Abstract
This study aimed to examine the structural, mechanical, thermal and barrier properties of the polyvinyl alcohol/casein (PVA/CA) films plasticized with poly(ethylene glycol) (PEG) and sorbitol (SOR). The films were prepared by solution casting technique at different loading levels of plasticizers (10-15-20-25%). The mechanical results indicated that PEG and SOR effectively plasticize the PVA/CA film while SOR efficiency was higher than PEG. The addition of plasticizers decreased the tensile strength while the increased elongation at break value of PVA/CA film. The maximum tensile strength was observed in 10% PEG addition as 37.5 MPa. On the other hand, maximum elongation at break value was observed in 15% SOR at 369% that was higher than the un-plasticized film about 58%. The water contact angle measurements showed that all films were hydrophilic structures (32–75°). Also, the water vapour permeability of the un-plasticized film was measured as 6.4 g m−1 s−1 Pa−1 × 10–11. This value decreased with the addition of PEG (5.5 g m−1 s−1 Pa−1 × 10–11) while increasing SOR addition (12.2 g m−1 s−1 Pa−1 × 10–11). The thermal degradation temperature of un-plasticized PVA/CA film was decreased with plasticization. The residual weight of un-plasticized PVA/CA was 12.5 wt. %, which decreased at 4.5 wt. % for PEG-25 and 4.8 wt. % for SOR-20 films. Also, the melting temperature of the films decreased by increasing the content of plasticizers. Melting temperatures of SOR containing films were lower than PEG containing the films. Consequently, un-plasticized and plasticized PVA/CA-based films successfully prepared and analyzed to determine the appropriate plasticizer. All prepared films exhibited acceptable properties for use as food packaging or biobased materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics are often found in many areas of our lives. According to The Plastic Europe 2019 report, 359 million tons of plastic products were produced in 2018 and approximately 44% of these was used in the packaging and agricultural (like mulch films) applications [1]. Only 1% of these produced 359 million tons of plastic are bioplastic [2]. With the increasing need for plastic materials, it is trying to replace biodegradable plastics instead of synthetic plastics that accumulate for a long time in the environment. Thus, this market is growing and diversifying with the increasing demand for bioplastics. Global bioplastic production, which was 2.11 million tons in 2019, is expected to increase to about 2.43 million tons in 2024. The majority of bioplastics are made up of 21.3% starch blends, 13.9% polylactic acid (PLA) and 13.4% polybutylene adipate terephthalate and the others [2].
Bio-based polymers can be divided into three main categories; directly extracted from biomass (polysaccharides, proteins, lipids), produced from microorganisms and synthesized from bio-based monomers (PLA, PVA, Polybutylene acrylate). Currently, among all the biopolymers proteins attract great attention. They are potential candidates for various applications like food or non-food packaging, mulching film, tissue engineering, reconstructive surgery, etc.[3, 4]. Large amounts of protein waste are constantly produced by the dairy industry. Most of the wastes emerge by mixing with water during the cleaning of milk tankers and facilities. This can lead to environmental problems such as intense algae blooms and increased water turbidity in the long term [5, 6]. The difficult and expensive disposal of this material has created new research areas and consequently, the production of casein and whey protein by-products was initiated [6]. As milk contains around 80% casein [7], the use of this protein to produce biodegradable films for food packaging, wound dressing, edible films, medical applications, etc. is a convenient route for casein utilization. Casein (CA) is a globular protein, which includes four major subunits as αs1-casein, αs2-casein, β-casein, and κ-casein. The film-forming property of casein is affected by these subunit’s fractions [8, 9]. CA exhibit a random-coil structure that has flexible, open and mobile conformation. It can easily form a film from its aqueous solution without more processing due to its random-coil open structure [8, 10]. The complete biodegradability, biocompatibility, nontoxicity, high thermal stability, transparency properties and low cost make casein appropriate material for biodegradable film applications [4, 8, 11, 12]. CA films include several polar functional groups like amino and hydroxyl. Thus, CA films exhibit high barrier to non-polar molecules such as O2, CO2 [8, 13]. On the other hand, virgin CA-based films are brittle and their tensile strengths are lower than other synthetic films. Besides, the moisture barrier of CA-based films are limited like most protein-based films due to its hydrophilic nature [8, 10, 12,13,14]. Therefore, various strategies have been reported to improve the properties of casein and its derivatives based films such as plasticization [4, 14,15,16,17,18], crosslinking [14, 19, 20], blending with polysaccharides [21] or biopolymers like poly(butylene adipate-co-terephthalate) [4], polyvinyl alcohol [11, 22, 23].
