Abstract
Inputs of glacial meltwater and changes in climate can profoundly influence lake ecosystems. Anderson and Seton lakes, two morphologically and chemically similar fjord lakes within the Fraser River Basin, British Columbia, experience a common biogeoclimatic setting, yet contrasting turbid-water influences from a hydroelectric development which diverts glacially-turbid water into Seton Lake, but not Anderson Lake. We conducted a comparative paleolimnological study of these two lakes to infer climatic and hydro-system influences affecting the freshwater algal community over the past ~ 200 years. Paleolimnological analysis of multiple cores for sedimentary diatom assemblages from Seton Lake revealed substantial diversion-related reductions in diatom concentrations and fluxes following the completion of the Bridge River Diversion (ca. 1950). Diatom compositional changes in Seton Lake were consistent with decreased light penetration due to increased turbidity. These changes did not occur in Anderson Lake, indicating the changes in the Seton Lake cores were likely driven by inflow of the glacially-turbid waters. Both lakes exhibited diatom compositional changes ca. 1980, with a rise in Lindavia comensis coincident with significant increases in local mean annual air temperatures and presumably associated limnological changes. Modern phytoplankton data, collected as part of this study, provides support for the occurrence of different L. comensis morphs throughout the sampling period (May–October) in Anderson Lake and in the fall in Seton Lake. The rise of L. comensis in both Anderson and Seton lakes is conceivably linked to the recent ice-free conditions enabling this taxon to persist throughout the year.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate warming is resulting in the rapid ablation of glaciers around the world (Milner et al. 2017; Moon 2017). Glacier runoff is an important source of freshwater and hydropower generation in British Columbia, with glaciers and ice fields covering ~ 3% of British Columbia (Bolch et al. 2010). The volume of glacial ice in western Canada is expected to be much reduced by 2100 (Clark et al. 2015), influencing flow to downstream lakes. Examination of changes in glacial input from hydro-development, such as presented in this study, can provide analogies to similar influences as the result of glacial melting in response to the warming climate.
Increased inputs of fine glacial sediments as the result of melting glaciers has led to higher turbidity in many aquatic systems worldwide (Slemmons et al. 2013; Rose et al. 2014; Sommaruga 2015). Increased turbidity in lakes can have profound effects on their physical and chemical properties, and as a consequence on biological communities (Bonalumi et al. 2011; Slemmons et al. 2013). For example, inputs of glacial flour influences light attenuation, and thereby the depth of the euphotic zone, which in turn affects thermal stratification, the distribution of plankton in the water column, primary production, and ultimately the flow of energy to higher trophic levels (Lloyd et al. 1987; Melack et al. 1997; Edmundson and Edmundson 2002; Hylander et al. 2011; Slemmons et al. 2013; Rose et al. 2014). Increased inputs of glacial flour also have the potential to alter the nutrient regime in lakes. Depending on the mineral content of the glacial flour, concentrations of phosphorus (Hodson et al. 2004) and nitrogen may increase with increased inflow of glacial meltwaters with the potential to influence algal growth (Slemmons et al. 2013).
Climate warming is also directly affecting lake-water temperatures, with surface waters often warming at a faster rate than air temperatures (Richardson et al. 2017). The extent and duration of ice cover, as well as a multitude of consequential limnological properties, including mixing depth and length of the growing season, are changing as climate warms (Richardson et al. 2017). Warming lake temperatures and increased duration of the ice-free period change light availability to algal communities, algal growth and seasonal plankton dynamics (Lotter and Bigler 2000; Huisman et al. 2004; Weyhenmeyer et al. 2008; Feuchtmayr et al. 2012). The period of ice cover has declined in numerous lakes in the Northern Hemisphere leading to numerous changes in algal production and community composition (Magnuson et al. 2000; Weyhenmeyer et al. 2008; Benson et al. 2012; Wang et al. 2012). The phenology of lake ice (timing of ice break up and freeze up) are driven largely by latitude, altitude and morphometric characteristics (Bernhardt et al. 2012; Kirillin et al. 2012; Magee and Wu 2017; Hewitt et al. 2018). Morphometric characteristics of lakes largely determine the degree of heat storage, while water clarity can also influence heat budgets through the degree of light penetration, with deeper, larger and clearer lakes often being more sensitive to increasing air temperatures due to their large thermal capacity and delay in forming ice (Bernhardt et al. 2012; Magee and Wu 2017; Richardson et al. 2017).
Light availability, which can be profoundly influenced by glacial inputs, plays an important role in phytoplankton composition and seasonal dynamics. The ability of diatoms to adapt to varying light conditions enables them to occupy a wide range of ecological niches, including low-light conditions during unstable water column periods and the deep chlorophyll maxima, DCM (Lavaud et al. 2007; Polimene et al. 2014; Shi et al. 2016). The existence and depth of the DCM is highly influenced by light availability, and can be affected by inputs of glacial water (Modenutti et al. 2013; Navarro et al. 2018).
Nutrient concentrations are also a major influence on diatom composition and seasonal dynamics. Both of the lakes in this study are Sockeye Salmon (Oncorhynchus nerka) nursery lakes and salmon-derived nutrients (SDN) in nursery lakes often account for a large proportion of the nutrient budget and diatom communities are responsive to these inputs (Finney et al. 2000; Gregory-Eaves et al. 2003; Chen et al. 2011). Glacial meltwaters and associated glacial flour can also be a source of nutrients (Slemmon et al. 2013), in addition to nutrient inputs from the watershed depending on vegetation, precipitation and other lake-watershed interactions (Hobbs and Wolfe 2007; Selbie et al. 2009).
The comparative paleolimnological study of Anderson and Seton lakes, two large, deep fjord lakes in the Fraser River drainage basin in British Columbia (BC) provides a unique opportunity to explore the influence of glacial-water inflow and climate on long-term diatom composition. Seton Lake has received influxes of glacial flour since the 1950s via the construction of the Bridge River Diversion (BRD) for hydropower generation. Anderson Lake flows into Seton Lake and consequently can act as a comparative reference system as it is not influenced by glacial input and resultant turbidity. Furthermore, the two lakes are located within the same biogeoclimatic zone, have similar morphological and chemical characteristics, and thus Anderson Lake can also act as a comparative reference system for climate influences apart from the BRD construction.
Paleolimnological records often provide the only means to assess long-term dynamics, as monitoring records of sufficient duration are rare. The ability to undertake comparative studies between lakes in the same biogeoclimatic zone with similar morphological and chemical characteristics is uncommon. The comparison of the sedimentary diatom records from Anderson and Seton lakes provides the rare opportunity to investigate the influence of both glacial turbidity and climate over the past ~ 200 years. Sub-decadal to decadal paleolimnological analyses of diatom assemblages, biological indicators that are sensitive to chemical and physical changes in the lake ecosystems (Smol and Cumming 2000; Cumming et al. 2015) were completed on multiple cores from Anderson and Seton lakes. Contemporary insights into the paleo-record were provided by the examination of seasonal changes in the diatom phytoplankton in both lakes. Analysis of the air temperature record from the Shalalth Station on the shore of Seton Lake facilitated an examination of the recent climate variation on these two sites over the last six decades.
Study lakes
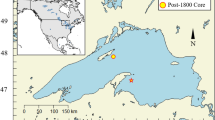
Seton and Anderson lakes are located in the Fraser River drainage basin British Columbia, Canada (Fig. 1). Anderson Lake (N 50°38. 089′ W 122°23.577′) flows into the west end of Seton Lake (N 50°41.758′ W 122°08.007′). The maximum depth of Seton Lake is 151 m with a mean depth of 85 m and volume of 21 × 108 m3. Anderson Lake is deeper with a maximum depth of 215 m, a mean depth of 140 m and volume of 37 × 108 m3. Anderson Lake has a slightly higher surface area of 28.6 km2 versus 24.6 km2 for Seton Lake (Geen and Andrew 1961). Lengths and average widths are similar at 21.9 km long × 1.1 km wide for Seton Lake and 21.3 km × 1.4 km for Anderson Lake. Neither lake freezes today, but did in the past (B. Aldoph personal communication, First Nation St’at’imc Territories). Modern limnological data were collected monthly (May-Oct) from 2014 to 2016 at two stations in each lake (Fig. 1). Both lakes are oligotrophic, with mean annual epilimnetic concentrations of total phosphorus (TP) of 2.1 µg L−1 for Anderson Lake and 2.6 µg L−1 for Seton Lake, mean annual total nitrogen (TN) of 50.4 µg L−1, and 42.2 µg L−1, respectively, and average chlorophyll a of ~ 1.1 µg L−1 for both lakes (Limnotek 2017; Barouillet et al. 2019). The mean euphotic zone depth is ~ 26 m in Anderson Lake and ~ 12 m in Seton Lake. Both Anderson and Seton lakes are slightly alkaline with average pH of 7.9 and 7.8, respectively.
