Abstract
The intention of this work is to study the impact of inclusion of Cashew nut shell powder (CNS) in the Poly vinylalcohol (PVA) based films. CNS is a biowaste material chosen as a particulate reinforcement, and the biodegradable polymer PVA is made as a matrix material. The films with various weight proportion was fabricated through solution casting method and characterized using FTIR, XRD, morphological examinations, mechanical and thermal property analysis, antibacterial evaluation, and degradation tests. Compared to films lacking essential oil, the biodegradable films showed significant enhancements in mechanical properties, with tensile strength increasing to 22 MPa (a 130% increase), tensile modulus increasing to 264 MPa, and elongation decreasing by 24.5%. However, the addition of essential oil significantly improved thermal stability, with the initiation of degradation showing at a significantly higher temperature of 215 °C, representing a significant 43% increase. The antimicrobial testing revealed a significant 93% reduction in bacterial growth, demonstrating CNS's potency as an antibacterial agent. These findings highlight the possibility of combining CNS with PVA to fabricate biocomposite films with higher biodegradability and antibacterial characteristics, along with higher tensile strength and thermal stability, confirming their viability for eco-friendly packaging materials and other sustainable applications.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Increase in the need to find alternates for plastic-based packaging materials, especially in food product wrappings, and to reduce the biowaste burden to municipal solid waste management; the attempt to analyze the novel biowaste Cashew nut shell powder along with biodegradable polymer-based wrappers. The unique combination of Polyvinyl alcohol and Cashew nut shell powder is expected to act as an alternative to traditional plastic-based wrappers. The novel biowaste and polyvinyl alcohol-based films can be prepared using a simple solution casting method. The novelty of this film is its biodegradability, antibacterial behavior, and enhanced mechanical properties as compared to raw Polyvinyl alcohol film. These behaviors will certainly help us to resolve ecological problems and potential for day-to-day use.
Introduction
Many applications of plastics prompt mankind to utilize them in our day-to-day life. Also, they become more reliable due to their abundant availability and flexibility in manufacturing [1]. However, on the other side, they pose environmental harm when used in consumables and at their end-use [2]. Plastics are employed in consumable applications like food packaging, containers, and wrappers. Additionally, after use, they are discarded as waste material, causing environmental issues due to their non-biodegradability [3]. Therefore, there is a need to find an alternative material that is environmentally friendly for food packaging. Formulating this as objective, researchers focused on developing a biodegradable polymer-based material. The importance of biodegradable polymers arises from their crucial role in the production of food packaging materials, such as composite films, multilayer films, active films, and more [4].
In the development of biodegradable polymer films, a chosen biodegradable polymer material serves as the matrix, combined with particulate reinforcements [5]. The commonly used biodegradable polymers include Polyglycolic acid (PGA), Polylactic acid (PLA), Poly(oxyethylene), Polyhydroxyalkanoates (PHA), Polypropylene carbonate, Polyvinyl alcohol (PVA), etc. [6, 7]. Out of these, water soluble polymers were utilized in large applications due to their reliability. So in this instance PVA was selected for this work due to its solubility in water and biodegradability. Additionally, it exhibits enhanced mechanical properties and film-forming capacity. PVA is essentially a synthetic material and water-soluble, obtained through vinyl acetate polymerization [8, 9].
As per studies [10, 11], PVA exhibits excellent mechanical and physical qualities. However, enhancing both physical and mechanical properties is essential when developing biopolymeric/biocomposite films intended for food packaging [12]. The augmentation of these properties is achieved through the addition of filler materials, such as organic and inorganic substances [13]. There are few natural materials were utilized along with PVA in the recent past research to improve the properties of PVA. Rathinavel et.al [14] attempted to utilize the PVA along with Orange peel powder and gained the thermal as well as tensile property improvement in the optimum inclusion of orange peel powder in the PVA. Balavairavan et.al [15] utilize the banana peel as a filler material to form banana peel/PVA film through solution casting method and analyzed its optical and water absorption property. Tripathy et.al [16] examined the physiochemical analysis and antibacterial activity of chitosan and PVA films. Similarly the idea is to utilize the focuses on biodegradable materials, organic or natural filler materials to improvise the properties of PVA with better anti bacterial activity and mechanical properties.