Polyvinyl alcohol (PVA) is a biodegradable synthetic polymer that is synthesized from the hydrolysis of polyvinyl acetate [24, 25]. It exhibits excellent film-forming property, mechanical strength, flexibility, chemical resistance. Also, PVA films are non-toxic, transparent and odourless as well as low cost [26,27,28]. PVA is water-soluble and highly polar, it is suitable for blending with natural raw materials like proteins [28]. PVA blends with natural materials may be used for various applications such as packaging, drug delivery, wound dressing, etc.[11, 24, 27]. There are several studies in the literature on the blending of PVA and natural materials like cellulose [29], whey protein isolate [25], chitosan and guar gum [27], eggshell powder [30]. In the present study, casein was chosen as a natural material to be a blend with PVA. Because while creating an alternative to other biodegradable blend films, it is thought that protein wastes produced in large quantities by the dairy industry can be used in this blend in the future. Also, in our previous studies [22, 23] for the first time, PVA/CA blend films were prepared by solution casting method at different blending ratios and the properties of the films were evaluated. It was observed that the mechanical properties of the CA film improved by adding PVA. Additionally, the water vapour barrier property of PVA and CA increased with blending. This study showed us that the mechanical properties of PVA/CA (80/20) films are better or worse than traditional materials such as polyethylene and nylon. On the other hand, the water vapour permeability of PVA/CA films was higher [31] or lower [32] than some biodegradable plastic films [23]. Based on this, it was decided to examine the effect of plasticizer type and amount on properties of PVA/CA films. No studies investigating the effects of different plasticizers on PVA/CA blend films have been found. Only in our previous study, the effects of glycerol were examined [22]. In the present study, different plasticizers that can be used instead of glycerol have been investigated.
The plasticizers are non-volatile small molecules that increase the flexibility, distensibility and processability of the polymer matrix material. They perform this function by increasing polymer chain mobility and decreasing intermolecular interactions [33, 34]. The effectiveness of plasticizer depends on its molecular weight (Mw), polarity and durability [14, 35]. For most of the biopolymers water is an effective plasticizer but volatile. Furthermore, other non-volatile common plasticizers have been used for protein, biopolymer-based films. The polyols such as glycerol (GLY) [12, 15, 18, 22, 29], sorbitol (SOR) [34,35,36] and polyethylene glycol (PEG) [16, 37, 38] are effective and most used plasticizers due to their water solubility and compatibility. The -OH groups of SOR make it suitable for -OH or -NH rich polymers like PVA and CA [36]. Chick and Ustunol reported that SOR is more effective mechanical and barrier properties than GLY for lactic acid casein films [17]. SOR has low Mw and it’s more effective than high Mw compounds as the plasticizer. Also, SOR is a non-toxic and chemically stable material that may be used in food contact materials [36]. Therefore, it is appropriate for food packaging applications and our study.
The objective of this research is to investigate the two different polyol-based plasticizers and their loading rates on the properties of PVA/CA (80/20) film. SOR and PEG were selected as plasticizers and added to PVA/CA at four different loading rates (10,15,20,25%). The structural, mechanical, thermal, barrier properties of the films were evaluated. The films obtained are thought to have the potential for packaging materials with superior properties.