Location of Anderson and Seton lakes in British Columbia. The two Anderson Lake core sites are identified as A1 and A2. The three Seton Lake cores are identified as S1, S2, and S3. The phytoplankton sampling sites are identified by the Secchi disk symbols (note one overlays the coring site S1 symbol). The Shalalth Station, next to the Bridge River Diversion from Carpenter Reservoir, is identified
The geology of the region is comprised of a mix of volcanic and sedimentary rocks of Carboniferous to Jurassic origin (Geen and Andrew 1961). A continental climate characterizes the region with cold winters and warm summers. The mean annual low in winter temperatures during December and January 1964–2016 was -1.0 °C, and was 21 °C during the warmest months of July and August at the British Columbia Hydro Shalalth Climate Station, Seton Lake (Fig. 1). The area which encompasses Anderson and Seton lakes is within the rain-shadow of the Coastal Mountains, with a semi-arid low mean annual precipitation (between ~ 300 and 400 mm) and a forest comprised mainly of Ponderosa Pine (Pinus ponderosa).
Before the Bridge River Diversion (BRD), the flow from Anderson Lake provided the majority of the water flow into Seton Lake. By the end of the BRD project in the mid-1950s, two-thirds of the water coming into Seton Lake originated from the Carpenter Reservoir. The water from Carpenter Reservoir is highly turbid and rich in glacial flour (Chernos 2014). This inflow resulted in decreased light transmission and visibility as the result of the increased turbidity (Geen and Andrew 1961). The average annual turbidity in Seton Lake from 2014–2016 was 3.3 NTU (Nephelometric Turbidity Units), whereas Anderson Lake was only 0.7 NTU (Limnotek 2017; Barouillet et al. 2019). The Carpenter Reservoir originates from the rapidly retreating Bridge Glacier (Chernos 2014), which erodes Mesozoic and Cretaceous bedrock rich in quartz diorite and low in phosphorus-bearing apatite (B.C. digital Geology, Geospatial Data, Government of British Columbia).
Methods
Sediment Core collection and sampling
A Glew gravity corer (Glew et al. 2001), equipped with a 1-m clear tube of 7.6 cm diameter, was used to retrieve sediment cores from the deep depositional basins of Seton and Anderson lakes in August 2014 (Fig. 1). Two cores from Anderson Lake were retrieved from depths of 203 m (core A1) and 205 m (core A2), and were 66.5 cm and 51.5 cm in length, respectively. Three cores were retrieved from Seton Lake at depths of 128 m (core S3), 118 m (core S2), and 110 m (core S1), and were 74 cm, 66.5 cm and 74.5 cm in length, respectively. Core S3 from Seton Lake was nearest to the discharge of the diversion, while cores S2 and S1 were located at increasing distance from the BRD (Fig. 1). The two cores from Anderson Lake were taken to span a similar spatial gradient as in Seton Lake, and were used as a temporal reference for Seton Lake because the water residence time and turbidity in Anderson Lake did not change as a result of the BRD, and because of the similar geomorphological and physical characteristics of the two lakes. The cores were sectioned into 0.5-cm intervals into 5 × 9 inch sterile bags, and were stored at ~ 4 °C. Chronological control is based on radioisotopic activities of 210Pb (lead), 137Cs (Cesium), 214Pb and 214Bi (Bismuth) using an Ortec gamma spectroscopy counter (Schelske et al. 1994) at the Paleoecological Environmental Assessment and Research Laboratory (PEARL) at Queen’s University and for Seton Lake independent markers of changes in grain size composition during pre- and post-diversion periods (Barouillet et al. 2019).
Sedimentary diatom analysis
Sub-samples for diatom analysis were processed every 1 cm from 0 to 40 cm in both of the Anderson Lake cores and at every 2 cm to the bottom of the core for each of the Seton Lake cores. This sampling strategy was chosen because it was suspected sedimentation rates would be much higher in Seton Lake. For each sediment interval, ~ 0.2–0.3 g of wet sediment was subsampled into a 20-ml glass vial and a 1:1 molar mixture of concentrated nitric (HNO3) and sulphuric (H2SO4) acid was added to remove organic matter. Samples settled ~ 24 h before the acid was aspirated and rinsed with deionized water. This settling and rinsing procedure was repeated ~ 7 times until the pH of the samples was that of the deionized water. Aliquots of each sample were pipetted onto coverslips, and air-dried overnight before heating on a warming plate and mounted with Naphrax® onto glass microscope slides. Diatoms were identified and counted along parallel slide transects using a Leica (DMRB model) microscope fitted with a 100 × fluotar objective (numerical aperture of objective = 1.3) and using differential interference contrast (DIC) optics at 1,000 × magnification. A minimum of 400 diatom valves (average of 420) were enumerated per slide. Diatoms were identified to the species level or lower, using the following taxonomic references: Krammer and Lange-Bertalot (1986,1988,1991a, b), Cumming et al. (1995), Lange-Bertalot and Melzeltin (1996), Camburn and Charles (2000) and Fallu et al. (2000).
Sedimentary concentrations of diatoms in each sample were determined following Battarbee and Kneen (1982). Briefly, an aliquot of a known concentration of microspheres was added to each diatom sample, prior to subsampling for mounting on coverslips. The microspheres were enumerated along with the diatoms and used to calculate estimates of the number of diatoms per gram dry weight of sediment. The estimated sediment accumulation rate was used to calculate diatom flux as number of diatom valves per cm2 per year.
The diatom assemblage zones in the down-core analyses were defined by a depth-constrained cluster analysis using a square-root transformation (Edwards and Cavalli-Sforza chord distance) on the relative abundance data of taxa that were > 4% abundance (Grimm 1987). Correspondence analysis (CA) was run with the computer program C2 v. 1.7.5 (Juggins 2003) as a means of simplifying the multivariate species data into the main directions of change in the diatom assemblage data. CA ordinations were run on each core from Anderson and Seton lakes using species data, expressed as percent abundance, of the major taxa or groups of similar taxa that reached > 10% minimum abundance in one sample of one of the lake sedimentary records. This was done to have the same taxa or groups of taxa included in the analyses of both lakes. The diatom nutrient preferences identified in the results and discussion sections are based on a 251-lake dataset in British Columbia (Cumming et al. 2015).
Diatom phytoplankton collection and analysis
Depth-integrated monthly water samples (20 mL from 6 equally spaced depths) were collected from the top 30 m of the water column using a Niskin bottle at two stations in each of Anderson and Seton lakes typically from May to October 2014–2016 and preserved with Lugols (Fig. 1). Counts of algal cells with chloroplasts, by taxa, were completed using an inverted microscope equipped with phase-contrast. Cells of large micro-plankton (20–200 μm) were counted at 250X magnification. All cells within one transect were counted at 1560X magnification. In total, 250–300 cells were counted in each sample (cells in filaments and colonies were enumerated equally to small cells). Taxonomic references were Canter-Lund and Lund (1995) and Prescott (1978).
A separate enumeration focused on diatom species present in the monthly samples was completed for the sampling years of 2015 and 2016, May and June sampling for 2014 were not available, nor October 2015. Approximately 120 mL of each water sample was settled in glass beakers for several days, 100 mL was then siphoned off and ~ 40 mL of 30% hydrogen peroxide was added to remove any organic material. Samples were warmed in a water bath at ~ 70 °C for ~ 4–5 h. Samples were settled for at least two days and the supernatant aspirated to 20 mL and rinsed with deionized water. Rinsing was repeated at least 5 times to remove any remaining hydrogen peroxide. Samples were plated onto coverslips, air dried and repeated on the same coverslip until the concentration of diatoms was sufficient for enumeration. A minimum of 400 diatom valves was counted when possible using the same microscope and taxonomic references as the sedimentary diatom analysis. For samples with a low concentration of diatoms at least 100 valves up to ~ 300 were enumerated within a maximum of 10 transects. Data are presented as percentages of diatom taxa, along with estimates of number of diatom valves per mL based on the quantified total algal enumeration data.
Climate analysis
Weather instrumentation near the Shalalth generating station on Seton Lake provided temperature records from 1964 to 2016 (Fig. 1). The weather instrumentation is located away from the effect of property lighting and included an Onset Hobo Micro Station Data logger (H21-002) recording data from a Photosynthetically Active Radiation (PAR) sensor (S-LIA) and a Solar Radiation sensor (S-LIB). An Onset Hobo Pro (U23) installed inside a solar radiation shield was used to measure air temperature and relative humidity.
Mean, minimum and maximum average monthly temperatures from 1964 to 2016 were calculated from the daily records (BC Hydro, unpubl. data). Mean annual temperature (MAT) was calculated for each year of the record, and a fast Fourier transform (FFT) 5-year smooth performed using Origin v. 6.1 (2000). Linear regressions between time and the mean, minimum and maximum monthly temperatures, were run to determine if any significant linear trends occurred over the length of the record. The average monthly temperature for each month over the record (1964–2016) was also calculated.
Results
Sedimentary diatoms
The average estimated temporal resolution between the samples analyzed for diatoms in the Anderson Lake cores was 6.2 years for core A1 and 6.9 years for core A2. In the Seton Lake cores, the average estimated temporal resolution between samples prior to the diversion ranged from ~ 12–13.7 years. The temporal resolution of post-diversion samples was higher due to the increased sedimentation rate and was estimated to be highest in core S3 (closest to the diversion) at 2.7 years, in core S2 at 3.5 years and core S1 at 4.8 years. The sub-decadal to decadal resolution of the analyses provided the ability to assess pre- and post-diversion changes, and enabled the evaluation of decadal-scale changes within Seton Lake in comparison to changes in Anderson Lake.
