Abstract
With an intention to replace the synthetic non-biodegradable films in packaging applications, the polyvinyl alcohol (PVA) blended with green banana peel filler (GBPF), the biodegradable films were prepared by solution casting method with varying the concentrations of GBPF (5–25 wt%) in PVA matrix. The bio films were characterized by Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), thermo gravimetric analysis, transmissibility, FESEM, tensile test, film solubility and water absorption, water vapour transmission (WVT), soil burial test. Based on results obtained, the changes evidenced in the FTIR spectrum of this PVA/GBPF biofilms suggest that strong hydrogen bonding is taking place due to interfacial exchanges of GBPF in PVA matrix. The XRD results showed that crystallinity of bio films are greater than PVA. Thermo gravimetric analyses predicted that PVA/GBPF bio films are thermally stable up to 300 °C. The light is 45% for transmittance in the visible light region for the PVA/GBPF (25 wt%) bio film. The FESEM micrographs of biofilms palpable that formation of good physical interaction and compatibility between polymer matrix and GBPF up to 20 wt% of GBPF in PVA Matrix. FESEM results also confirmed that higher loading of GBPF (25 wt%) in PVA matrix, observed voids and agglomerations in film surface. The PVA/GBPF bio films with 20% of GBPF gave the highest tensile strength and young’s modulus 44.5 MPa and 66.7 GPA respectively compared to other samples. The elongation at break decreases with increases the GBPF in PVA Matrix up to 20 wt%.The slight decrease in mechanical properties perceived due to higher loading of GBPF (25 wt%) with PVA matrix. The solubility, water absorption and WVT of the PVA/GBPF bio films increased upon increasing the GBPF content. The biodegradation test results discovered that he highest weight loss at 42.3% (25 wt% of GBPF) probably due to the hydrophilic nature of GBPF in PVA matrix. On the whole, the present investigation confirmed that the PVA/GBPF bio films potential for possible utilization in active packaging applications attributable to its better mechanical, thermal, optical, water absorption and biodegradation properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petrochemical-based plastics are being replaced by bio based materials because of being widely eco-friendly. The plastics produced from sources (e.g., polyesters and polyolefin) have been commonly used in the packaging industry due to their potential features. Plastic is used as packing material on daily life. They are obtainable in large quantities and at low cost, displaying advantageous properties (i.e., good tensile strength, enriched barrier properties, and heat sealing) and applicability in the industry [1]. Even though plastic has those advantages, a high dependence on plastic could make bad effect not only on human health but also for the environment. However, these plastics are totally non-biodegradable and expose a serious ecological problem due to hydrophobic properties and very low water vapor transmission rate [2]. Due to that reason, there is an urgent need to find a new alternative packing material which is save for our body and environmentally friendly to replace plastics [3].
The growing public interest on the environment is induced the considerable research to develop biofilms from biopolymers. The harmful effect of conventional petroleum-based plastic packaging materials overcomes by environment friendly biodegradable alternatives [4]. Various types of commercial polymers as well as composites are utilized for a number of industrial and daily purposes which are mainly constructed from non-biodegradable components [5]. Now-a-days, water soluble and biodegradable polymers got importance as an alternative source of material for using it in many purposes. Natural fibers are cheap, biodegradable and available in our surroundings and can be used to produce biodegradable composites, therefore assist to assured our environment. Manufacturing of biodegradable composites from such biodegradable plastics will enhance the demand of such materials [6]. The renewable natural polymers can be obtained from several sources such as starch, cellulose, chitosan, etc., while biodegradable synthetic polymers such as polyvinyl alcohol [7], polycaprolactone [8], polybutylene succinate [9], and copolymers are produced by using natural or petroleum-based monomers have been used. However; synthetic biopolymers have some drawbacks like a poor mechanical, barrier and thermal properties when compared to those of the petroleum –based non-biodegradable polymers. Hence, in order to overcome these drawbacks, the biopolymers are blended with fillers.
Polyvinyl alcohol (PVA) is a biodegradable polymer that has received significant attention because of its ability to decompose in relatively short timeframe. PVA is an important polymer having superior gas barrier properties along with higher strength, tear, and flexibility than those of natural bio based polymers [10]. PVA is a synthetic biodegradable polymer with the technological potential as a water-processible polymer, thus allowing for film production by solution casting [11]. The excellent chemical resistance, good thermal barrier, good mechanical properties and physical properties of PVA have been employed in various applications, including dip coating, adhesives and solution casting film. PVA was combined with other polymer or filler, can increase the physical, thermal and barrier properties [12]. PVA is a costly commercial raw material. To lower down the cost of product without any compromise or partial compromise with its properties, the composites of PVA have been used. Composites are produced by blending biodegradable polymers with natural fillers to produce bio-based or biodegradable polymer composite that exhibit unique and stable characteristics. PVA has an excellent compatibility with the addition of nanocellulose filler which produces environmental friendly Nano composite. In this direction many researchers have attempted to use waste fillers such as tamarind nut powder [13], spent tea-leaf powder [14], Orange peel powder [15], coconut shell powder [16], and Finger millet powder [17] etc., to make biocomposites for packaging applications.
Biodegradable plastics are a new generation of polymers emerging on the world market. Biodegradable plastics have an expanding range of potential applications, and driven by the growing use of plastics in packaging and the perception that biodegradable plastics are ‘environmentally friendly’; their use is predicted to increase [18]. However, issues are also emerging regarding the use of biodegradable plastics and their potential impacts on the environment and effects on established recycling systems and technologies. Polyvinyl alcohol and starch are biodegradable polymers. Starch is one of the major sources in the development of biodegradable plastics. Owing to its large availability, low cost, renewability and biodegradability starches are commonly used in the production of biodegradable plastics. One of the most common waste forms of starch is the banana peels. The banana fruit’s peel was selected for this experiment because it is a waste material rich in starch, the proximate composition [19] of a banana peel is shown Table 1.