Experimental Section
Materials
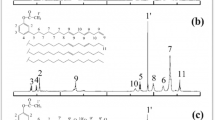
Polyvinyl alcohol (degree of hydrolysis = 86.7–88.7 mol %) was purchased by Kuraray Co. Ltd. (Chiyoda, Tokyo, Japan) with the trade name of Kuraray Poval 47–88. Casein powder (C3400) was supplied from Sigma Aldrich Co. (St. Louis, MO, USA). Triethanolamine (TEA; purity 99%, d = 1.12 kg/l) was used as the solvent and supplied from Merck (Darmstadt, Germany). Poly (ethylene glycol) (P2139, Mw = 8000 g/mol) and sorbitol (S1876, Mw = 182.17 g/mol) were used as the plasticizers and they were procured from Sigma-Aldrich (St. Louis, MO, USA). The structures of materials are given in Fig. 1.
Film Preparation Procedure
The solution casting method was used to obtain the PVA/CA/plasticizer blend films. Firstly, CA and plasticizers (PEG, SOR) were dissolved in 5% TEA solution under magnetic stirring for 1 h at the 35 °C to prepare 6.0 wt % plasticized CA solutions. The CA/PEG and CA/SOR ratio were changed as 100/0, 90/10, 85/15, 80/20 and 75/25 (wt/wt). Also, PVA was dissolved separately in distilled water under magnetic stirring for 1 h at 80 °C to prepare a 6.0wt % solution. After that, plasticized CA and PVA solutions were blended at the ratio of 20/80 (v/v). This ratio was determined based on the results of the pretest and our previous studies [23]. The determined ratio PVA/CA was blended under magnetic stirring for 15 min to obtain a homogeneous blend solution. Finally, the same amounts of film-forming solutions were cast onto polystyrene petri plates (120 × 120 mm) and dried under ambient conditions for 2 days to get a uniform film thickness. Then the dried films were peeled off. The thickness of the films was measured using a Mitutoya digital caliper they were about 0.20 ± 0.02 mm. The detailed compositions and codes of prepared blend films can be shown in Table 1.
Characterization of the Films
The Fourier transform infrared spectra (FTIR) of samples were recorded on a PerkinElmer Spectrum 100 instrument in the range from 650 to 4000 cm−1 with a diamond crystal.
The thermal stability of the samples was evaluated by thermogravimetric analysis (Mettler Toledo TGA1). The sample weight was approximately 5–10 mg and the experiments were carried out in a nitrogen atmosphere (50 ml/min). Heating scans from 25 to 600 °C with 20 °C min−1 heating rate were performed for each sample. The residual mass at 600 °C, the onset temperature (Tonset), the end temperature (Tendset) and the temperature at the maximum rate of weight loss (Tmax) were determined.
Differential scanning calorimetry (DSC) analysis was performed on Mettler Toledo DSC 1 instrument under the nitrogen atmosphere. The samples heated from 25 to 250 °C with 20 °C min−1 heating rate. The melting temperatures (Tm) of the samples were examined.
The mechanical properties of the films, including tensile strength and elongation at break were determined using a computer-controlled Instron universal testing machine following the ASTM-D 882 standard. The test was carried out at 10 mm/min crosshead speed. The films were cut into 20 mm wide and 80 mm long strip and five samples were tested for each composition. The results were reported as the average value with standard deviations.
Contact angle measurements of films were carried out using a goniometer (Attention Theta Lite) with the sessile drop technique, by depositing a drop of deionized water onto the film surface. Ten measurements were taken at 1 s intervals for each drop. At least five measurements were made on each specimen to obtain the average contact angle value at different positions at ambient temperature.