Diatom assemblages from both lakes were comprised of ~ 80% planktonic diatom taxa, reflecting the vast open-water conditions of each lake and limited littoral zones. Diatom valves were well preserved, with very few valves showing any evidence of silica dissolution. In Anderson Lake, the percent planktonic composition of the assemblages ranged from ~ 73–90%. In Seton Lake, planktonic composition ranged from ~ 62–93%, with a few samples in core S3 having a lower % planktonic composition of ~ 50%. The most common planktonic species present in both lakes were: Discostella stelligera (Cleve and Grunow) Houk and Klee; Lindavia ocellata (Pant.) Nakov, Guillory, Julius, Theriot and Alverson; Stephanodiscus minutulus (Kütz.) Round and Aulacoseira subarctica (Müller) Haworth.
Anderson Lake
Approximately 160 diatom species were encountered in the core samples from Anderson Lake, however, only 16 of these reached abundances > 4%. The diatom assemblages had small compositional change prior to ~ 1980 (Fig. 2) with small fluctuations in oligotrophic (i.e. L. ocellata, D. stelligera), meso-eutrophic (i.e. A. subarctica, S. minutulus) and eutrophic Stephanodiscus oregonicus (Ehrenb.) Håkansson planktonic taxa.
Relative abundance of the dominant diatom taxa in Anderson Lake sediment cores in a core A1, and b core A2. Total diatom concentration is to the right of the taxa. Zones (A, B1, B2) are based on depth-constrained cluster analyses. Pennate planktonic (Plank.) taxa consist of A. formosa and F. crotonensis
The earliest part of the Anderson Lake sediment records (Zone B2; ca. pre-1870 in core A1 and ca. pre-1850 in core A2) generally had higher percent abundance of A. subarctica, whereas S. minutulus was generally higher in Zone B1 (Fig. 2). In the recent sediments (Zone A; post-1980 in cores A1 and A2), increases of ~ 20–40% were observed in the oligotrophic to slightly mesotrophic planktonic Lindavia comensis (Grunow) Nakov, Guillory, Julius, Theriot and Alverson (includes small amounts of Cyclotella gordonensis Kling and Håkansson, a similar taxon). Mesotrophic pennate planktonic taxa (i.e. Asterionella formosa Hassall; Fragilaria crotonensis Kitton) also increased slightly in both cores post- 1980 (Zone A), and L. ocellata and S. minutulus declined. Diatom concentrations remained relatively stable throughout, with a small increase in core A1 in the upper sediments (upper portion Zone A). and varied around a mean of 46.7 valves g−1 dry weight × 108 in core A1 and 48.4 valves g−1 dry weight × 108 in core A2.
Seton Lake
The number of diatom species encountered in the samples for Seton Lake was ~ 165 taxa, however, only 16–19 of these taxa reached abundances > 4%. In the Seton Lake cores, meso-eutrophic planktonic taxa were often the most common, particularly S. minutulus and A. subarctica, in contrast to the Anderson Lake cores in which the more common planktonic taxa were oligotrophic, D. stelligera and L. ocellata.
Comparing across all of the Seton Lake cores there is a high similarity in the diatom assemblages (Fig. 3). For example, L. ocellata was most abundant ca. pre-1950 (Zone B2, and also B3 in core S1), and L. comensis increased to between 5–10% ca. post-1980 in all cores (Zone A), similar to the increase seen in the Anderson Lake cores, but of a lower magnitude. In all cores, post ca. 1950 (Zone B1) L. ocellata declined in percent abundance, whereas meso-eutrophic planktonic Fragilaria (F. capucina Desmaziéres, F. capucina v. gracilis (Østrup) Hustedt, F. nanana Lange-Bertalot, F. tenera (W. Sm.) Lange-Bertalot and F. crotonensis only in core S3) and A. formosa increased. Total benthic taxa also increased post ca. 1950, possibly as a result of increased transport of littoral taxa with the increased flow from the BRD, and was most pronounced in core S3. These changes in the diatom assemblage post ca. 1950 corresponded to a distinct decrease in total diatom concentration in all cores from Seton Lake compared to earlier years (Fig. 3). The sediment record from core S1 provided a longer temporal perspective and indicated distinct fluctuations in the eutrophic planktonic, A. subarctica, and oligotrophic planktonic taxa such as L. ocellata and D. stelligera (Zone B3).
Relative abundance of the dominant diatom taxa in Seton Lake sediment cores in a core S3, b core S2 and c S1. Total diatom concentration is to the right of the taxa. Zones (A, B1, B2, B3) are based on depth-constrained cluster analyses. Planktonic (Plank) Fragilaria consists of F. capucina, F. capucina v. gracilis, F. nanana and F. tenera. Taxa names were further shortened in (b) and (c), but are in same order as in (a) (e.g. O. Plank. is Other Planktonic)
In the Seton Lake cores, diatom concentrations precipitously declined post ca. 1950 to ~ 4 to 7.6 valves g−1 dry weight × 108. A similar pattern is seen in the diatom flux rates (Online Resource 1). Prior to this decline, concentrations in cores S1 and S2 varied around means of 66.4 valves g−1 dry weight × 108 and 75.7 valves g−1 dry weight × 108, respectively (slightly higher than in the Anderson Lake cores). The mean concentration in core S3, prior to the decline, was lower at 35 valves g−1 dry weight × 108.
Correspondence analysis
The axis-1 scores of a correspondence analysis (CA) on the diatom assemblages from the Anderson and Seton lake cores provides a simplification of the species-rich records, accentuating the major underlying changes over the past 200 years. The CA axis-1 scores of the Anderson Lake data highlights the relative stability of the diatom assemblages from ca. 1800 to 1970 and the prominent change in the diatom assemblages at ca. 1980, driven primarily by the large increase in L. comensis (Fig. 4). In the Seton Lake cores, diatom assemblages exhibited small variations pre-1950, followed by a distinct two-step change in the diatom assemblages exemplified first at ca. 1950, and subsequently at ca. 1980. Of further notable interest is that while variability pre-1950 in the Seton Lake cores was low, it was higher than what was observed in the Anderson cores pre-1980, further emphasizing the large post-1980 changes in Anderson Lake.
Seasonal diatom dynamics
The primary focus of the analysis of the limited modern diatom data was to explore whether any seasonal patterns in dominant diatom taxa could be discerned, particularly L. comensis, to provide insight into this taxon’s distinct post-1980 rise in the sediment records from Anderson and Seton lakes. Measured monthly limnological variables generally did not significantly vary within each lake. For example, seasonal variation in TP and TN indicated no significant difference within or between lakes (Barouillet et al. 2019). While, the depth of the euphotic zone was significantly shallower in Seton Lake compared to Anderson Lake, ~ 12 m vs ~ 26 m, respectively (t-test, p < 0.005), seasonal variation within each lake was limited (Barouillet et al. 2019). As a consequence, the ability to decipher the influence of these variables on diatom seasonality in 2015 and 2016 was difficult. Hence, the focus in this paper is on the seasonal patterns of individual diatom species, and how these observations help with the interpretation of the observed changes in the species assemblages in the cores.
Diatom algal counts (#cells mL−1) were typically highest in the spring and late-summer/early-fall in both Anderson and Seton lakes, with the exception of 2016 in which the late-summer/early-fall counts in Seton Lake were very low (Fig. 5). In Anderson Lake, D. stelligera was more prominent in the spring sampling; whereas L. comensis was found throughout the sampling season, but typically comprised a greater percentage of the late summer/fall assemblage (Fig. 5a, b). Lindavia bodanica (Grunow ex Håkansson) Nakov, Guillory, Julius, Theriot and Alverson and F. crotonensis were also of higher relative abundance in the summer/fall sampling. In 2015, A. subarctica had its’ highest relative abundance in July; whereas in 2016 this taxon was dominant in May and June.
Relative abundance of the dominant diatom taxa in monthly water samples for Anderson Lake a 2015 and b 2016 and Seton Lake c 2015 and d 2016. Results from each month consist of two sampling stations with one replicate at each station. Absolute numbers (diatom algal count) are based on the total algal enumeration. (O. Plank. Fragilaria = Other Planktonic Fragilaria)
In Seton Lake, D. stelligera was present in all of the monthly samples, whereas L. comensis was a smaller component of the phytoplankton assemblage and present later in the sampling season (Fig. 5c, d). In both sampling years, F. tenera had its’ highest relative abundance in June and July, but with absolute numbers lower than in the May sampling. In 2016, A. formosa and F. crotonensis were prominent in terms of relative abundance, but total number of diatoms was very low. A. subarctica was present in all monthly samples from both years, but comprised a small proportion of the assemblage.