Banana is a fruit plant grown plenty in tropical countries like India, Sri Lanka, Malaysia, and Thailand etc. The banana peel is thrown as a waste after consumption of the fruit. These peels are also used as feed for calves. However, if they are not properly disposed, they create environmental pollution by emitting toxic gases like hydrogen sulphide and ammonia [20]. Banana peels are also used in the production of methane [21], bio-adsorbent for dyes in wastewater and gelling agent in food processing industries. Banana peels account for 30–40% weight of the fruit and contain proteins, carbohydrates, dietary fibers, ash and traces of fat and moisture [22]. They also contain mono and disaccharides, phenolic compounds and minerals [23]. The banana peels are mainly composed of carotenoids, phenolic compounds, flavonolsanols, biogenic amines and dietary fibers. These components are prospective antioxidants that possess a wide range of biological effects including antibacterial, anti-viral, anti-inflammatory, anti-allergenic, antithrombotic, and vasodilatory actions etc [24]. The photo chemicals present in banana peels are generally antioxidants and can help in preventing the oxidation in food materials [25]. Banana peels exhibit excellent antimicrobial activity against bacteria, fungi, and yeast. Since banana peels contain phenolic compounds, they can be used as a source to develop the desired properties and to extend the food safety and the shelf life [26]. Banana peels consists high source of starch. As banana peels ripen, the glucose level increases. Therefore, banana peels can be suggested as a suitable source for the manufacturing of biodegradable plastics. It is also understandable through the literature that banana peels could be a value addition to biomaterials thereby helping the reduction in environment pollution, and banana peels have enormous potential for its industrial use [27].
The main objective of this work is to prepare biocomposites films from PVA and banana peel powder with different concentrations (5, 10, 15, 20 and 25 wt%) and characterize the fabricated bio composite films by, FTIR, XRD, TGA, FESEM, transmissibility, % water absorption and water vapour permeability, tensile testing and soil burial test. These bio composite films are appropriate for potential use in food packaging.
Materials and Methods
Materials
Poly (vinyl alcohol), PVA, 98% hydrolyzed, (MW of 1,25,000 and 1% ash max) and distilled water was purchased from Sigma–Aldrich chemicals Private limited (Bengaluru). Before the use, poly (vinyl alcohol), PVA was kept in desiccated atmosphere to do away with moisture content. Green Banana Peel Powder (GBPP) used as reinforcing filler was obtained from Venus Starch Suppliers, Salem, Tamil nadu, India. The average particle size of the GBPP was 333.3 nm measured by using Malvern Particle size Analyzer Instrument.
Fabrication of PVA/GBPF Bio Films
The PVA/GBPF bio films were fabricated using a solution casting method. First, the PVA was dissolved in distilled water by magnetic stirring for 3 h at 80 °C. Second, the GBPF (5, 10, 15, 20 and 25 wt%) added in PVA solution and magnetically stirred for about 6 h. The polymer solution (PVA/GBPF) cured at 50 °C for 30 min. The Prepared solution was casted on Petri plates of 130 and 60 mm diameter and dried at desiccators for 12 h. Ten samples were prepared for each composition of poly (vinyl alcohol) and green banana peel filler. Finally prepared PVA/GBPF bio films were characterized and analyzed.
Characterization of PVA/GBPF Bio Films
Fourier Transform Infrared Spectroscopy (FTIR)
Fourier transformation infrared spectrographs (FTIR) were recorded to categorize a number of peaks and an organic functional group on the exterior of the green banana peel filler, PVA and the prepared PVA/GBPF bio-films. A small piece was cut from the prepared film samples and placed onto the germanium plate. Infrared spectra in attenuated total reflectance accessory of GBPF, PVA controlled films and PVA/GBPF dry films were recorded in the mid-IR region 4000–500 cm−1 at room temperature, using an RXI Perkin Elmer FTIR Spectrophotometer at a resolution of 4 cm−1.
X-ray Diffraction Test (XRD)
The crystalline structures of thin film samples were determined by XRD technique. XRD equipment is used for recording the film diffractograms in 25 °C. X-ray diffraction patterns of the GBPF, PVA controlled film and PVA/GBPF bio-films were carried out by using a Bruker D8 X-ray diffractometer. The X-ray source was Ni-filtered Cu Kα radiation (40 kV and 30 mA). The composite films were mounted on a sample holder, and the pattern was recorded in the reflection mode at an angle 2θ over a range of 5.000°–80.009° at a scan rate of of 5° min−1. The conventional peak height method used to calculate the crystallinity index (IC) of films.
\({\text{I}}_{002}\) is the intensity of crystalline peak (around 25°) and \({\text{I}}_{{{\text{am}}}}\) is the intensity of amorphous peak (around 18°).
Thermo Gravimetric Analysis (TGA)
TG/DTG curves were obtained using thermograms Thermogravimetric analyzer (TA Instruments, TGA Q500). The thermograms of green banana peel filler, PVA matrix and PVA/GBPF bio films were recorded using at a heating rate of 10 °C min−1 under a dynamic flow of nitrogen (50 ml min−1) in the 50–600 °C range. Approximately 10.2 mg of samples were placed on an aluminum pan for testing. Weight losses of the samples were noted for every 10 °C temperature rise.
Surface Morphology (FESEM)
The surface morphology and element mapping of the prepared films were investigated by capturing micrographs of the representative samples using Nova Nano SEM 450, a field—emission scanning electron microscope (FE–SEM) equipped with energy dispersive X-ray (EDX) analyzer. The operating voltage 2 kV, (Make: carl zeiss (USA), Model: sigma with Gemini column), and resolution of 1.5 nm. The samples of the prepared films were prior coated with gold for 2 min using EMITECH SC7620 Mini Sputter Coater.
Optical Absorption (Transmissibility) Analysis
The barrier properties of PVA/GBPF composite films against ultraviolet (UV) and visible light were measured at selected wavelength between 200 and 800 nm, using a UV–visible spectrophotometer (model 8415A, Hewlett–Packard Co., Santa Clara, CA, USA).