The selection of filler material is based on its abundant availability and biodegradable properties [17]. Additionally, the choice of filler material is made considering it is a biowaste material. A report on municipal solid waste indicates that waste from natural products, during the conversion to its byproducts, creates ecological problems such as landfill and disposal challenges due to their degradability period [18, 19].Additionally, owing to their non-toxicity, permeability, bioactivity, and biodegradable qualities, the utilization of biopolymeric composite materials with natural fillers is extensive in the food packaging industry [20]. Consequently, we focused on using fillers made from waste natural materials in this study. Due to consumer awareness, there is now a greater demand for packaging material reinforced with natural fillers made from plant extracts [21]. The filler materials already explored are Napier grass, rice husk and Tamarind (Tamarindus indica) seeds by divya.et.al [22].
Cashew is a tropical cash crop, and its nut is consumed by mankind, leaving the remaining as waste material [23]. The shell of the cashew is about 2–3 mm in thickness and can be utilized as biomass, allowing us to avoid wastage [24]. The cashew shell is left unused and dumbed as waste material. This waste material crates ecological problems like landfill and affects environment during disposal through incineration. Aside of the disposal problem these Cashew nut shells have useful constituents listed in Table 1 [25] made the choice of filler material. The reduced hemicellulose content and high cellulose content will help the polymer matrix to bind with them. Already researchers tried the cashew dust powders as a friction dust materials to find an application in brake pads as an alternative to asbestos materials [26].
In this study, biocomposite films were fabricated by incorporating particulate CNS into PVA. The weight percentage of CNS in the films varied from 0 to 20%. Subsequently, the generated films underwent comprehensive characterizations like surface morphology, thermal analysis and tensile test. In addition to these assessments, the films were scrutinized for antibacterial efficacy and degradability, aiming to assess their compatibility. The study sought to comprehend how Cashew nut shell powder, when integrated into biofilms along with PVA, can be effectively employed, especially in the realm packaging of materials.
Materials and Methods
Materials
Cashew nut shells discarded as waste were collected from the cashew nut processing unit located in Kollam, Kerala. The collected cashew nut shells are dried in the sunlight for a week. Once the moisture in the shells is completely dried, they are then crushed down to the fine nano-sized cashew nut shell powder (CNS).
The required PVA was purchased in Bangalore, India, with 120,000 g/mol as molecular weight. The provided PVA is hot water-soluble to achieve better film-forming capacity. Figure 1
Methodology
Preparation of PVA/CNS Biocomposite Films
Following the literature [1, 14], the PVA/CNS biocomposite films were fabricated by solution casting method. The films with various weight percentages of CNS along with PVA. To obtain these films, the hot water-soluble PVA crystals were converted into a solution by 3 h of magnetic stirring of PVA along with water at 80 ºC. Then, the CNS of the respective weight percentages was added to the completely dissolved PVA solution and stirred continuously until completely dispersed solutions were obtained. After achieving a completely dissolved PVA/CNS solution, it was spread onto a glass plate and left to cure at room temperature. Then, the cured films were carefully removed from the glass plate. Table 2 shows the percentage of CNS by weight for fabricating PVA/CNS biocomposite films.
Characterization and Determination Of Properties
Microscopic Analysis
The microscopic analysis on the surface of PVA/CNS biocomposite films was done by Field Emission Scanning Electron Microscope (FESEM). The samples were analyzed after a sputtering procedure to coat the PVA/CNS biocomposite films with a layer of gold coating [27].
FTIR Analysis
The presence of various functional groups on differently weighed PVA/CNS films was analyzed by spectrophotometer. The spectral measurements were in the range of 4000 cm−1 to 500 cm−1 at a scanning rate of 4 cm−1. Before initiating the procedure of spectral recording, the biofilms were compressed using KBr pellets.
XRD
With the range of 2Ɵ = 10°–80°, X-ray diffractograms was observed with the assistance of an EXPERT-PRO Diffractometer using step-scan mode. The process was conducted with a generator setup of 30 mA and 40 kV. The crystalline size was determined using Scherrer's equation [15, 28].
where: DA = Average crystallite size (nm); K = Scherrer's constant (typically 0.89); \(\uplambda\) = wavelength of X-ray; B = Full Width at half maximum (FWHM) of generated peaks; Ɵ = Angle of Diffraction.
where I002 is maximum intensity of the crystalline materials and IAM refers to the diffraction rate of amorphous materials.