Shalalth climate station data
There was a significant increase in the mean annual temperature (MAT) from 1964 to 2016 recorded at the Shalalth station (r = 0.45, p < 0.001) (Fig. 6). The FFT- smoothed record exemplifies sub-decadal variability with an overall increasing trend particularly evident following the mid-1980s. Analysis of the mean monthly air temperatures indicated this was largely related to increases in summer/early-fall, as well as January temperatures (Online Resource 2). Both the mean and maximum July and September temperatures exhibited significant, increasing trend from 1964 to 2016. January temperatures indicated a significant increase since 1964 in the mean, maximum and minimum air temperatures. Other significant increasing trends were mean April air temperature and minimum May temperature. While the significant monthly temperature trends were not strong, the analyses provided information on which months were the prominent drivers of the changes in the MAT (Online Resource 3).
Discussion
Influence of glacial turbidity on diatom dynamics
In western Canada, surface runoff from glaciers provides a large source of freshwater (Bolch et al. 2010) and varying turbidity inputs can influence downstream lakes. Glacial flour and meltwater can alter levels of phosphorus, acting either as a source or further limit phosphorus availability through colloidal binding (Hodson et al. 2004). In addition, glacial meltwaters may be enriched in nitrate and thereby have the potential to alter algal assemblages (Slemmons et al. 2013). The influence of glacial meltwater on turbidity in lakes can also impact the depth of light penetration (Slemmons et al. 2013).
The euphotic zone of Seton Lake was significantly shallower than Anderson Lake and was negatively correlated with turbidity from 2014–2016 associated with the glacier inflow (Barouillet et al. 2019). Total diatom concentrations rapidly declined post-1950 in all of the Seton Lake cores, coincident with flow from the BRD and concurrent increase in clay-sized clastic material in the cores (Online Resource 4). This is in stark contrast to the relative stability of diatom concentrations throughout the Anderson Lake cores. In Kootenay Lake, a large mountain lake in British Columbia, turbidity varied in the three arms of the lake as a result of drainage from different watersheds providing a unique opportunity to assess the influence of light and nutrients on algal production (Northcote et al. 2005). The South Arm of Kootenay Lake exhibited decreased light penetration and shallower euphotic zone associated with the high inflow of glacial meltwater in comparison to the West and North Arms, which resulted in decreased primary production during the late spring and summer diatom blooms, despite higher phosphorus levels (Northcote et al. 2005). In Seton Lake, declines in Cladocera remains were also contemporaneous with the influx of glacial turbidity, hypothesized to be largely related to decreased primary production, but also to potential physiological impairment of filter feeding, and differential sensitivity of zooplankton to glacial flour (Barouillet et al. 2019).
A response by primary producers to potential new nutrient loads into Seton Lake compared to before the BRD might be expected, but the degree of turbidity associated with glacial meltwater can often be of greater influence on aquatic biota than nutrient inputs from the meltwater (Slemmons et al. 2013). Glacial runoff from the Bridge Glacier via the BRD into Seton Lake would be expected to be low in phosphorus as the parent materials are low in phosphorus-bearing apatite, but nonetheless may still be an important nutrient source that did not exist before the BRD. The post-diversion increase in percent of S. minutulus and subsequent decline post-1980, is consistent with a shorter-term response to nutrients with the initial glacial flow. However, the influence of glacial turbidity on reduced light penetration in Seton Lake may generally exceed the potential bio-stimulatory effects of added nutrient loading and on a long-term perspective reduce biological production compared to pre-BRD conditions. In a survey of 18 glacially-fed lakes in Chili, New Zealand and the Rocky Mountains of the U.S. and Canada, the extent of glacial flour was found to be the primary driver of photosynthetically activated radiation (PAR) and light attenuation (Rose et al. 2014).
The ability of diatoms to adapt to varying light conditions is thought to be a key factor in their seasonal succession and vertical distribution within lake ecosystems. The adaptive strategies to light are postulated to be related to varying photoprotection capacity and varying pigment concentrations of different diatom species (Lavaud et al. 2007; Polimene et al. 2014; Shi et al. 2016) and believed to be an essential component of why the largest blooms of diatoms most often occur during the turbulent spring and fall conditions (Huisman et al. 2004; Sommer et al. 2012). Diatom species that persist through the summer are often found in the denser metalimnion where sinking is reduced (Reynolds 1984; Longhi and Beisner 2009) and may become a component of the deep chlorophyll maximum (DCM). While light tends to be limited in the DCM, nutrient availability is often higher than in the epilimnion in large oligotrophic lakes (Saros et al. 2005; Caballero et al. 2016). Lindavia ocellata (previously Cyclotella ocellata) has been found to be abundant in the DCM (Stoermer et al. 1996; Wang et al. 2015; Caballero et al. 2016) and under other low-light conditions (Malik and Saros 2016). The decline of L. ocellata in Seton Lake (albeit a smaller component of the assemblage) coincident with the onset of the BRD is consistent with a reduction of light associated with higher turbidity leading to a reduction or elimination of the DCM (Stoermer et al. 1996; Hylander et al. 2011); although other studies suggest the DCM depth becomes shallower with increased turbidity but is not eliminated (Navarro et al. 2018). In Lake Superior, the abundance of L. ocellata was not found to be related to turbidity or thermocline depth (proxies of light availability), but the maximum turbidity only reached 0.6 NTU from 2001 to 2011 (Kireta and Saros 2019), much lower than in Seton Lake. The post-diversion increase in planktonic Fragilaria and A. formosa may in part be related to their ability to occur in light-limited conditions under higher nutrient conditions in the epilimnion of lakes (Reynolds et al. 2002); increases in these taxa have also been associated with nitrogen enrichment in alpine lakes (Yang et al. 1996; Saros et al 2011).
The sharp decline in diatom concentrations in Seton Lake coincident with the BRD and the lack of a similar pattern in Anderson Lake provides substantial evidence that inputs of glacial flour had a profound impact on the primary production of diatoms. Furthermore, adaptability to low-light conditions and short-term nutrient pulse from the initial glacial turbidity inputs may help to explain the trends of a number of diatom taxa in Seton Lake. The lack of similar changes in the temporal reference system of Anderson Lake supports unique internal dynamics within Seton Lake, exemplified by the initial post-1950s shift in species composition (Fig. 4).
Influence of climate on diatom community structure
Changes in climate may alter the physical attributes of a lake over time (e.g. water temperature, mixing depth, ice cover and length of growing season), which in turn could influence the abundance and composition of diatom taxa. One of the most profound influences on a lake ecosystem is going from being seasonally ice-covered to a year-round open system (Weyhenmeyer et al. 2011). As the length of the ice-free season and number of ice-free years increases in many lakes (Assel et al. 2003; Weyhenmeyer et al. 2008; Wang et al. 2012; Magee et al. 2016), algal growth during the winter would be expected to expand, even under low temperature and low-light conditions. Although there are no ice records for Anderson Lake, the native elders recall skating on Anderson Lake in the late 1960s, consistent with photos of ice cover during this time, whereas today neither Anderson nor Seton lakes freeze in the winter (B. Aldoph pers com, First Nation St’at’imc Territories).
Globally, winter temperatures have had larger increases than other seasons (IPCC 2013), with warming most pronounced since 1979 (Hartmann et al. 2013). The number of ice-free winters in Swedish lakes was related to significantly higher January and February temperatures (Weyhenmeyer et al. 2008). The timing of ice formation is generally correlated with air temperature and local winds and is also dependent on the morphometric characteristics of lakes which determine the degree of heat storage (Arp et al. 2010, 2013; Bernhardt et al. 2012; Kirillin et al. 2012; Magee and Wu 2017). Water clarity can also influence the heat budget of lakes, as it controls the degree of light penetration (Bernhardt et al. 2012; Magee et al. 2016). Deeper, larger, and clearer lakes, often with large thermal capacity, can be sensitive to increasing air temperatures, particularly exemplified by the delay in the onset of ice formation (Bernhardt et al. 2012). January temperatures significantly increased since 1964 at the Shalalth climate station, as well as MAT, most notably post ca. 1980 (Online Resources 2 & 3). This trend holds more broadly across the British Columbia Southern Interior Ecoprovince in which these lakes are situated, which has experienced the most pronounced warming in winter relative to other seasons over the period 1900–2013 (Mean Winter Temperature + 1.5 °C, Maximum Winter Temperature + 1.2 °C, Minimum Winter Temperature + 2.4 °C, BC Ministry of the Environment, MoE 2016). A number of other regions have experienced a post-1980 warming, including the Canadian Rockies (Hobbs et al. 2011) and central Europe (Woolway et al. 2017). On a global perspective, the rate of increase in average land and ocean temperature since 1880 has further increased since 1981 to more than double the rate prior to this time (NOAA 2019).