Mechanical Properties of PVA/GBPF Bio Films
Tensile strength (TS) and elongation at break (EAB) of the prepared films were conducted by using an Instron 3369 testing machine. Tensile test of (PVA and PVA/GBPF bio films) the samples were conducted according to the ASTMD882The test was performed in the controlled room at 25 °C and 50% relative humidity (RH). Five film samples in composition (10 × 100 mm) with the initial grip length of 3 cm were used for testing for each film. The film samples were clamped and deformed under tensile loading using a 100 N load cell with the cross head speed of 2 mm s−1 until the samples were broken. TS values were calculated by dividing the maximum load (N) by the initial cross-sectional area (m2) of the specimen. EAB was calculated as the ratio of the final length at the point of sample rupture to the initial length of the specimen and expressed as a percentage.
Film Solubility in Water (FS %)
The film Solubility was determined as the percentage of dissolved dry matter after immersion in water. Three randomly selected specimens of each type of film (3 × 3 cm) were first dried at 60 °C for 24 h to determine the initial dry matter (W1). Each film was immersed into 30 ml of distilled water in a 50 ml for 24 h with slow mechanical stirring. The film samples were removed by filtration after 24 h and dried in a drying oven at 105 °C for 24 h to determine the undissolved final dry weight (W2). The initial dry weight was determined from the sample moisture content (determined by gravimetric analysis), and the difference in weight was used to calculate the water soluble matter as percentage of the initial weight. Then FS of the film sample was calculated as follows:
Water Absorption
The swelling test was performed to determine water uptake ability of the prepared films (PVA and PVA/GBPF). The water absorbing capacity of films was conducted in accordance with ASTM D570-98 [28]. The film samples of each composition were cut into the dimensions of 24 × 10 mm2. Thin film samples were dried in on oven at 80 °C prior to measurement. The dried films were weighed and immersed in 20-ml distilled water for 12 h at 25 °C. The excessive water on the surface of the films was removed with filter paper and then weighed immediately. The average of five values was recorded. The percentage of water absorption, \(W_{a}\) (%) was calculated.
where \(W_{e}\) was the weight of the samples at equilibrium and \(W_{0}\) was the initial weight of the dried samples.
Water Vapour Permeability or Water Transmission (WVP)
The water vapor permeability (WVP) of PVA/GBPF films was determined by the standard method of ASTM E96-80 [29]. The WVP measuring cup was filled with 18 ml of distilled water and film sample (10 × 10 cm) was placed on the top of cup and sealed tightly to prevent the leakage of water vapor. The assembled WVP cup was weighed and subsequently placed in a controlled environmental chamber set at 25 °C and 50% RH. Weight change of the cup was determined every1 h for 10 h. The water vapor transmission rate (WVP; g m−2 h) of the film was calculated by using the slope of the steady-state (linear) portion of weight loss versus time plot. Then, the WVP (g m−2 H pa) of the film was calculated as follows:
where \(\Delta W\) was the weight gain, t was the time tested (h) and A was the area exposed to water.
Bio Degradation Test—Soil Burial Test
The soil burial test was carried out on a laboratory scale to examine the biodegradability using the method reported by [30]. The Average initial weight and dimensions of the dry specimens were 0.65 g and (40 × 10 mm).The dry specimens were buried by random pattern in a perforated box to allow the samples to be attacked by the microorganisms and moisture. The moisture content was maintained at 40–50% of the soil’s maximum water holding capacity. In addition, the pots were covered with plastic film to avoid water evaporation from the soil surface. The pot containing the soil and samples were incubated at almost constant temperature of 25 °C for one month. Biodegradation was estimated by monitoring changes in weight as a function of burial time. The samples were removed from the soil every 10 days. The specimens were then carefully washed with water several times in order to remove debris and soil from the film and to ensure that the degradation stopped. After that, the samples were dried at room temperature to a constant weight. After drying, they were weighed using a GR200 model analytical balance in order to determine the percentage values of weight loss as by the following equation:
where \(W_{0}\) is the initial mass and \(W_{t}\) is the remaining mass at any given time, t.
Results and Discussion
FTIR Analysis
The FTIR spectra of green banana peel filler (GBPF), PVA matrix and PVA/GBPF bio films with 5, 10, 15, 20 and 25 wt% of GBPF loading are presented in Fig. 1a. Figure 1b represents the FTIR spectrum of a GBPF. Figure 1b indicated the bands in the region of 3418 cm−1 indicates the presence of a stretching of strong hydroxyl groups. The band at 2980 cm−1 is assigned to CH2 stretching. Also, the band at 1735 cm−1 corresponding to the stretching of a carbonyl group C=O (hemicellulose band). The band at 1647 cm−1 represents absorbed water and the band at 1420 cm−1 assigned to –C–H bending. In addition, the band at 1281 cm−1 corresponds to C–O stretching (lignin band) and the band at 1026 cm−1 represents C–OR stretching [31]. The characteristics peak of PVA could be observed at 3327 cm−1 for stretching vibration of (–OH) and additional peaks of 2931 cm−1 (–CH3 vibration) and 1725 cm−1 (C=O stretching) while PVA having the additional bands at 1240 cm−1 (–CO of carbonate), 1082 cm−1 (–O–C=O) and 855 cm−1 (C–C methyl group). It is clearly understood that all the spectra are almost similar except for a few changes. Comparing the spectra of the GBPF with PVA matrix indicates that the intensity of the OH groups (3318 cm−1 of the filler is very less when compared to that of the matrix indicating lower α-cellulose content in the filler). Further, the GBPF has the additional peaks corresponding to the CH2 group in the range 3010–2853 cm−1 with higher intensity compared to the matrix. This indicates the presence of more number of flexible CH2 groups in the filler than in the matrix. There is a strong peak observed at 1735 cm−1 for the filler corresponding to the CO stretching vibrations. The intensity of this peak in the matrix is shifted to 1725 cm−1 and it is very low peak, this indicates that more amount of CO group containing hemicelluloses in the GBPF [32]. Thus, it clearly indicates that the filler is more amorphous than the matrix due to the presence of a large amount of amorphous hemicellulose in it. Another peak observed in the GBPF at 1610 cm−1 which is shifted to 1630 cm−1 for the PVA matrix corresponds to the observed water molecules which are more in the filler. Similarly, the peak at around 1385 cm−1 corresponds to CH2 and CH3 vibration of the filler which is having higher intensity when compared to that of the matrix. The intensity of the peak observed at 1071 cm−1 corresponding to the C–O–H and C–O–R vibrations is lower than that of the matrix indicating lower OH groups. There is an additional peak in the filler at 1281 cm−1 corresponding to the O–H phenolic of lignin.
