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-011-9576-z