The distinct shift in diatom assemblages in both Anderson and Seton lakes ca. 1980, as exemplified by the CA axis-1 scores, was coeval with the continued rise in mean annual air temperatures at the Shalalth Station. The increase in L. comensis was more pronounced in Anderson Lake where it reached nearly 40% of the assemblage from being rare or non-existent prior to ca. 1980. Similarly, L. comensis (previously Cyclotella comensis) increased since 1985 in a northern Swedish lake coincident with increasing air temperatures (Bigler and Hall 2003). Rises in small oligotrophic centric planktonic diatoms (Cyclotella, Lindavia, Discostella) have been related to increased stratification in lakes with warming conditions (Sorvari et al. 2002; Rühland et al. 2008; Winder et al. 2009; Shaw Chraïbi et al. 2014). The mixing depth of the water column can also be an important factor in the distribution of Cyclotella species (Saros et al. 2012). Sedimentary records from the Great Lakes indicated L. comensis increased ca. 1970–1980, whereas D. stelligera declined or had no discernible trend (Reavie et al. 2017), similar to what was observed in Anderson Lake. A significant increase in the minimum annual temperature showed the most consistent relationship to Cyclotella across all of the Great lakes (Reavie et al. 2017). Accumulating evidence indicates the rise in L. comensis and other Cyclotella taxa can be indirectly linked to increasing air temperature, which would influence lake-water temperature (Schneider and Hook 2010; O’Reilly et al. 2015; Richardson et al. 2017; Woolway et al. 2017), ice duration (Magnuson et al. 2000), light regimes, and thereby lake thermal structure and mixing depths (Edmundson and Mazumder 2002; Saros et al. 2016).
In Anderson Lake, the post 1980 rise of L. comensis occurred while D. stelligera comprised a large component of the diatom assemblage for at least the past ~ 200 years. The appearance or increased presence of D. stelligera is one of the most cited taxon for inferring increased strength and length of stratification in sedimentary records (Rühland et al. 2008, 2015; Saros and Anderson 2015). In this study, inferring the rise of L. comensis primarily to such a mechanism seems an inadequate explanation with the long-term presence of D. stelligera. Various forms of L. comensis have been documented in alpine lakes in the Alps and Canadian Rockies (Wunsam et al. 1995; Yang et al.1996; Hausmann and Lotter 2001), and in temperate lakes in southern Ontario (Werner and Smol 2005). Hausmann and Lotter (2001) found no clear relationship of the L. comensis complex with environmental variables; whereas, summer temperature was found to be related to the distribution of six defined morphs. Scheffler and Morabito (2003) documented morphs of L. comensis that were found in the late-fall and in the early-spring, suggesting it may have overwintered. Five morphs of L. comensis were defined in the Anderson and Seton lake samples. In the 2015 phytoplankton samples from Anderson Lake there was a clear separation between the spring, summer and fall morphs (Online Resource 5) that were similar to the cold and warm morphs defined by Hausmann and Lotter (2001); whereas the 2016 sampling did not have as clear a distinction of the different morphs. Phytoplankton biomass has been found to be significantly higher after winters with ice-free conditions compared to those with ice coverage; diatoms often outcompeted other phytoplankton groups under the well-mixed, ice-free conditions (Weyhenmeyer et al. 2008). Thackeray et al. (2008) found that the spring dynamics of L. comensis was dependent on the magnitude of the over-wintering population, with large populations sometimes found under the ice (Kienel et al. 2017), providing a competitive advantage over other algal groups at the onset of open-water conditions.
The coeval rise in L. comensis in both Anderson and Seton lakes suggest a regional driver. We hypothesize that the rise in L. comensis was related to a recent absence of ice cover (B. Aldoph pers com, First Nation St’at’imc Territories) due to climate warming, and the adaptability of different L. comensis morphs to various light and mixing conditions over the spring to fall periods. Phytoplankton data provides insights into the potential of a higher continual contribution of frustules to the sediments, that is plausibly related to overwintering populations of L. comensis. The higher variability in assemblage composition post-1980 in Seton Lake than in Anderson Lake (exemplified in the CA axis-1 scores) and the smaller increase in L. comensis, suggests the interaction between glacial turbidity and climate influences can result in unique responses.
Insights from seasonal diatom dynamics on recent sedimentary changes
Seasonal phytoplankton data can provide insights into species-specific patterns in paleolimnological records (Boeff et al. 2016). In our study, we examined whether L. comensis and D. stelligera had different timing of blooms, which could provide insight into the recent rise of L. comensis, as both of these taxa are often grouped into Cyclotella sensu lato in sediment records (Rühland et al. 2008; Reavie et al. 2017). Deciphering specific environmental variables (i.e. light, nutrients, lake temperature) related to these seasonal dynamics is more difficult and often complex. For example, detailed analyses of phytoplankton dynamics in the Great Lakes of individual Cyclotella sensu lato species have concluded that multiple interacting physical and chemical variables often only explain part of the variation in the distribution of various taxa (Reavie et al. 2017; Kireta and Saros 2019). Nonetheless, information on seasonal dynamics can be beneficial for interpreting sedimentary records. In the Experimental Lakes Area, D. stelligera was found to be primarily a spring or early-summer bloomer (Wiltse et al. 2016); whereas other studies have shown variability in the timing of blooms across lakes in the same climatic region (Boeff et al. 2016). The occurrence of L. comensis in various light and mixing regimes, including the DCM of the Great Lakes during summer stratification (Stoermer et al. 1996; Bramburger and Reavie 2016) and blooming in the late-winter, either under the ice or under ice-free winter conditions (Thackeray et al. 2008; Kienel et al. 2017) indicates this taxon (or particular morphs) can be tolerant of low light, including cold, low-light conditions during the winter.
In Anderson Lake, the phytoplankton data indicate that D. stelligera tended to bloom in the spring, whereas L. comensis was commonly found across the sampling season. In contrast, D. stelligera was prominent throughout much of the sampling season in Seton Lake, whereas L. comensis was less common, and generally present in the summer. This is consistent with the sedimentary record where remains of D. stelligera were low post-1960 in Seton Lake, but have increased in relative abundance in the recent sediments to as high or higher than in the past. The relative dominance of L. comensis across the main growing season in Anderson Lake (May–October) provides evidence that the increase in the sedimentary record could in part be related to an overall greater annual production, particularly in the late summer/fall. We hypothesize that a decrease in ice cover and an increased length of the growing season, enabled L. comensis to have a unique niche from D. stelligera. Furthermore, more of the late summer/fall bloom may reach the sediments in comparison to the spring bloom and the subsequent clear-water phase brought about by increased zooplankton populations and associated grazing pressures (Sommer et al. 2012). This is supported by the highest density of zooplankton in Anderson and Seton lakes occurring in the spring (Barouillet et al. 2019). Complex interactions related to the glacial flour input and the influence on light availability in Seton Lake may explain the lower magnitude increase of L. comensis in comparison to Anderson Lake. However, interaction between various variables, particularly temperature, light and nutrients is complex and it is often difficult to disentangle the specific influences on the various co-occurring small Cyclotella (Lindavia, Discostella) species (Malik and Saros 2016; Kireta and Saros 2019).
Comparative paleolimnological studies
Comparative paleolimnological studies of lakes with similar chemical, morphological and biogeoclimatic settings can provide unique opportunities to examine long-term environmental impacts, but rarely do such circumstances exist (Hadley et al. 2010). Here we examined the influence of increased inputs of glacial flour into Seton Lake post ca. 1950, a phenomenon that certainly will increase with glacial melting, but then abate as glaciers disappear. The largest changes in concentrations and fluxes of diatoms over ~ 200 years occurred in Seton Lake coincident with the onset of the Bridge River Diversion and influx of glacial turbidity. Total diatom concentrations sharply declined ca. post-1950 in all three Seton Lake cores. Commensurate changes in the composition of the diatom assemblages were consistent with decreased light penetration and increased turbidity. Similar changes did not occur in Anderson Lake, suggesting the changes in Seton Lake post ca. 1950 were primarily driven by internal dynamics as a result of the diversion and not a regional forcing such as climate. This comparative study also enabled an uncommon circumstance to examine the influence of climate with that of climate and glacial turbidity interaction in two similar lakes. This comparison revealed that while both Anderson and Seton lakes underwent a compositional change in their diatom assemblages ca. 1980 that is consistent with climatic forcing, and coincident with increasing mean annual temperatures at the Shalalth Climate Station, the strength of the response varied. This recent assemblage change was most pronounced in Anderson Lake, with a dramatic rise in L. comensis; whereas the more muted response in Seton Lake is likely a result of interaction of climate forcing with glacial inputs leading to a novel assemblage from those in Anderson Lake. The comparative seasonal phytoplankton data provided insights into the timing of blooms of L. comensis in the two lakes and led to the hypothesis of ice-free conditions being a major driver of this recent assemblage change.
Data availability
The data in this current study can be made available from the corresponding author upon request.