Thermogravimetric Analysis (TGA)
Thermal degradation concerning the weight of the fabricated PVA/CNS films was recorded with a NETZSCH STA instrument at nitrogen atmosphere with 25 ml/min flow rate. With increase of 10 ºC/min of temperature over a range of 50 °C to 550 °C to analyze the biofilm's thermal stability. Using the finite difference equation [29], derivative thermograms were derived from the recorded thermograms as
where:
Wt + Δt = Residual weight samples at time t + Δt.
Wt-Δt = Residual weight samples at time t-Δt.
Δt = Time for reading a sample of residual weight.
Mechanical Properties
Thickness and the tensile properties were evaluated in the mechanical properties. The thickness of the fabricated PVA/CNS composite films was evaluateded using a digital micrometer (Quantu Mike IP65) at ten different locations on the films, and the average value was considered. Meanwhile, the tensile test of PVA/CNS biofilms was conducted according to ASTM D882-2018, where the films were sized to 100 mm × 10 mm using a Zwick Roell instrument. The tensile properties were measured with preset of speed 2 mm/min and at preload of 0.1 N [30]. The five samples of each composition was tested and standard deviations are reported.
Antibacterial Activity
Antibacterial test was done by using the disc diffusion method [31]. To make nutrient agar plates with agar broth, 100 ml of distilled water were mixed with nutrient agar medium. These agar plates were then autoclaved for 15 min at 120 °C under 15 psi of pressure. After autoclaving, the medium was transferred to 10 cm Petri dishes. Petri plates with 20 ml of additional agar medium were individually prepared for a whole day at 37 °C, using separate Gram-positive (S. aureus, S. oralis) and Gram-negative (E. coli, P. aeroginosa) agar plates. The antibacterial activity was assessed by measuring the diameter of the inhibition zone [32, 33].
Degradation Property
The soil burial test was used to determine the degradation properties [34] of the PVA/CNS biofilms. The samples were buried in pots containing soil, each with a drain hole in the bottom. Sized to 30 mm × 100 mm, the films were buried at 5 cm depth. The soil burial setup was shielded from direct sunlight to maintain the temperature at ambient levels. Additionally, watering was performed every 30 days to keep the soil surface at 40–50% humidity. After the 30-day degradation period, the samples were removed and thoroughly cleaned to eliminate any remaining soil particles. Following the cleaning process, the degradation weight percentage was determined by calculating the weight fraction concerning its initial weight.
Results and Discussions
Microstructure Analysis
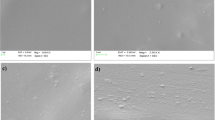
The surface morphology of PVA/CNS biocomposite films with varied CNS weight percentages ranging from 5 to 20% is illustrated in Fig. 2(a–d). Figure 2(a) depicts the PVA/5%CNS surface, revealing a smooth surface with a minimal quantity of CNS, as indicated by the dot-like structure [34]. A rough surface is evident in Fig. 2(b) and Fig. 2(c) for PVA/10%CNS and PVA/15%CNS biofilms, respectively, reflecting a slightly higher occurrence of dots due to increased CNS inclusion during fabrication. Compared to the PVA/5%CNS film, a significantly higher incidence of rough surface development is observed. Figure 2(d) showcases the PVA/20%CNS film with a greater CNS content, revealing CNS agglomerates on the film's surface. These agglomerates contribute to rough surfaces that are evenly distributed. In comparison to films with lower rough surfaces, PVA/20%CNS exhibits higher agglomerated CNS and rougher surfaces, potentially enhancing the film's mechanical properties [35].
FTIR Analysis
The various weight % of CNS incorporated PVA/CNS biocomposite films FTIR Spectra is illustrated in Fig. 3 and Table 3. From Fig. 3, it is evident that all the films exhibit almost identical peaks with varying intensity, indicating that the inclusion of CNS in the PVA does not significantly affect the functional groups present. In all four combinations of PVA/CNS films, a wider band between 3750 cm−1 to 3000 cm−1 is observed, encompassing acme peaks of 3612 cm−1 and 3043 cm−1 [36]. These sharp peaks indicate the presence of –OH groups. Another distinct peak appeared in 2936 cm−1, attributed as cellulosic -C-H band. Peaks appeared in 1742 cm−1 and 1640 cm−1 are observed due to phenolic components (C = O stretching vibration) [37]. Peaks at 1250 cm−1 and 1100 cm−1 corresponded to C = C stretching, associated with the acetyl group caused by presence of hemicellulose in CNS [38]. In the fingerprint region, peaks attributed to glucosidic linkages are observed at 850 cm−1 and 920 cm−1 [39].