The FTIR analysis of the GBPF indicates its lignocellulose nature. The –OH, C=O, CH2 and CH3 groups are available in both PVA and GBPF that may be possibly from compatibility and hydrogen bonding matrix and filler. It is evident from the FTIR spectra that the intensity of the bands of the PVA/GBPF bio films mainly consist of the functional groups in between that of the matrix and the filler. It is noted that C=O peak slightly shifted toward the right side from 1725 to 1690 cm−1 for the incorporation of GBPF (5–25 wt%) in the PVA Matrix. These observations confirmed that all PVA/GBPF composite films having assimilation peak at 1690 cm−1 were authorized to the vibration of carbonyl (C=O) group, which facilitated the compatibility between two components. With the enhancement of GBPF (5–25 wt%) with PVA, the characteristic peak at 3327 cm−1 shifted toward lower wave numbers in bio films. The intensity of the peak gradually decreased due to GBPF loading in the PVA matrix. There is no notable high intensity peaks in PVA/GBPF bio films spectra due to GBPF loading in the PVA matrix. Both PVA and GBPF are hydrophilic [33] in nature, so good at dispersion of Nano particles occurred in bio films.
XRD Analysis
X-ray diffraction is a proven tool to study crystal lattice arrangements and yields very useful information on degree of sample crystallinity. The X-ray patterns of GBPF, PVA and PVA/GBPF bio films are shown in figure, from Fig. 2a–e, it is understandable that the matrix and the bio films have almost similar peaks. The main common peaks at 2θ = 14.3°, 18.8° and 19.4°. The GBPF has the peak at 2θ = 20.9°. From Fig. 3a, b, it is clearly evident that GBPF has higher crystallinity than PVA. Also it can be noticed that the intensity of peaks of the filler is lower than the intensity of the matrix and the intensity of PVA/GBPF bio films reduced with increase in the filler content. The PVA diffractograms shows that higher the intensity, which divulges that semi crystallinity, crosslinking property and amorphous nature [34]. The diffractograms of PVA/GBPF bio films have higher intensity than that of GBPF. It is clearly evident that the PVA/GBPF bio film consists of amorphous (PVA) and high crystalline (GBPF) components. There by fabricated PVA/GBPF bio films have crystallinity index in between the crystallinity of filler and matrix.
Table 2 shows the peak values and calculated CI value of PVA, GBPF and PVA/GBPF bio films. The CI value of Bio films augmented when increasing the filler content which may be due to the higher CI value of GBPF. The Peak value and crystallinity Index (CI) of PVA, GBPF and PVA/GBPF bio-films are shown in Table 2. The high crystallinity value of PVA/GBPF bio films strongly predisposed in the thermal capacity and betterment in tensile properties.
Thermal Analysis (TGA and DTG)
The thermal stability of the poly vinyl alcohol (PVA), green banana peel filler (GBPF) and PVA/GBPF bio films were tested using Thermogravimetric analysis and the resulting TGA and DTG curves are shown in Fig. 4a, b.
The TGA curves of the PVA/GBPF bio films showed the weight loss pattern with increasing temperature and the DTG curves evidently exhibited the decomposition temperature at each stage of thermal degradation. The entire bio film sample exhibited three stages of thermal degradation. The initial stage of decomposition observed around below 60—150 °C corresponds to the evaporation of moisture content with about 7–15% of weight reduction. Even though the GBPF was dried before the usage of ample amputation of moisture content is difficult due to its hydrophilic nature [35]. The second stage of thermal degradation from 200 to 380 °C which indicated the putrefaction of polymeric network of lignin, cellulous and wide range organic molecules. The third of thermal dilapidation occurs between 400 and 550 °C indicated the deprivation of wax and ash content in PVA/GBPF bio films 85–96% of weight reduction. PVA exhibited a two-step degradation at about 280 and 430 °C, which were attributed to the acetate group elimination at lower temperatures followed by a breakdown of polymer backbone at higher temperatures [36]. The main stage of weight loss or the maximum thermal decomposition (Tmax) exhibited around 300, 333, 310, 313, 316, and 320 °C for PVA, GBPF, PVA/GBPF (5–25 wt%) bio films respectively. The DTG curve shows maximum degradation temperature (300 °C) of PVA got shifted to 320 °C in Fig. 4b, mainly due to higher thermal stability of filler content (25 wt%). Further, it is evident that the GBPF demonstrated higher thermal stability than matrix and bio films. The inflection temperature corresponding to the maximum degradation rate for the PVA was found to be at 300 °C and it was increased for the PVA/GBPF bio films with increase in the filler concentration from 5 to 25 wt%. The increased thermal stability of the PVA/GBPF bio films may be due to presence of rigid polyphenols in the GBPF [37]. This improvement in thermal stability of PVA/GBPF bio films is attributed to the strong interfacial interaction and chemical bonding between PVA and GBPF. It is also evident that lowr glass transition temperature of the PVA/GBPF bio films due to many methylene groups available in GBPF. The above declared verdicts ensured that PVA/GBPF bio films are appropriate materials to fabricate the application with employed temperature of 300 °C.
Transmissibility
Figure 5a, shows that the PVA/GBPF bio film has low light transmittance and the observation on the transparency level of the film. On the surface, PVA/GBPF bio film the green banana peel filler is spread evenly, for higher concentration of GBPF in PVA matrix it seems rough, and appears to be porous. The PVA/GBPF bio films photographic images clearly shown in Fig. 5b. Although the value of light transmission is low, the composite film still showed a good result. This condition causes a high chance for vapor to interact with the surface of the film [38]. Thus the light is not only reflected and refracts by both film and vapour surface but also absorbed toward the film [39].