References
Arp CD, Jones BM, Whitman M, Larsen A, Urban FE (2010) Lake temperature and ice cover regimes in the Alaskan subarctic and arctic: Integrated monitoring, remote sensing, and modeling. J Am Water Res Assoc 46:777–791. https://doi.org/10.1111/j.1752-1688.2010.00451.x
Arp CD, Jones BM, Grosse G (2013) Recent lake ice-out phenology within and among lake districts of Alaska, U.S.A. Limnol Oceanogr 58:2013–2028. https://doi.org/10.4319/lo.2013.58.6.2013
Assel R, Cronk K, Norton D (2003) Recent trends in Laurentian Great Lakes ice cover. Clim Change 57:185–204. https://doi.org/10.1023/A:1022140604052
Barouillet C, Cumming BF, Laird KR, Perrin CJ, Selbie DT (2019) Influence of glacial flour on the primary and secondary production of Sockeye Salmon nursery lakes: a comparative modern and paleolimnological study. Can J Fish Aquat Sci. https://doi.org/10.1139/cjfas-2018-0372
Battarbee RW, Kneen MJ (1982) The use of electronically counted microspheres in absolute diatom analysis. Limnol Oceanogr 27:184–188. https://doi.org/10.4319/lo.1982.27.1.0184
BC-MoE (British Columbia, Ministry of Environment) (2016) Indicators of Climate Change for British Columbia: 2016 Update. 57pp
Benson BJ, Magnuson JJ, Jensen OP, Card VM, Hodgkins G, Korhonen J, Livingstone JKM, Stewart GA et al (2012) Extreme events, trends, and variability in Northern Hemisphere lake-ice phenology (1855–2005). Clim Chang 112:299–323. https://doi.org/10.1007/s10584-011-0212
Bernhardt J, Engelhardt C, Kirillin G, Matschullat J (2012) Lake ice phenology in Berlin-Brandenburg from 1947–2007: observations and model hindcasts. Clim Chang 112:791–817. https://doi.org/10.1007/s10584-011-0248-9
Bigler C, Hall RI (2003) Diatoms as quantitative indicators of July temperature: a validation attempt at century-scale with meteorological data from northern Sweden. Palaeogeogr Palaeoclimatol Palaeoecol 189:147–160. https://doi.org/10.1016/S0031-0182(02)00638-7
Boeff KA, Strock KE, Saros JE (2016) Evaluating planktonic diatom response to climate change across three lakes with differing morphometry. J Paleolimnol 56:33–47. https://doi.org/10.1007/s10933-016-9889-z
Bolch T, Menounos B, Wheate R (2010) Landsat-based inventory of glaciers in western Canada, 1985–2005. Remote Sens Environ 114:127–137. https://doi.org/10.1016/j.rse.2009.08.015
Bonalumi M, Anselmetti FS, Kaegi R, Wüest A (2011) Particle dynamics in high-Alpine proglacial reservoirs modified by pumped-storage operation. Water Resour Res 47:W09523. https://doi.org/10.1029/2010WR010262
Bramburger AJ, Reavie ED (2016) A comparison of phytoplankton communities of the deep chlorophyll layers and epilimnia of the Laurentian Great Lakes. J Great Lakes Res 42:1016–1025. https://doi.org/10.1016/j.jglr.2016.07.004
Caballero M, Vázquez G, Ortega B, Favila ME, Lozano-García S (2016) Responses to a warming trend and ‘“El Ninõ”’ events in a tropical lake in western Mexico. Aquat Sci 78:591–604. https://doi.org/10.1007/s00027-015-0444-1
Camburn KR, Charles DF (2000) Diatoms of low-alkalinity lakes in the Northeastern United States. Academy of Natural Sciences, Philadelphia
Canter-Lund H, Lund JWG (1995) Freshwater Algae – their microscopic world explored. BioPress Ltd., Bristol
Chen G, Saulnier-Talbot E, Selbie DT, Brown E, Schindler DE, Bunting L, Leavitt PR, Finney BP, Gregory-Eaves I (2011) Salmon-derived, nutrients drive diatom beta-diversity patterns. Freshwat Biol 56:292–301. https://doi.org/10.1111/j.1365-2427.2010.02496.x
Chernos M (2014) The relative importance of calving and surface ablation at a lacustrine terminating glacier: a detailed assessment of ice loss at Bridge Glacier, British Columbia. MSc Thesis. University of British Columbia
Clarke GKC, Jarosch AH, Anslow FS, Radic V, Menounos B (2015) Projected deglaciation of western Canada in the twenty-first century. Nat Geosci 8:372–377. https://doi.org/10.1038/NGEO2407
Cumming BF, Wilson SE, Hall RI, Smol JP (1995) Diatoms from British Columbia (Canada) Lakes and their relationship to salinity, nutrients and other limnological variables BibliothecaDiatomologica Vol.31 (Stuttgart), 207. Davidson,T.A., & Jeppesen, E.. 2013.
Cumming BF, Laird KR, Gregory-Eaves I, Simpson KG, Sokal MA, Nordin RN, Walker IR (2015) Tracking past changes in lake-water phosphorus with a 251-lake calibration dataset in British Columbia: tool development and application in a multiproxy assessment of eutrophication and recovery in Osoyoos Lake, a transboundary lake in Western North America. Front Ecol Evol 3:84. https://doi.org/10.3389/fevo.2015.00084
Edmundson JM, Edmundson JA (2002) Sockeye Salmon production relative to changes in rearing capacity of Crescent Lake, Upper Cooke Inlet. Alaska Department of Fish and Game Regional Information Report. 2A02–08.
Edmundson JA, Mazumder A (2002) Regional and hierarchical perspectives of thermal regimes in subarctic, Alaskan lakes. Freshwat Biol 47:1–17. https://doi.org/10.1046/j.1365-2427.2002.00775.x
Fallu M, Allaire N, Pienitz R (2000) Freshwater Diatoms from Northern Que´bec and Labrador (Canada). Bibliotheca Diatomologica Band 45. Gebru¨der Borntraeger, Berlin.
Feuchtmayr H, Thackeray SJ, Jones ID, de Ville M, Fletcher J, James B, Kelly J (2012) Spring phytoplankton phenology-are patterns and drivers of change consistent among lakes in the same climatological region? Freshwat Biol 57:331–344. https://doi.org/10.1111/j.1365-2427.2011.02671.x
Finney BP, Gregory-Eaves I, Sweetman J, Douglas MSV, Smol JP (2000) Impacts of climatic change and fishing on Pacific Salmon abundance over the past 300 years. Science 290:795–799. https://doi.org/10.1126/science.290.5492.795
Geen GH, Andrew FJ (1961) Limnological changes in Seton Lake resulting from hydroelectric diversions. International Pacific Salmon Fisheries Commission Progress Report 8.
Glew JR, Smol JP, Last WM (2001) Sediment core collection and extrusion, p. 73–105. In Last WM, Smol JP [eds.], Tracking Environmental Change Using Lake Sediments, Vol. 1., Basin Analysis, Coring, and Chronological Techniques Kluwer Academic Publishers, Dordrecht.
Gregory-Eaves I, Smol JP, Douglas MSV, Finney BP (2003) Diatoms and sockeye salmon (Oncorhynchus nerka) population dynamics: Reconstructions of salmon-derived nutrients over the past 2,200 years in two lakes from Kodiak Island, Alaska. J Paleolimnol 30:35–53. https://doi.org/10.1023/A:1024792831385
Grimm EC (1987) CONISS: a FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Comput Geosci 13:13–35. https://doi.org/10.1016/0098-3004(87)900227
Hadley KR, Douglas MSV, McGhee R, Blais JM, Smol JP (2010) Ecological influences of Thule Inuit whalers on high Arctic pond ecosystems: a comparative paleolimnological study from Bathurst Island (Nunavut, Canada). J Paleolimnol 44:85–93. https://doi.org/10.1007/s10933-009-9388-6
Hartmann DL, Klein Tank AMG, Rusticucci M, Alexander LV, Brönnimann S, Charabi Y, Dentener FJ, Dlugokencky EJ et al (2013) Observations: atmosphere and surface in climate change 2013. In: Stocker T, Qin D, Plattner GK, Tignor M, Allen S, Boschung KJ, Nauels A, Xia Y, Bex V, Midgley PM (eds) Working Group I contribution to the Fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York, NY, USA, pp 187–197
Hausmann S, Lotter AF (2001) Morphological variation within the diatom taxon Cyclotella comensis and its importance for quantitative temperature reconstructions. Freshwat Biol 46:1323–1333. https://doi.org/10.1046/j.1365-2427.2001.00752x
Hewitt BA, Lopez LS, Gaibisels KM, Murdoch A, Higgins SN, Magnuson JJ, Paterson AM, Rusak JA, Yao H, Sharma S (2018) Historical trends, drivers, and future projections of ice phenology in small north temperate lakes in the Laurentian Great Lakes region. Water 10(1):70. https://doi.org/10.3390/w10010070
Hobbs WO, Wolfe AP (2007) Caveats on the use of paleolimnology to infer Pacific salmon returns. Limnol Oceanogr 52:2053–2061, www.jstor.org/stable/4502355
Hobbs WO, Vinbrooke RD, Wolfe AP (2011) Biogeochemical responses of two alpine lakes to climate change and atmospheric deposition, Jasper and Banff National parks, Canadian Rocky Mountains. Can J Fish Aquat Sci 68:1480–1494. https://doi.org/10.1139/f2011-058
Hodson A, Mumford P, Lister D (2004) Suspended sediment and phosphorus in proglacial rivers: bioavailability and potential impacts upon the P status of ice-marginal receiving waters. Hydrol Process 18:2409–2422. https://doi.org/10.1002/hyp.1471
Huisman J, Sharples J, Stroom JM, Visser PM, Kardinaal WEA, Verspagen JMH, Sommeijer B (2004) Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85:2960–2970. https://doi.org/10.1890/03-0763
Hylander S, Jephson T, Lebret K, vonEinem J, Fagerberg T, Balseiro E, Modenutti B, Souza MS et al (2011) Climate-induced input of turbid glacial meltwater affects vertical distribution and community composition of phyto- and zooplankton. J Plankton Res 33:1239–1248. https://doi.org/10.1093/plankt/fbr025
IPCC (2013): Summary for Policymakers, p. 3–29. In Stocker T, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM [eds.], Climate Change 2013: The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, New York, NY, USA
Juggins S (2003) C2 version 1.7.5 Software for ecological and Palaeoecological data analysis and visualization. Newcastle upon Tyne: University of New Castle.