XRD Analysis
The fabricated films underwent X-ray diffraction analysis, as depicted in Fig. 4. Similar to the FTIR spectra, the XRD diffractograms also exhibit nearly identical peaks, regardless of the weight percentage of CNS included during fabrication. While the peaks appear similarly, their intensity increases with the rise in CNS content in the films. A common cellulose peak is observed in all the fabricated samples at 2Ɵ = 19.2°, and this cellulose peak is confirmed through the plane of reflection (1 1 0) [40]. Notably, the peak intensity of the PVA/20%CNS film is comparatively higher than in all other films. This increase in peak intensity is attributed to the higher weight percentage of included CNS in PVA/20%CNS. The intensity of the peak increases with crystalline size, aligning with the FESEM result of the PVA/20%CNS film. This film exhibits more agglomerated CNS on its surface, consequently increasing the crystalline size of the CNS. The increase in the intensity might help in the increase of tensile properties.
Through Eq. (1), the crystalline size of the films were calculated, and as aforementioned the agglomerated CNS in the PVA/20%CNS film has higher crystalline size, measuring 14.70 nm. Table 4 further illustrates that an increase in the inclusion of CNS contributes to achieving a higher crystalline size in the biocomposite films. The crystallinity index of PVA/CNS films which are reported in Table 5 are almost similar to those of the earlier report on Pomegranate peel powder[1], orange peel powder[14] and Tapioca Starch[13] reinforced PVA films.
TGA and DTG
Thermograms for both PVA and PVA/CNS biocomposite films are illustrated in Figs. 5 and 6. Figure 5 displays the primary thermograms, while Fig. 6 depicts the derivative thermograms. The curves exhibit three major peaks, indicating points of inflection. These major peaks indicate the three stages of degradation. The initial degradation corresponds to the vaporization of volatile components. The second degradation stage indicates the degradation of hemoicellulose components and linkages of cellulose components. The third stage is the last stage where the thermal stability of the films will be determined, the complete degradation of cellulose components where degraded. Three such points of inflection are observed in Fig. 6. The first point of inflection, around 115 °C, corresponds to moisture loss absorbed by the composite films during the fabrication process. The second point of inflection, observed between 180 °C and 200 °C, signifies a weight loss of approximately 15% due to the degradation of hemicellulose and cellulose links. For the PVA film, the third point of inflection is observed at 290 °C, indicating the thermal stability of the film. Remarkably, the thermal stability point for PVA/20%CNS is 367 °C, the highest among all fabricated films. Table 5 highlights that the CNS content contributes to enhanced thermal performance, with increasing content correlating to higher thermal stability. Peaks around 450 °C suggest the presence of other components in the CNS, and the natural oils in CNS degrade at this temperature, contributing to the films' elasticity. At 550 °C, the residual masses of the films were recorded, as tabulated in Table 6. Pure PVA has very low residual masses compared to all other films with 4.64%. The PVA/15%CNS has the highest residual mass of 12%, the higher weight CNS shows the residual mass of 9.7%. This reduction of residual weight might from the loss of oil content in CNS, the CNS of 20% film has more amount of CNS compare to 15% CNS film and the reduction of oil and other substance might result in the reduced residual mass. These thermally withstanding films can be used for food packaging wraps [41].
Mechanical Properties
The mechanical properties were influenced by the thickness of the films. As shown in Table 6, an increase in CNS content correlates with an inflation of thickness. The maximum thickness of 0.148 mm is observed for the film with the highest CNS content. This increase in thickness may positively impact the tensile properties.
The tensile properties were investigated for the various contents of CNS in the PVA films. The properties, including tensile strength, tensile modulus, and elongation%, are illustrated in Fig. 6(a), (b), and (c), respectively.
Figures 7(a) and (b) display consistent patterns in the bar graph, illustrating that as the content of CNS increases, both tensile strength and modulus show an upward trend. Specifically, the PVA/20%CNS exhibits the highest tensile strength at 26 MPa and a tensile modulus of 264 MPa. Conversely, PVA alone shows the lowest tensile properties, and the inclusion of CNS consistently enhances these properties.