Hence, only a few of the light transmit into this film. PVA film is transparent with nearly 70% light transmittance at a wavelength of 800 nm as shown in Fig. 5a. However, after the green banana peel filler addition into PVA, the light transmission significantly decreases. As the concentration of GBPF from 5 to 25 wt% in PVA matrix, the light transmission of bio film decreased. The light is 70% for transmittance in the visible light region for the controlled PVA film and 38% for the film containing 25 wt% of GBPF. All the PVA/GBPF biofilm samples are characterized by high transmission in the visible spectral range. Visible light is composed of a range of frequencies. The frequency of the radiation is proportional to its energy and the wavelength of the radiation is inversely proportional to the energy. Red is the lowest energy visible light and violet is the highest. The PVA/GBPF samples transmit predominantly in the brown region of the spectrum (700–800 nm). Figure 5a shows that, all these films have low light transmission % (high light absorption). Increase of light absorption threshold of biodegradable films in the UV region of the spectrum offers prospects for their use in the production of packaging material for food industry [40]. Although the value of light transmission is low, the composite film still showed a good result and has appropriate optical clarity for using in packaging application.
Surface Morphology
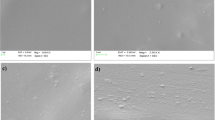
FE-SEM micrographs of Green Banana Peel Filler (GBPF) are shown in Fig. 6a, b with the magnification of 100 and 10 µm respectively. The FE-SEM images at a magnification of 100 µm of the composites with lowest (5 wt%) to highest (25 wt%) of GBPF loading in PVA matrix were presented in Fig. 6d–h respectively. From Fig. 6c, it is evident that the controlled PVA films exhibits a very flat and smooth surface and does not show any favored alignments.
The GBPF exhibited the irregular surface morphology and a porous surface. This porous surface can cause a high absorption of water soluble PVA polymer solution [41]. The PVA/GBPF bio films are showing the highly layered structure and the nanoparticles of fillers dispersed in PVA matrix due to strong intermolecular hydrogen bonding [42]. From Fig. 6d–g, it is evident that in the case of the bio films with 5, 10 and 15 wt% of filler loading, the GBPF was fairly uniformly distributed in the PVA matrix. However, when the filler loading was increased to 20 wt%, more agglomeration of the GBPF was evidenced in Fig. 6g, h. The portion of the composite where agglomeration was identified is shown in circles in Fig. 6g. At high loading of GBPF, some parallel alignment and rough texture are also observed on the addition of GBPF which results in transition occurs from ductile to brittle. This will indirectly affect the mechanical properties at higher loading of GBPF and films will become more brittle further incorporation of GBPF in PVA Matrix, The similar results were observed in the case of PVA/surface treated bagasse fiber composites [43]. It is also evident that N2 functional groups in filler promote the homogeneous spreading of GBPF into polar PVA matrix. From the mentioned results, it is obvious that the GBPF molecules have good compatibility with PVA and gives small voids, and agglomerations observed due to higher loading of filler and its high crystalline nature.
Mechanical Properties
In order to study the effect of the GBPF on the mechanical properties of the PVA/GBPF bio films the tensile test was carried out and the variation in tensile strength, % elongation at break and tensile modulus are presented in Fig. 7a–c. From Fig. 7a, b it is clearly evident that the incorporation of GBPF increased the tensile strength and modulus of elasticity of the bio films. From Fig. 7, it is also noted that the tensile strength of the PVA/GBPF bio films increased from 27.1 (Controlled PVA films) to 44.5 MPa (PVA/GBPF 20 wt%), 39.10% of increase of tensile strength. Similarly, the tensile modulus of the PVA/GBPF increased from 5.34 to 66.7 GPa, 91.99% of increase in tensile modulus from pure PVA film to PVA/GBPF (20wt %) biofilm. Figure 7c shows the % of Elongation at break is reduced with filler loading in PVA matrix. It is evident from the results that the GBPF truly acted as reinforcements, as tensile properties of the PVA/GBPF bio films improved with increased filler content. This may be ascribed to the even distribution of the GBPF in PVA matrix and good adhesion between the components. The existence of polyphenols, fibers, proteins, carbohydrates, mono and disaccharides in the GBPF, may be the reason for the bonding and enhanced properties of the PVA/GBPF bio films. Further, the increase in filler content also has increased the number of interfacial region between filler and the matrix which leads to higher load-bearing capacity. Moreover, in the case of random filler reinforcement, the strength and young’s modulus increases only for some proportion of the weight fraction of filler [44].
Subsequently, there was a small reduction in the tensile strength and modulus with 25 wt% GBPF reinforcement but the values were higher than that of the matrix. This may be ascribed to some agglomeration of the GBPF particles in PVA matrix when the concentration was further increased. The agglomeration of the GBPF particles is evident from the SEM images.
It is also understandable that the stress transfer from the matrix to the fillers was effective which improved the tensile properties. A drop in the % elongation of the blends could be seen with increased filler particles. This indicates that the GBPF acted as a rigid component in the PVA/GBPF bio films. It is a common trend when there is an increase in the tensile modulus and vice versa. This shows the enhanced toughness of the bio films. Similar results were also reported earlier [45]. The above stated result reveals that PVA/GBPF bio films (5–20 wt% of GBPF in PVA matrix) have Good mechanical properties and it is suitable material for packaging applications.
Film Solubility in Water (FS)
The Water solubility (FS) of bio film which indicates the water resistance of the film, Fig. 8 shows the % of film solubility in water for the controlled PVA film and PVA/GBPF (25 wt %) bio film were 20.5 and 25.5%, respectively.
The FS of fabricated bio films increased linearly with increase in amalgamation of GBPF in PVA matrix. This result indicates that the GBPF based bio films absorb water and hydrophilic more than the controlled PVA film, which might be attributed to the hydrophilic components in the green banana peel filler such as fat, fiber, and resistant starch [39, 46] also suggested that the water sensitivity of controlled PVA film could be improved by adding the GBPF.