Kienel U, Kirillin G, Brademann B, Plessen B, Lampe R, Brauer A (2017) Effects of spring warming and mixing duration on diatom deposition in deep Tiefer See, NE Germany. J Paleolimnol 57:37–49. https://doi.org/10.1007/s10933-016-9925-z
Kireta AR, Saros JE (2019) Contemporary abundance patterns of Cyclotella sensu lato diatom taxa in Lake Superior: Assessing responses to physical and chemical gradients and potential links to climate change. J Great Lakes Res 45:119–128. https://doi.org/10.1016/j.jglr.2018.11.014
Kirillin G, Leppäranta M, Terzhevik A, Granin N, Bernhardt J, Engelhardt C, Efremova T, Golosov S et al (2012) Physics of seasonally ice-covered lakes: a review. Aquat Sci 74:659–682. https://doi.org/10.1007/s00027-012-0279-y
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae. 1: Teil: Naviculaceae. In Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D [eds.], Süßwasserflora von Mitteleuropa, Band 2/1. Gustav Fischer Verlag, Stuttgart/New York.
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae. 2: Teil: Bacillariaceae, Epithmiaceae, Surirellaceae. In Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D [eds.], Süßwasserflora von Mitteleuropa, Band 2/2. Gustav Fischer Verlag, Stuttgart/New York.
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae. 3:Teil: Centrales, Fragilariaceae, Eunotiaceae. In Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D [eds.], Süßwasserflora von Mitteleuropa, Band 2/3. Gustav Fischer Verlag, Stuttgart/Jena.
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae. 4:Teil: Achnanthaceae. In Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D [eds.], Süßwasserflora von Mitteleuropa, Band 2/4. Gustav Fischer Verlag, Stuttgart/Jena.
Lange-Bertalot H, Melzeltin D (1996) Indicators of oligotrophy, vol 2. Koeltz Scientific Books, Königstein, Iconographia Diatomologica
Lavaud J, Strzepek RF, Kroth PG (2007) Photoprotection capacity differs among diatoms: Possible consequences on the spatial distribution of diatoms related to fluctuations in the underwater light climate. Limnol Oceanogr 52:1188–1194. https://doi.org/10.4319/lo.2007.52.3.1188
Limnotek (2017) Seton Lake aquatic productivity monitoring (BRGMON6): Final Report. Report prepared for BC Hydro. 215p.
Lloyd DS, Koenings JP, Laperriere JD (1987) Effects of turbidity in fresh waters of Alaska. N Amer J Fisher Manag 7:18–33. https://doi.org/10.1577/1548-659%281987%297%3c34%3ATAAWQS%3e2.0.CO%3B2
Longhi ML, Beisner BE (2009) Environmental factors controlling the vertical distribution of phytoplankton in lakes. J Plankton Res 31:1195–1207. https://doi.org/10.1093/plankt/fbp065
Lotter AF, Bigler C (2000) Do diatoms in the Swiss Alps reflect the length of ice-cover? Aquat Sci 62:125–141. https://doi.org/10.1007/s000270050002
Magee MR, Wu CH, Robertson DM, Lathrop RC, Hamilton DP (2016) Trends and abrupt changes in 104 years of ice cover and water temperature in a dimictic lake in response to air temperature, wind speed, and water clarity drivers. Hydrol Earth Syst Sci 20:1681–1702. https://doi.org/10.5194/hess-20-1681-2016
Magee MR, Wu CH (2017) Effects of changing climate on ice cover in three morphometrically different lakes. Hydrol Process 31:308–323. https://doi.org/10.1002/hyp.10996
Magnuson JJ, Robertson DM, Benson BJ, Wynne RH, Livingston DM, Arai T, Assel RA, Barry RG et al (2000) Historical trends in lakes and river ice cover in the Northern Hemisphere. Science 289:1743–1746. https://doi.org/10.1126/science.289.5485.1743
Malik HI, Saros JE (2016) Effects of temperature, light and nutrients on five Cyclotella sensu lato taxa assessed with in situ experiments in arctic lakes. J Plankton Res 38:431–442. https://doi.org/10.1093/plankt/fbw002
Melack JM, Dozier J, Goldman CR, Greenland D, Milner AM, Naiman RJ (1997) Effects of climate change on inland waters of the Pacific Coastal Mountains and Western Great Basin of North America. Hydrol Process 11:971–992. https://doi.org/10.1002/(SICI)1099-1085(19970630)11:8%3c971::AIDHYP514%3e3.0CO;2-Y
Milner AM, Khamis K, Battin TJ, Brittain JE, Barrand NE, Füreder L,, Cauvy-Fraunié S, Gíslason GM, Jacobsen D, Hannah DM, Hodson AJ, Hood E, Lencioni V, Ólafsson JS, Robinson CT, Tranter M, Brown LE (2017) Glacier shrinkage driving global changes in downstream systems. Proc Natl Acad Sci 114: 9770–9778. https://doi.org/10.1073/pnas.1619807114
Modenutti B, Balseiro E, Navarro MB, Laspoumaderes C, Souza MS, Cuassolo F (2013) Environmental changes affecting light climate in oligotrophic mountain lakes: the deep chlorophyll maxima as a sensitive variable. Aquat Sci 75:361–371. https://doi.org/10.1007/s00027-012-0282-3
Moon T (2017) Saying goodbye to glaciers. Science 356:580–581. https://doi.org/10.1126/science.aam9625
Navarro MB, Martyniuk N, Balseiro E, Modenutti B (2018) Effect of glacial lake outburst floods on the light climate in an Andean Patagonian lake: implications for planktonic phototrophs. Hydrobiologia 816:39–48. https://doi.org/10.1007/s10750-016-3080-4
NOAA (2019) National Centers for Enviromental Information, State of the Climate: Global Climate Report for Annual 2019, published online January 2020. https://www.ncdc.noaa.gov/sotel/global/201913
Northcote TG, Pick FR, Fillion DB, Salter S (2005) Interactions of nutrients and turbidity in the control of phytoplankton in a large Western Canadian lake prior to major watershed impoundments. Lake Reserv Manag 21(3):261–276. https://doi.org/10.1080/07438140509354434
O’Reilly CM, Sharma S, Gray DK, Hampton SE, Read JS, Rowley RJ, Schneider P, Lenters JD et al (2015) Rapid and highly variable warming of lake surface waters around the globe. Geophys Res Lett. https://doi.org/10.1002/2015GL066235
Origin (2000) Origin Lab Cooperation, North Hamptom, MA. www.OriginLab.com
Polimene L, Brunet C, Butenschön M, Martinez-Vicente V, Widdicombe C, Torres R, Allen JI (2014) Modelling a light-driven phytoplankton succession. J Plankton Res 36:214–229. https://doi.org/10.1093/plankt/fbt086
Prescott GW (1978) How to Know the Freshwater Algae, 3rd edn. Wm. C. Brown Company, Dubuque, IA
Reavie ED, Sgro GV, Estepp LR, Bramburger AJ, Shaw Chraïbi VL, Pillsbury RW, Cai M, Stow CA, Dove A (2017) Climate warming and changes in Cyclotella sensu lato in the Laurentian Great Lakes. Limnol Oceanogr 62:768–783. https://doi.org/10.1002/lno.10459
Reynolds CS (1984) Phytoplankton periodicity: the interactions of form, function and environmental variability. Freshwat Biol 14:111–142. https://doi.org/10.1111/j.1365-2427.1984.tb00027.x
Reynolds CS, Huszar V, Kruk C, Naselli-Flores L, Melo S (2002) Towards a functional classification of the freshwater phytoplankton. J Plankton Res 24:417–428. https://doi.org/10.1093/plankt/24.5.417
Richardson DC, Melles SJ, Pilla RM, Hetherington AL, Knoll LB, Williamson CE, Kraemer BM, Jackson JR et al (2017) Transparency, geomorphology and mixing regime explain variability in trends in lake temperature and stratification across Northeastern North America (1975–2014). Water 9:442. https://doi.org/10.3390/w9060442
Rose KC, Hamilton DP, Williamson CE, McBride CG, Fischer JM, Olson MH, Saros JE, Allan MG, Cabrol N (2014) Light attenuation characteristics of glacially-fed lakes. J Geophys Res Biogeosci 119:1446–1457. https://doi.org/10.1002/2014JG002674
Rühland K, Paterson AM, Smol JP (2008) Hemispheric-scale patterns of climate-related shifts in planktonic diatoms from North American and European lakes. Global Change Biol 14:2740–2754. https://doi.org/10.1111/j.1365-2486.2008.01670.x
Rühland K, Paterson AM, Smol JP (2015) Lake diatom responses to warming: reviewing the evidence. J Paleolimnol 54:1–35. https://doi.org/10.1007/s10933-015-9837-3
Saros JE, Interlandi SJ, Doyle S, Michel TJ, Williamson CE (2005) Are the deep chlorophyll maxima in alpine lakes primarily induced by nutrient availability, not UV avoidance? Arctic Antarc Alpine Res 37:557–563. https://doi.org/10.1657/1523-0430(2005)037[0557:ATDCMI]2.0.CO;2