The incorporation of CNS significantly enhances the tensile properties of PVA. This improvement is attributed to the agglomeration of CNS during the fabrication of biocomposite films. The agglomerated CNS effectively blocks porosity and fills cracks in the PVA films. This blockage of porosity and the agglomeration process contribute to reducing flexibility and enhancing the inter-linkage strength of the films. As the CNS content increases, the agglomeration on the film surfaces intensifies, leading to a proportional increase in strength [42]. Notably, at lower CNS content, the biocomposite film exhibits a tensile strength of 14 MPa and a tensile modulus of 232 MPa.
In Fig. 7(c), the analysis of elongation for the biocomposites reveals an inverse relationship between strength and flexibility. As mentioned earlier, in comparison to Figs. 7(a) and (b), Fig. 7(c) displays an opposite trend in the bar graph. This reduction in elongation is attributed to the strong interaction between the matrix and the agglomerated CNS particulates. The increasing CNS content leads to particulate agglomeration, effectively blocking porosity and improving bonding strength [43]. Although there is a noticeable reduction in elasticity, it is not drastic, constituting a 16% decrease compared to films with lower CNS content. This moderated reduction in elasticity is influenced by the oil content present in the CNS shells. The outcomes were similar to those of the earlier report on Pomegranate peel powder [1], orange peel powder [14] and Tapioca Starch [13] reinforced PVA films.
Based on the above discussion, it is evident that the strength of PVA films increases with the content of CNS, supporting the suitability of these films for use as wrappers.
Antibacterial Activity
The various CNS-weighted films were subjected to two different gram-positives and two different gram-negative pathogens. The resultant of test indicates, the CNS content in the films produces more substantial zone of inhibition [44,45,46], as illustrated in Fig. 8. The diameter of the inhibition zone is detailed in Table 7, demonstrating that CNS contributes to a more effective inhibition zone compared to PVA films alone. Notably, PVA/20% CNS films exhibit a robust zone of inhibition, making them recommended for use in food packaging applications. This reduction in antibacterial activity may also contribute to an extended shelf life for packaged food.
Degradation Property
To assess the decomposition capabilities of the films, the soil burial test [38, 45], was employed. For 30 days, the samples are buried in the ground. Numerous microorganisms participate in the breakdown process. The intended period's weight loss has been identified and is depicted in this test's weight loss% to beginning weight. Figure 9 depicts the manufactured films' degrading behavior. The deterioration of PVA film is 2.5% of its initial weight [1]. The 20%CNS film weighs11% less than it did at first. The PVA/20%CNS film also has the greatest degree of deterioration when compared to the other films. Similar to this, other PVA/CNS films exhibit better degrading characteristics than PVA films.
Conclusions
The study was intended to analyze the impact of CNS in PVA films using the solution casting process to create biocomposite films with varying weight proportions of CNS. Despite the addition of CNS to PVA and film formation, no changes or alterations in the functional groups were observed, as confirmed by FTIR analysis. As the level of CNS incorporation increased, agglomeration of CNS at the film surface occurred, leading to an augmentation in crystalline size. XRD analysis further confirmed the presence of cellulose content. This agglomeration positively influenced the tensile properties of films with higher CNS percentages. Thermal analysis demonstrated a notable 30% enhancement in the thermal stability of the PVA/CNS biocomposite films. Additionally, the glass transition temperature of biocomposite films containing CNS exhibited improvement compared to pure PVA films. The incorporation of CNS at higher percentages introduced rough surfaces, resulting in significant improvements in tensile characteristics, including a 22 MPa increase in tensile strength and a substantial 264 MPa increase in tensile modulus. Moreover, the inclusion of 20% CNS reduced porosity caused by CNS particles on the film's surface. Importantly, the addition of CNS improved the antibacterial activity and degrading properties of the films. As compared to all other fabricated films the 20% CNS PVA film posses better results in cellulose content, crystallinity index, thermal stability, tensile property as well as antibacterial activity. These observed impacts of 20% CNS in PVA films make them promising for applications in food packaging and wrapping materials, providing a sustainable alternative to conventional plastic materials.
Data availability
Any data related to this manuscript can be made available on request.