Water vapour permeability (WVP) of the selected films was determined according to the ASTM E96 method described in our previous study [23]. Test cups of 8 cm in diameter were filled with distilled water to expose the films to 100% relative humidity inside the test cups. The films were mounted in the cups. The permeability cups containing films were placed into pre-equilibrated 34–35% relative humidity desiccator cabinets at 25 °C. The test cups were weighed to obtain the initial weight and after the steady-state condition was obtained, cups were weighted at 2 h intervals. The experiment took 60–72 h after equilibrium was reached. The water vapour transmission rate (WVTR) of the films was determined from the slope of weight loss versus time. The WVP of the films was calculated using Eq. (1):
where WVTR is in gm−2 s−1, P1 is the partial pressure (Pa) of water vapour at the inner surface of the film inside the cup, P2 is the partial pressure (Pa) of water vapour at the outer surface of the film outside the cup and Δx is the thickness of the film (m).
Results and Discussion
FTIR
FTIR spectroscopy is an analytical technique that is used to determine the changes in the chemical structures of the materials by identifying the characteristic functional groups of the components [19, 27]. If the film components interact at the molecular levels, there may be seen some changes in the FTIR spectra like shifting, changes in peak heights. These can be attributed to the miscibility of the components [28].
The FTIR spectra of the films and pristine plasticizers were shown in Fig. 2a, b. The spectrum of un-plasticized PVA/CA film (PLAST-0) showed a major broad peak at 3286 cm−1 attributed to the hydroxyl group (-OH) stretching vibration. Peaks at 2937, 1730, 1072, 1030 cm−1 correspond to symmetrical-asymmetrical vibrations of C-H, C=O stretching of the carbonyl group, C-O is stretching vibration from an acetate group of PVA and C-C-C stretching vibration, respectively [23, 25, 31]. Also, the spectrum showed some peaks at 1651 and 1547 cm−1 assigned to amide I and amide II characteristic bands of the CA protein network [19, 23]. As can be seen in Fig. 2a, there are some changes in the spectrum of PLAST-0 film with the addition of 25 PEG and 25 SOR. The -OH peak of PLAST-0 shifted to high wavenumbers and intensity decreased at 25 PEG spectrum suggesting the hydrogen bond interactions. Moreover, the C-H peak shifted to lower wavenumber with the addition of 25 PEG. This may be a result of conformational changes in the matrix [12]. Additionally, a new C-O peak at 1104 cm−1 was seen in 25 PEG film. When 25 SOR spectra are examined, it is seen that the peak intensity of -OH increases because of forming new hydroxyl bonds [34]. On the other hand, as it can be shown in Fig. 2b pure SOR includes more hydroxyl bond intensity than PEG. This may show that it can be made more hydrogen bond interaction with the PVA-CA matrix. An excess of -OH in 25 SOR films revealed that the plasticizing effect of SOR could be greater. As will be mentioned later, the mechanical property results also support this prediction. Consequently, the FTIR spectra results demonstrated the compatibility of PVA/CA with both PEG and SOR.
Mechanical Properties
The mechanical properties of the films give information about their physical integrity and durability. The films must exhibit sufficient flexibility and strength that are important to estimate whether the films can be used for packaging or other applications [12, 37]. The tensile strength (TS) and elongation at break (E %) are critical parameters for the evaluation of mechanical properties. The mechanical properties of the film effect by the thickness, which depends on the preparation method, solid content and drying. In our study, these parameters were kept as same, so thicknesses of all films were almost the same. Thus, just the composition and type of films (plasticizer properties, contents) affected the film property.