Water Absorption
Water absorption properties of the PVA/GBPF bio films after immersion in a water tank at 25 °C for 12 h are shown in Fig. 9. Water absorption testing provided the water absorption characteristics of the film. The tendency of film to absorb moisture cannot be overlooked; especially for packaging applications, because the slightest amount of moisture or water can significantly change the other properties. The percentage of water absorption PVA/GBPF films is shown in Table 3.
The perceived increase in water absorption for PVA/GBPF films, with increasing GBPF composition, was due to the existence of hydroxyl groups [47], which are extremely susceptible to water or moisture absorption. This was proven by hydrogen bonding observed in the FTIR spectra. A decrease in water sensitivity for bio film is required for most applications of films. However, due to the hydrophilic nature of the PVA and water sensitivity of the GBPF, the percentage of water uptake was increased slightly for PVA/GBPF bio films. The same behaviour was also observed in previous studies by Liang et al. [48].
Water Vapour Permeability (WVP)
WVP is a measurement used to determine the ability of moisture to penetrate and pass through a material. The WVP was calculated and is shown in Table 3. WVP testing determined the ability of water or moisture to penetrate and pass through the film. Gontard et al. [49] mentioned that the rate of WVP of a hydrophilic film depends on both the diffusivity and solubility of water molecules in the matrix of the film. Figure 10, shows the rate of WVP of the Controlled PVA film is lower than that of PVA/GBPF (5–25 wt%) bio films.
The hydrophilic nature of PVA and the water sensitivity behaviour of GBPF owing to absorption of water molecules, thus resulting in a better WVP of PVA/GBPF bio films than pure PVA films. Bio films, with the\ highest content of GBPF, showed the highest value of WVP. This may have been caused by the poor compatibility between PVA, GBPF during higher loading which allows more water molecules to diffuse through the films. Water molecules preferably diffuse through amorphous areas of the polymer matrix, so the degree of crystallinity is also important in the permeability behavior of Films [50]. The WVP of PVA/GBPF bio films is increased when permeable nano fillers (GBPF) are dispersed in the polymer matrix to induce tortuous path for diffusion and some discontinuities were observed in higher composition of green banana peel filler in PVA matrix. It is also observed in SEM results.
Soil Burial Test
The biodegradation behavior of controlled PVA film and PVA/GBPF bio films are shown in Fig. 11. The soil burial provides qualitative indications of biodegradation. Most of the studies on the biodegradability have been based on the weight loss [51]. In this investigation, the degradation behavior of PVA/GBPF bio film was evaluated by measuring its weight loss, which refers to the erosion of molecules from the solid phase.
The initial biodegradation step involves the enzymatic oxidation of secondary alcohol groups in PVA to ketone groups; then the hydrolysis of ketone groups results in chain cleavage [52]. The degradation behavior of the films is dependent on the presence of green banana peel filler reinforced in the PVA matrix. Figure 11 shows the PVA/GBPF bio films have the highest biodegradability rate than the controlled PVA films. The PVA/GBPF bio films deserve the highest weight loss at 42.3% probably due to the hydrophilic nature of GBPF itself. Hydrophobicity increases water absorbability and hence increases degradation. In fact, the degradability also can be related to its appearance which is diminished in size and this contributed to the weight loss of the samples. Moreover, the addition of GBPF reinforcement improved properties of the PVA based film as a whole. Both GBPF and PVA are hydrophilic. The incorporation of both components (PVA and GBPF) is believed to absorb water due to formation of hydrogen bonding between PVA and green banana peel filler evidenced by FTIR analysis. It can be concluded that the weight loss of the PVA/GBPF bio films at 30 days proved that they are biodegradable.
Conclusion
In the present report, Poly (vinyl alcohol) PVA, the agricultural waste Green Banana Peel Filler (GBPF), and PVA/GBPF biofilms with various compositions of GBPF (5–25 wt%) with the PVA matrix were prepared by solution casting method. Based on this study, investigating the preparation and properties of PVA/GBPF bio films, the following conclusions can be drawn. FTIR spectra designated that the presence of—OH groups on PVA and GBPF resulted in hydrogen bonding interaction which could improve the compatibility of the two components. The degree of crystallinity of all the PVA/GBPF bio film was found to be superior to the PVA. It is manifest from the thermal analysis that the PVA/GBPF bio films are appropriate materials to withstand the temperature of 300 °C. The light is 45% for transmittance in the visible light region for the PVA/GBPF (25 wt%) bio films at a wavelength of 800 nm. The FESEM result revealed a good compatibility between PVA and GBPF upto PVA/20 wt% GBPF biofilms. The small size of voids, agglomeration and cracks exhibited in the film surface in PVA/25 wt% GBPF biofilms can be attributed to high crystalline nature of GBPF. The tensile strength and young’s moduls of the PVA/GBPF bio films were enriched by 39.10 and 91.99% respectively. The elongation at break decreased with increase in the GBPF content up to 20 wt% in PVA matrix. The FESEM results clearly show that the incorporation of 25 wt% GBPF in PVA matrix the voids, agglomeration and micro cracks are present in surface of the film. So it is the transition stage of biofilm from ductile to brittle nature, thereby tensile properties of PVA/GBPF biofilms reduced after incorporation of 25 wt% of GBPF in PVA matrix. The water absorption test showed that the hydrophilic nature of starch molecules of GBPF increases the water uptake for all the PVA/GBPF biofilms. The presence of GBPF in the PVA matrix influenced the water uptake of the PVA/GBPF bio films. Both water absorption and water vapour transmissibility increased with incorporation of GBPF in PVA matrix. % of Water absorption upturns from 10.1 to 31.6% and water vapour transmissibility increased from 7.2 to 11.5%, compared with the pure PVA film. The degradation behaviour of PVA/GBPF bio films was enhanced at 42.3% at higher composition of GBPF in PVA/GBPF bio films. High water absorption capacity of PVA/GBPF bio films is supportive for augmentation in biodegradation properties. Hence, with better tensile, thermal, optical, water absorption and biodegradation properties, the PVA/GBPF bio films can be suitable for food packaging and enfolding applications. The study also deliberated to show the biodegradable film from amalgamation of polymer and organic waste has potential to become alternative resources in plastic making industries, to reduce the amount of discarded organic wastes and to contribute to waste-to-wealth industry development.