Saros JE, Michel TJ, Interlandi SJ, Wolfe AP (2011) Resource requirements of Asterionella formosa and Fragilaria crotonensis in oligotrophic alpine lakes: implications for recent phytoplankton community reorganizations. Can J Fish Aquat Sci 62:1681–1689. https://doi.org/10.1139/f05-077
Saros JE, Stone JR, Pederson GT, Slemmons KEH, Spanbauer T, Schliep A, Cahl D, Williamson CE, Engstrom DR (2012) Climate-induced changes in lake ecosystem structure inferred from coupled neo- and paleoecological approaches. Ecology 93:2155–2164. https://doi.org/10.1890/11-2218.1
Saros JE, Anderson NJ (2015) The ecology of the planktonic diatom Cyclotella and its implications for global environmental change studies. Biol Rev 90:522–541. https://doi.org/10.1111/brv.12120
Saros JE, Northington RM, Osborn CL, Burpee BT, Anderson NJ (2016) Thermal stratification in small arctic lakes of southwest Greenland affected by water transparency and epilimnetic temperatures. Limnol Oceanogr 61:1530–1542. https://doi.org/10.1002/lno.10314
Scheffler W, Morabito G (2003) Topical observations on centric diatoms (Bacillariophyceae, Centrales) of Lake Como (N. Italy). J Limnol 62:47–60. https://doi.org/10.4081/jlimnol.2003.47
Schelske CL, Peplow A, Brenner M, Spencer CN (1994) Low-background gamma counting: applications for 210pb dating of sediments. J Paleolimnol 10:115–128. https://doi.org/10.1007/2FBF00682508
Schneider P, Hook SJ (2010) Space observations of inland water bodies show rapid surface warming since 1985. Geophys Res Lett 37:L22405. https://doi.org/10.1029/2010GL045059
Selbie DT, Finney BP, Barto D, Bunting L, Chen G, Leavitt PR, MacIsaac EA, Schindler DE et al (2009) Ecological, landscape, and climatic regulation of sediment geochemistry in North American sockeye salmon nursery lakes: Insights for paleoecological salmon investigations. Limnol Oceangr 54:1733–1745. https://doi.org/10.4319/10.2009.54.5.1733
Shaw Chraïbi VLS, Kireta AR, Reavie ED, Cai M, Brown TN (2014) A paleolimnological assessment of human impacts on Lake Superior. J Great Lakes Res 40:886–897. https://doi.org/10.1016/j.jglr.2014.09.016
Shi P, Shen H, Wang W, Yang Q, Xie P (2016) Habitat-specific differences in adaptation to light in freshwater diatoms. J Appl Phycol 28:227–239. https://doi.org/10.1007/s10811-015-0531-7
Slemmons KEH, Saros JE, Simon K (2013) The influence of glacial meltwater on alpine aquatic ecosystems: a review. Environ Sci Processes Impacts 15:1794–1806. https://doi.org/10.1039/c3em00243h
Smol JP, Cumming BF (2000) Tracking long term changes in climate using algal indicators in lake sediments. J Phycol 36:986–1011. https://doi.org/10.1046/j.1529-8817.2000.00049.x
Sommaruga R (2015) When glaciers and ice sheets melt: consequences for planktonic organisms. J Plankton Res 37:509–518. https://doi.org/10.1093/plankt/fbv027
Sommer U, Adrian R, De Senerpont DL, Elser JJ, Gaedke U, Ibelings B, Jeppesen E, Lürling M et al (2012) Beyond the plankton ecology group (PEG) model: Mechanisms driving plankton succession. Annu Rev Ecol Evol Syst 43:429–448. https://doi.org/10.1146/annurev-ecolsys-110411-160251
Sorvari S, Korhola A, Thompson R (2002) Lake diatom response to recent Arctic warming in Finnish Lapland. Global Change Biol 8:171–181. https://doi.org/10.1046/j.1365-2486.2002.00463.x
Stoermer EF, Emmert G, Julius ML, Schelske CL (1996) Paleolimnologic evidence of rapid recent change in Lake Erie’s trophic status. Can J Fish Aquat Sci 53:1451–1458
Thackeray SJ, Jones ID, Maberly SC (2008) Long-term change in the phenology of spring phytoplankton: species-specific responses to nutrient enrichment and climatic change. J Ecol 96:523–535. https://doi.org/10.1111/j.1365-2745.2008.01355.x
Wang J, Bai X, Hu H, Clites A, Colton M, Lofgren B (2012) Temporal and spatial variability of Great Lakes ice cover, 1973–2010. J Clim 25:1318–1329. https://doi.org/10.1175/2011JCLI4066.1
Wang Q, Yang X, Anderson NJ, Ji J (2015) Diatom seasonality and sedimentation in a subtropical alpine lake (Lugu Hu, Yunnan-Sichuan, Southwest China). Arctic Antarct Alpine Res 47:461–472. https://doi.org/10.1657/AAAR0014-039
Werner P, Smol JP (2005) Diatom–environmental relationships and nutrient transfer functions from contrasting shallow and deep limestone lakes in Ontario, Canada. Hydrobiologia 533:145–173. https://doi.org/10.1007/2Fs10750-004-2409-6
Weyhenmeyer GA, Westöö A, Willén E (2008) Increasingly ice-free winters and their effects on water quality in Sweden’s largest lakes. Hydrobiologia 599:111–118. https://doi.org/10.1007/s10750-007-9188-9
Weyhenmeyer GA, Livingstone DM, Meili M, Jensen O, Benson B, Magnuson JJ (2011) Large geographical differences in the sensitivity of ice-covered lakes and rivers in the Northern Hemisphere to temperature changes. Global Change Biol 17:268–275. https://doi.org/10.1111/j.1365-2486.2010.02249.x
Wiltse B, Paterson AM, Findlay DL, Cumming BF (2016) Seasonal and decadal patterns in Discostella (Baccillariiophyceae) species from bi-weekly records of two boreal lakes (Experimental Lakes Area, Ontario, Canada). J Phycol 52:817–826. https://doi.org/10.1111/jpy.12443
Winder M, Reuter JE, Schladow SG (2009) Lake warming favours small-sized planktonic diatom species. Proc R Soc B 276:427–435. https://doi.org/10.1098/rspb.2008.1200
Woolway RI, Dokulil MT, Marszelewski W, Schmid M, Bouffard D, Merchant CJ (2017) Warming of Central European lakes and their response to the 1980s climate regime shift. Clim Chang 142:505–520. https://doi.org/10.1007/s10584-017-1966-4
Wunsam S, Schmidt R, Klee R (1995) Cyclotella-taxa (Bacillariophyceae) in lakes of the Alpine region and their relationship to environmental variables. Aquat Sci 57:360–386. https://doi.org/10.1007/2FBF00878399
Yang J, Pick FR, Hamilton PB (1996) Changes in the planktonic diatom flora of a large mountain lake in response to fertilization. J Phycol 32:232–243. https://doi.org/10.1111/j.0022-3646.1996.00232.x
Acknowledgements
This study was funded by BC Hydro, with administration provided by Teri Neighbour, Ahmed Gelchu, Darin Nishi, and Jeff Walker. We thank Bonnie Adolf, Gilda Davis, and Jude Manahan of St’aět’imc Eco-Resources Ltd who managed the study as part of a larger water use planning project. Special thanks goes to Dr. Dave Levy who facilitated and negotiated inclusion of this study within the planning process. Access to BC Hydro field facilities was provided by Dorian Turner. Garett Lidin of Fisheries and Oceans Canada is thanked for assistance in the field during collection of cores. Staff of the BC Ministry of Environment and Climate Change Strategy including Shannon Harris, Allison Hebert, and Petra Wykpis are thanked for boat support and assistance in the field during collection of the phytoplankton samples. Other assistance in the field was provided by Dani Ramos, Annika Putt, Marc Laynes, Tyler Creasey, L.J Wilson, Caroline Melville, John Goes, and Frank Richings. Data compilations were completed by Mike Chung and Shauna Bennett. Lab support was provided by Danusia Dolecki, Lech Dolecki, staff of Fisheries and Oceans lab at Cultus Lake, British Columbia, Canada, and ALS Environmental, Burnaby, Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare. Funding was provided by BC Hydro and managed by St’aět’imc Eco-Resources Ltd. Further details are provided in the acknowledgements.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Laird, K.R., Barouillet, C., Cumming, B.F. et al. Influence of glacial turbidity and climate on diatom communities in two Fjord Lakes (British Columbia, Canada). Aquat Sci 83, 13 (2021). https://doi.org/10.1007/s00027-020-00767-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-020-00767-3