The effects of SOR and PEG amounts on the TS of the PVA/CA film were illustrated in Fig. 3. The error bars on the plots show the standard deviations. TS is the maximum tensile stress that the film can resist and sustain. It is significantly affected by the plasticizer. As seen in Fig. 3, TS of PVA/CA film decreased with increasing amounts of SOR and PEG. This is a result of reduced interactions of biopolymer chains. The plasticizers are small molecules and have low viscosity. They can easily diffuse between biopolymer chains and cause an increment of fluidity. As a result, the film structure softens and a reduction in TS is seen [37]. Similar results regarding this effect of plasticizers have been reported in several studies [17, 18, 22, 35,36,37]. TS of the PVA/CA film without plasticizer was 38.8 MPa which gradually decreased to 24.7 MPa with increasing of PEG amount from 10 to 25wt %. The maximum reduction was observed in 25 PEG film as 36%. In the case of samples containing SOR, the maximum reduction was observed in 25 SOR film as 45%. It is seen that films containing SOR exhibit lower TS than PEG containing films at all loading rates. The same result reported by Sun et. al. for chitosan/zein film plasticized by SOR and PEG [37]. TS values of our un-plasticized and plasticized PVA/CA films were in the range of 21–39 MPa that comparable to those many biobased and synthetic films. For example, it was reported that LDPE, LLDPE, HDPE films exhibited TS in the range of 8–31 MPa, 13–27 MPa, 22 MPa, respectively [39]. These values are very close or lower than our results. Additionally, the TS values of PVA/CA films are higher than whey protein isolate/PVA [25], glycerol-plasticized sodium caseinate/lipid fraction of tomato pomace by-product films [12], tara gum/PVA films [31]. These comparisons may not be entirely accurate since different films have varying compositions and were tested under different conditions. But they provide an idea in terms of comparison.
On the other hand, elongation at break value of PVA/CA film (234.8%) increased considerably with the addition of both plasticizers (Fig. 4). This is the plasticization effect of SOR and PEG that increased the chain mobility and finally the stretchability and flexibility of the films. The situation that plasticizers increase flexibility has been observed for many biobased films such as sodium caseinate [14], PVA-casein [22], PVA [22], β-lactoglobulin [40], whey protein-carboxymethyl cellulose [38], cassia gum [34]. According to Fig. 4, the maximum elongation was observed for 15% plasticizer loading rate as 369% for SOR and 326% for PEG. Above this loading rate, elongation value decreased, but it was still higher than PLAST-0 film. This behavior related to excess content of plasticizers. Audic and Chaufer reported similar effects of excess amount of triethanolamine and glycerol plasticizers on the elongation value of sodium caseinate film and also Siew and coworkers reported similar results for sodium caseinate films [14, 16].
SOR is a more effective plasticizer than PEG for PVA/CA film as seen from tensile strength and elongation graphs (higher elongation—lower TS). These results are related to the molecular characteristics of plasticizers. The molecular weight (Mw), number hydroxyl group units, shape of molecules of plasticizers affects the plasticization efficiency [40]. It was reported that in the literature low Mw plasticizers were more efficient than the high one [40, 41]. Tapia-Blacido and coworkers reported that, glycerol containing amaranth flour films more flexible and less resistant than SOR containing films. Because of Mw of glycerol lower than SOR [42]. In our study, the Mw of SOR (182 g/mol) is lower than PEG (8000 g/mol). Therefore, small molecules of SOR can easily diffuse the biopolymer chains and increase mobility and flexibility. Moreover, -OH groups in plasticizer chains are believed to develop polymer-plasticizing hydrogen bonds that replace polymer–polymer interactions in biopolymer films and increase plasticizing ability [14, 41]. According to reported studies [37, 38] and our FTIR results (Fig. 2) SOR involves more -OH groups than PEG. SOR tends to more water molecules due to these -OH groups. So, lower TS and higher elongation are achieved as more water will remain in the structure during the drying of the film [37].
Water Contact Angle
Measurement of the water contact angles (WCA) of the film surfaces is a useful method to estimate the hydrophilicity/hydrophobicity of the film. The contact angle value increase with increasing hydrophobicity. Generally, if a film has a WCA value greater than 90°, that film is called hydrophobic [31].