References
Sadanand V, Rajini N, Rajulu AV, Satyanarayana B (2016) Preparation of cellulose composites with in situ generated copper nanoparticles using leaf extract and their properties. Carbohyd Polym 150:32–39
Tian H, Wang K, Liu D, Yan J, Xiang A, Rajulu AV (2017) Enhanced mechanical and thermal properties of poly (vinyl alcohol)/corn starch blends by nanoclay intercalation. Int J Biol Macromol 101:314–320
Basu A, Kundu S, Sana S, Halder A, Abdullah MF, Datta S, Mukherjee A (2017) Edible nano-bio-composite film cargo device for food packaging applications. Food Packag Shelf Life 11:98–105
Khan A, Khan AAP, Asiri AM, Gupta V, Rathore M (2016) Preparation, properties and applications of organic–inorganic hybrid nanocomposite poly (aniline-co-o-toluidine) tungstomolybdate. J Mol Liq 216:646–653
Wróblewska-Krepsztul J, Rydzkowski T, Borowski G, Szczypiński M, Klepka T, Thakur VK (2018) Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int J Polym Anal Charact 23(4):383–395
Neto JSS, Lima RAA, Cavalcanti DKK, Souza JPB, Aguiar RAA, Banea MD (2019) Effect of chemical treatment on the thermal properties of hybrid natural fiber-reinforced composites. J Appl Polym Sci 136(10):47154
Cano AI, Cháfer M, Chiralt A, González-Martínez C (2015) Physical and microstructural properties of biodegradable films based on pea starch and PVA. J Food Eng 167:59–64
Pan L, Pei X, He R, Wan Q, Wang J (2012) Multiwall carbon nanotubes/polycaprolactone composites for bone tissue engineering application. Colloids Surf B 93:226–234
Zhang Y, Yu C, Chu PK, Lv F, Zhang C, Ji J, Wang H (2012) Mechanical and thermal properties of basalt fiber reinforced poly (butylene succinate) composites. Mater Chem Phys 133(2–3):845–849
Khan A, Rangappa SM, Jawaid M, Siengchin S, Asiri AM (eds) (2020) Hybrid fiber composites: materials, manufacturing, process engineering. Wiley, Hoboken
Vehapi M, Yilmaz A, Özçimen D (2020) Fabrication of oregano-olive oil loaded PVA/chitosan nanoparticles via electrospraying method. J Nat Fibers. https://doi.org/10.1080/15440478.2020.1774463
Nayak S, Khuntia SK, Mohanty SD, Mohapatra J (2020) Investigation and fabrication of thermo-mechanical properties of Ceiba Pentandra bark fiber/poly (vinyl) alcohol composites for automobile dash board and door panel applications. J Nat Fibers. https://doi.org/10.1080/15440478.2020.1745124
SenthilMuthu Kumar T, Rajini N, Jawaid M, VaradaRajulu A, WinowlinJappes JT (2018) Preparation and properties of cellulose/tamarind nut powder green composites: (green composite using agricultural waste reinforcement). J Nat Fibers 15(1):11–20
Xia G, Reddy KO, Maheswari CU, Jayaramudu J, Zhang J, Zhang J, Rajulu AV (2015) Preparation and properties of biodegradable spent tea leaf powder/poly (propylene carbonate) composite films. Int J Polym Anal Charact 20(4):377–387
Rathinavel S, Saravanakumar SS (2020) Development and analysis of poly vinyl alcohol/orange peel powder biocomposite films. J Nat Fibers. https://doi.org/10.1080/15440478.2019.1711285
Balavairavan B, Saravanakumar SS, Manikandan KM (2020) Physicochemical and structural properties of green bio films from poly (vinyl alcohol)/nano coconut shell filler. J Nat Fibers. https://doi.org/10.1080/15440478.2020.1723778
Manikandan KM, Yelilarasi A, Senthamaraikannan P, Saravanakumar SS, Khan A, Asiri AM (2019) A green-nanocomposite film based on poly (vinyl alcohol)/Eleusinecoracana: structural, thermal, and morphological properties. Int J Polym Anal Charact 24(3):257–265
Eltayeb NE, Khan A (2020) Preparation and properties of newly synthesized polyaniline@ graphene oxide/Ag nanocomposite for highly selective sensor application. J Mater Res Technol 9(5):10459–10467
Vu HT, Scarlett CJ, Vuong QV (2017) Effects of drying conditions on physicochemical and antioxidant properties of banana (Musa cavendish) peels. Drying Technol 35(9):1141–1151
Astuti P, Erprihana AA (2014) Antimicrobial edible film from banana peels as food packaging. Am J Oil Chem Technol 2(2):66–70
González-Montelongo R, Lobo MG, González M (2010) Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem 119(3):1030–1039
Tibolla H, Pelissari FM, Martins JT, Vicente AA, Menegalli FC (2018) Cellulose nanofibers produced from banana peel by chemical and mechanical treatments: characterization and cytotoxicity assessment. Food Hydrocoll 75:192–201
Rattanavichai W, Cheng W, Chang CC (2017) Simplified processing method of banana (Musa acuminata) peels possess the improvement in immunological responses of Macrobrachium rosenbergii. Aquac Res 48(10):5202–5213
Xue M, Lu W, Chen C, Tan Y, Li B, Zhang C (2019) Optimized synthesis of banana peel derived porous carbon and its application in lithium sulfur batteries. Mater Res Bull 112:269–280
Emaga TH, Andrianaivo RH, Wathelet B, Tchango JT, Paquot M (2007) Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem 103(2):590–600
Kong FB, He QL, Peng W, Nie SB, Dong X, Yang JN (2020) Eco-friendly flame retardant poly (lactic acid) composites based on banana peel powders and phytic acid: flame retardancy and thermal property. J Polym Res 27(8):1–12