References
Rathinavel S, Senthilkumar TS, Saravanakumar SS, et al (2023) Development and analysis of environmentally friendly biocomposite films with pomegranate peel as filler for conventional applications. Biom. Conv. Bioref. https://doi.org/10.1007/s13399-023-04658-z.
Awad, M.A., Hendi, A.A., Ortashi, K.M.O., Elradi, D.F.A., Eisa, N.E., Al-lahieb, L.A., Al-otiby, S.M., Merghani, N.M., Awad, A.A.G.: Int. J. Phys. Sci. 9, 34–40 (2014)
Rathinavel, S., Saravanakumar, S.S.: Development and Analysis of Silver Nano Particle Influenced PVA/Natural Particulate Hybrid Composites with Thermo-Mechanical Properties. J. Polym. Environ. 29, 1894–1907 (2021). https://doi.org/10.1007/s10924-020-01999-y
Davis, G., Song, J.H.: Biodegradable packaging based on raw materials from crops and their impact on waste management. Ind. Crops and Prod. 23(2), 147–161 (2006). https://doi.org/10.1016/j.indcrop.2005.05.004
Gross, R.A.: Biodegradable Polymers for the Environment. Science 297(5582), 803–807 (2002). https://doi.org/10.1126/science.297.5582.803
Rathinavel, S., Saravanakumar, S.S.: Synthesis of Silver Nanoparticles Through Orange Peel Powder for Antibacterial Composite Filler Applications. J. Polym. Environ. 30, 1407–1414 (2022). https://doi.org/10.1007/s10924-021-02276-2
Panou, Andreas, Karabagias, Ioannis Konstantinos: Biodegradable Packaging Materials for Foods Preservation: Sources, Advantages, Limitations, and Future Perspectives. Coatings 13(7), 1176 (2023). https://doi.org/10.3390/coatings13071176
Li, M., Sun, Y., Feng, D., et al.: Thermally conductive polyvinyl alcohol composite films via introducing hetero-structured MXene@silver fillers. Nano Res. 16, 7820–7828 (2023). https://doi.org/10.1007/s12274-023-5594-1
Silva, G.G.D., Sobral, P.J.A., Carvalho, R.A., et al.: Biodegradable Films Based on Blends of Gelatin and Poly (Vinyl Alcohol): Effect of PVA Type or Concentration on Some Physical Properties of Films. J. Polym. Environ. 16, 276–285 (2008). https://doi.org/10.1007/s10924-008-0112-9
Hajji, S., Chaker, A., Jridi, M., et al.: Structural analysis, and antioxidant and antibacterial properties of chitosan-poly (vinyl alcohol) biodegradable films. Environ. Sci. Pollut. Res. 23, 15310–15320 (2016). https://doi.org/10.1007/s11356-016-6699-9
Suganthi, S., Vignesh, S., Kalyana Sundar, J., et al.: Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications. Appl Water Sci 10, 100 (2020). https://doi.org/10.1007/s13201-020-1162-y
Emran, M.Y. et al. (2023). Biowaste Materials for Advanced Biodegradable Packaging Technology. In: Ali, G.A.M., Makhlouf, A.S.H. (eds) Handbook of Biodegradable Materials. Springer, Cham. https://doi.org/10.1007/978-3-031-09710-2_46
Ismail, H., Zaaba, N.F.: Effect of Additives on Properties of Polyvinyl Alcohol (PVA)/Tapioca Starch Biodegradable Films. Polym Plast Technol Eng 50(12), 1214–1219 (2011). https://doi.org/10.1080/03602559.2011.566241
Rathinavel, S., Saravanakumar, S.S.: Development and Analysis of Poly Vinyl Alcohol/Orange peel powder biocomposite films. J. Nat. Fibers. 18(12), 2045–2054 (2020)
Balavairavan, B., Saravanakumar, S.S.: Characterization of Ecofriendly Poly (Vinyl Alcohol) and Green Banana Peel Filler (GBPF) Reinforced Bio-Films. J. Polym. Environ. 29, 2756–2771 (2021). https://doi.org/10.1007/s10924-021-02056-y
S. Tripathi; G.K. Mehrotra; P.K. Dutta (2009). Physicochemical and bioactivity of cross-linked chitosan–PVA film for food packaging applications. , 45(4), 0–376. doi:https://doi.org/10.1016/j.ijbiomac.2009.07.006