Khawas P, Das AJ, Deka SC (2016) Production of renewable cellulose nanopaper from culinary banana (Musa ABB) peel and its characterization. Ind Crops Prod 86:102–112
Hassan MM (2018) Enhanced antimicrobial activity and reduced water absorption of chitosan films graft copolymerized with poly (acryloyloxy) ethyltrimethylammonium chloride. Int J Biol Macromol 118:1685–1695
Aguirre A, Borneo R, León AE (2011) Properties of triticale flour protein based films. LWT-Food Sci Technol 44(9):1853–1858
Laxmeshwar SS, Madhu Kumar DJ, Viveka S, Nagaraja GK (2012) Preparation and properties of biodegradable film composites using modified cellulose fibre-reinforced with PVA. ISRN Polym Sci. https://doi.org/10.5402/2012/154314
Kamel NA, Abd El-messieh SL, Saleh NM (2017) Chitosan/banana peel powder nanocomposites for wound dressing application: preparation and characterization. Mater Sci Eng C 72:543–550
Suki FM, Ismail H, Hamid ZA (2014) Preparation and properties of polyvinyl alcohol/banana frond flour biodegradable film. Prog Rubber Plast Recycl Technol 30(2):103–114
Zhong OX, Ismail H, Abdul Aziz NA, Abu Bakar A (2011) Preparation and properties of biodegradable polymer film based on polyvinyl alcohol and tropical fruit waste flour. Polym Plast Technol Eng 50(7):705–711
Wang H, Fang P, Chen Z, Wang S (2007) Synthesis and characterization of CdS/PVA nanocomposite films. Appl Surf Sci 253(20):8495–8499
Tahir MH, Zhao Z, Ren J, Rasool T, Naqvi SR (2019) Thermo-kinetics and gaseous product analysis of banana peel pyrolysis for its bioenergy potential. Biomass Bioenerg 122:193–201
Peng Z, Kong LX (2007) A thermal degradation mechanism of polyvinyl alcohol/silica nanocomposites. Polym Degrad Stab 92(6):1061–1071
Vu HT, Scarlett CJ, Vuong QV (2018) Phenolic compounds within banana peel and their potential uses: a review. J Funct Foods 40:238–248
Silva VDM, Macedo MCC, Rodrigues CG, dos Santos AN, Loyola ACDF, Fante CA (2020) Biodegradable edible films of ripe banana peel and starch enriched with extract of Eriobotrya japonica leaves. Food Biosci 38:100750
Pelissari FM, Andrade-Mahecha MM, Amaral Sobral PJ, Menegalli FC (2013) Comparative study on the properties of flour and starch films of plantain bananas (Musa paradisiaca). Food Hydrocoll 30(2):681–690
Vorobyeva OV, Andrusenko SF, Volosova EV, Avanesyan SS, Ivanova AM, Kadanova AA (2011) Modification of natural polymers for synthesis of biodegradable materials. Chem Sustain Dev 19(2):131–134
El Bourakadi K, Merghoub N, Fardioui M, Mekhzoum MEM, Kadmiri IM, Essassi EM, Bouhfid R (2019) Chitosan/polyvinyl alcohol/thiabendazoluim-montmorillonite bio-nanocomposite films: mechanical, morphological and antimicrobial properties. Compos B 172:103–110
Rajini N, Alavudeen A, Siengchin S, Rajulu V, Ayrilmis N (2019) Development and analysis of completely biodegradable cellulose/banana peel powder composite films. J Nat Fibers. https://doi.org/10.1080/15440478.2019.1612811
Guo G, Xiang A, Tian H (2018) Thermal and mechanical properties of eco-friendly poly (vinyl alcohol) films with surface treated bagasse fibers. J Polym Environ 26(9):3949–3956
Perumal AB, Sellamuthu PS, Nambiar RB, Sadiku ER (2018) Development of polyvinyl alcohol/chitosan bio-nanocomposite films reinforced with cellulose nanocrystals isolated from rice straw. Appl Surf Sci 449:591–602
Asad M, Saba N, Asiri AM, Jawaid M, Indarti E, Wanrosli WD (2018) Preparation and characterization of nanocomposite films from oil palm pulp nanocellulose/poly (Vinyl alcohol) by casting method. Carbohyd Polym 191:103–111
Orsuwan A, Sothornvit R (2015) Effect of miniemulsion cross-linking and ultrasonication on properties of banana starch. Int J Food Sci Technol 50(2):298–304
Ismail H, Zaaba NF (2011) Effect of additives on properties of polyvinyl alcohol (PVA)/tapioca starch biodegradable films. Polym Plast Technol Eng 50(12):1214–1219
Chen L, Imam SH, Gordon SH, Greene RV (1997) Starch-polyvinyl alcohol crosslinked film—performance and biodegradation. J Environ Polym Degrad 5(2):111–117
Gontard N, Duchez C, Cuq JL, Guilbert S (1994) Edible composite films of wheat gluten and lipids: water vapour permeability and other physical properties. Int J Food Sci Technol 29(1):39–50
Guimarães M, Vagner RB, Kátia MN, Fábio GT, Gustavo HDT (2015) High moisture strength of cassava starch/polyvinyl alcohol-compatible blends for the packaging and agricultural sectors. J Polym Res 22(10):192
Guohua Z, Ya L, Cuilan F, Min Z, Caiqiong Z, Zongdao C (2006) Water resistance, mechanical properties and biodegradability of methylated-cornstarch/poly (vinyl alcohol) blend film. Polym Degrad Stab 91(4):703–711
Campos AD, Marconato JC, Martins-Franchetti SM (2011) Biodegradation of blend films PVA/PVC, PVA/PCL in soil and soil with landfill leachate. Braz Arch Biol Technol 54(6):1367–1378
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balavairavan, B., Saravanakumar, S.S. Characterization of Ecofriendly Poly (Vinyl Alcohol) and Green Banana Peel Filler (GBPF) Reinforced Bio-Films. J Polym Environ 29, 2756–2771 (2021). https://doi.org/10.1007/s10924-021-02056-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02056-y