G. Davis, J.H. Song: Biodegradable packaging based on raw materials from crops and their impact on waste management. 23 147-161 (2006)https://doi.org/10.1016/j.indcrop.2005.05.004
N.H. Mutha, M. Patel, V. Premnath: Plastics materials flow analysis for India. 47 222-244 (2006) https://doi.org/10.1016/j.resconrec.2005.09.003
S. Gupta, K. Mohan, R. Prasad, S. Gupta: Solid waste management in India : options and opportunities. 24 137-154 (1998)
Soni, B., Mahmoud, B., Chang, S., El-giar, E.M., Barbary, E.: Physicochemical, antimicrobial and antioxidant properties of chitosan / TEMPO biocomposite packaging fi lms. Food Packag. Shelf Life 17(June), 73–79 (2018). https://doi.org/10.1016/j.fpsl.2018.06.001
Kicińska-Jakubowska, Anna, Bogacz, Edyta, Zimniewska, Ma.łgorzata: Review of Natural Fibers Part I—Vegetable Fibers. J Nat Fib 9(3), 150–167 (2012). https://doi.org/10.1080/15440478.2012.703370
Divakaran, D., Suyambulingam, I., Gapsari, F., Vijay, R., Ayyappan, V., & Siengchin, S: Isolation and characterization of microcrystalline cellulose from an agro-waste tamarind (Tamarindus indica) seeds and its suitability investigation for biofilm formulation. Intl. J. Biol. Macromol. (2024) https://doi.org/10.1016/j.ijbiomac.2023.127687.
Dineshkumar, J., Jesudas, T.: Hybrid polymer matrix development using cashew nut shell liquid as an additive into epoxy resin. J. Chin. Inst. Eng. 46(4), 380–388 (2023)
Ike, D.C., Ibezim-Ezeani, M.U., Akaranta, O.: Cashew nutshell liquid and its derivatives in oil field applications: an update. Green Chem. Lett. Rev. 14(4), 620–633 (2021)
Nuithitikul, K., Phromrak, R., Saengngoen, W.: Utilization of chemically treated cashew-nut shell as potential adsorbent for removal of Pb(II) ions from aqueous solution. Sci. Rep. 10, 3343 (2020). https://doi.org/10.1038/s41598-020-60161-9
Singaravelu, D. L., Vijay, R., & Filip, P: Influence of various cashew friction dusts on the fade and recovery characteristics of non-asbestos copper free brake friction composites. Wear. (2019) https://doi.org/10.1016/j.wear.2018.12.036
Sadanand, V., Rajini, N., Varada Rajulu, A., Satyanarayana, B.: Effect of Sunlight on the Preparation and Properties of Cellulose/Silver Nanoparticle Composite Films by in Situ Method Using Ocimum Sanctum Leaf Extract as a Reducing Agent. Int. J. Polym. Anal. Charact. 23(4), 313–320 (2018). https://doi.org/10.1080/1023666X.2018.1440915
Saravanakumar, S.S., Kumaravel, A., Nagarajan, T., Sudhakar, P., Baskaran, R.: Characterization of a Novel Natural Cellulosic Fiber from Prosopis Juliflora Bark. Carbohyd. Polym. 92(2), 1928–1933 (2013). https://doi.org/10.1016/j.carbpol.2012.11.064
Santhanam, K., Kumaravel, A., Saravanakumar, S.S., Arthanarieswaran, V.P.: Characterization of New Natural Cellulosic Fiber From Ipomoea Staphylinaplant. Int. J. Polym. Anal. Charact. 21(3), 267–274 (2016). https://doi.org/10.1080/1023666X.2016.1147654
Soni, B., Mahmoud, B., Chang, S., et al.: Physicochemical, antimicrobial and antioxidant properties of chitosan/TEMPO biocomposite packaging films. Food Pack. and Shelf Life 17(June), 73–79 (2018). https://doi.org/10.1016/j.fpsl.2018.06.001
Mathew, S., Snigdha, S., Mathew, J., Radhakrishnan, E.K.: Poly(vinyl alcohol): Montmorillonite: Boiled rice water (starch) blend film reinforced with silver nanoparticles; characterization and antibacterial properties. Appl. Clay Sci. 161, 464–473 (2018). https://doi.org/10.1016/j.clay.2018.05.009
Palai, B., Sarangi, S.K., Mohapatra, S.S.: Characterization of silver nano-particle coated Eichhornia crassipes fiber for antibacterial applications. J. Nat. Fibers 19, 1828–1836 (2022). https://doi.org/10.1080/15440478.2020.1788492
Lee, S.W., Phillips, K.S., Gu, H., Kazemzadeh-Narbat, M., Ren, D.: How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 268, 120595 (2021). https://doi.org/10.1016/j.biomaterials.2020.120595
Rajini, N., Alavudeen, A., Siengchin, S., Rajulu, V., Ayrilmis, N.: Development and analysis of completely biodegradable cellulose/banana peel powder composite films. J Nat Fibers (2019). https://doi.org/10.1080/15440478.2019.1612811
Senthilkumar, P., Yaswant, G., Kavitha, S., et al.: Preparation and characterization of hybrid chitosan-silver nanoparticles (Chi-Ag NPs); A potential antibacterial agent. Int. J. Biol. Macromol. 141, 290–298 (2019)
Suteewong, T., Wongpreecha, J., Polpanich, D., et al.: PMMA particles coated with chitosan-silver nanoparticles as a dual antibacterial modifier for natural rubber latex films. Coll. Sur. B: Biointerfaces 174, 544–552 (2018)
Gupta, M.K., Manimaran, P., Suresha, B., et al.: Investigation of mechanical and dynamic mechanical properties of novel Acacia arabica fiber polyester hybrid composites. Polym. Compos. (2022). https://doi.org/10.1002/pc.26569
Indira Devi, M.P., Nallamuthu, N., Rajini, N., et al.: Tensile, thermal, and antibacterial characterization of composites of cellulose/ modified Pennisetum purpureum natural fibers with in situ generated copper nanoparticles. Int. J. Poly. Anal. Charact. 23(6), 502–508 (2018). https://doi.org/10.1080/1023666X.2018.1485201
Cano, A.I., Cháfer, M., Chiralt, A., González-Martínez, C.: Physical and microstructural properties of biodegradable films based on pea starch and PVA. J. Food Eng. 167, 59–64 (2015)
Baskaran, P.G., Kathiresan, M., Senthamaraikannan, P., Saravana Kumar, S.: Characterization of new natural cellulosic fiber from the bark of dichrostachys cinerea. J Nat Fibers 15, 1–7 (2017). https://doi.org/10.1080/15440478.2017.1304314
Prithivirajan, R., Narayanasamy, P., Al-Dhabi, N.A., et al.: Characterization of Musa Paradisiaca L. cellulosic natural fibers from agro-discarded blossom petal waste. J Nat Fibers 17, 1640–1653 (2020). https://doi.org/10.1080/15440478.2019.1588826
Shanmugasundaram, N., Rajendran, I., Ramkumar, T.: Characterization of untreated and alkali treated new cellulosic fiber from an Areca palm leaf stalk as potential reinforcement in polymer composites. Carbohydr. Polym. 195, 566–575 (2018). https://doi.org/10.1016/j.carbpol.2018.04.127
Bhanu, Priya, Vinod Kumar, Gupta, Deepak, Pathania, Amar Singh, Singha: Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohyd. Polym. 109, 171–179 (2014). https://doi.org/10.1016/j.carbpol.2014.03.044
Kumar, Deepak; Kumar, Pramendra; Pandey, Jyoti (2018). Binary grafted chitosan film: Synthesis, characterization, antibacterial activity and prospects for food packaging. International Journal of Biological Macromolecules, S0141813017342599–. doi:https://doi.org/10.1016/j.ijbiomac.2018.04.084
Rayna Bryaskova, Daniela Pencheva, Girish M. Kale, Umesh Lad, T. Kantardjiev : Synthesis, characterisation and antibacterial activity of PVA/TEOS/Ag-Np hybrid thin films. , 349(1), 77–85 (2010). https://doi.org/10.1016/j.jcis.2010.04.091
Thiagamani, S.M.K., Rajini, N., Siengchin, S.: Influence of silver nanoparticles on the mechanical, thermal and antimicrobial properties of cellulose-based hybrid nanocomposites. Compos. Part B. Engineering 165, 516–525 (2019)
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahanta, G.K., Joardar, H. Study on the Impact of Bio Waste Cashew Nut Shell Powder in the Polyvinyl Alcohol Bio Films. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02576-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02576-3