The results of WCA measurements for PVA/CA films plasticized by different loading rates of SOR and PEG are illustrated in Fig. 5. When the graph is examined, it is seen that all films exhibited hydrophilic character (< 80°). The WCA of un-plasticized PVA/CA film was 25.5° which increased by adding plasticizer at all loading rates. It means that although the surface hydrophobicity of PVA/CA film by plasticization, still they have a hydrophilic surface that could be wetted by water. Similar plasticizer effects were reported in various studies such as; sage seed gum-glycerol [35], PVA/CA-glycerol [22], cassia gum-glycerol/sorbitol [34]. As can be seen in Fig. 5, SOR increased the WCA of un-plasticized film maximum to 75° at 10% loading rate. This value showed a decline in the rising content of SOR. This effect may be the result of the formation of hydrogen bonds between SOR and matrix chains. With these interactions, the number of hydroxyl groups might be decreased [34]. Although the interactions are somewhat weakened when the loading rate exceeds 10%, high WCA values are still observed compared to un-plasticized film. When PEG is added to the structure, the WCA values are lower in all loading rates than SOR. This may be due to decreased PVA-CA-PEG hydrogen bond interactions since PEG contains fewer hydroxyl groups than Sorbitol (FTIR section Fig. 2). Additionally, Cao et.al. reported that high Mw PEG has fewer hydrogen bond interactions [41]. As a result of these interactions being less, more -OH groups will remain in the structure and this will increase hydrophilicity. On the other hand, the WCA may also be affected by the surface roughness of the films.
High WCA value and hydrophobic character are important most of the packaging applications. The WCA values of our films are range from 25 to 75° which are comparable with other biobased films. Overall, the films containing films are more hydrophobic than glycerol plasticized PVA/CA film [22], sodium caseinate/lipid fraction of tomato pomace by-product film [12].
Water Vapour Permeability
WVP is defined as the amount of water vapour diffusing through per unit area of matrix per unit time [19]. It can be used to determine the water vapour barrier property of the materials that has critical importance for natural-based films. Especially food packaging applications WVP value of the material should be as low as possible to protect the food quality and extend the shelf life [19, 31]. The WVP is related to the hydrophilicity/hydrophobicity of the materials. Biodegradable polymer films have higher hydrophilicity as well as WVP than the synthetic one. Several factors affect WVP like environmental conditions, polymer morphology, chain mobility, molecular interaction, amorphous/crystalline structure. The plasticizers are also effective on WVP as they affect polymers molecular interactions and chain mobility [16, 19, 28].
In the present study, WVP analysis was applied to selected films and the results are given in Table 2. WVP values of the pure PVA and CA films are 13.2 × 10–11 and 10.9 × 10–11 g m−1 s−1 Pa−1 respectively. According to the results, it can be said that casein has a more hydrophobic character than PVA. The WVP value of the un-plasticized PVA/CA film appears to be lower than the pure states of both materials. This may be due to hydrogen bond interactions between PVA and CA [23]. WVP of the un-plasticized film is lower than previously reported PVA/Alyssum homolocarpum seed gum (80/20) [28], sodium caseinate [15, 19] films. However, it is higher than plasticized cassia gum [34], extruded CA-based films [18].
The addition of PEG and SOR to the PVA/CA film affected the permeability differently. The addition of PEG decreased WVP of PLAST-0 the film by 14%, while the addition of SOR increased by 90% in the same amount. The increasing of WVP of biodegradable films with the addition of plasticizer has been reported in different studies [22, 35, 37]. These results can be attributed to the plasticizing efficiencies of PEG and SOR. As mentioned in the mechanical property section, the plasticizing effect of SOR is higher than that of PEG. Because Mw of the SOR is lower than PEG and it increases free volume by easily diffusing between the PVA-CA network. Consequently, a larger space will be created in the film structure to allow water to pass through [37]. A small drop in the situation where PEG is added may be due to the weak plasticization effect and intermolecular interactions of PEG. Also, according to the water contact angle values, 25 SOR films are more hydrophilic than 25 PEG.
Thermal Properties
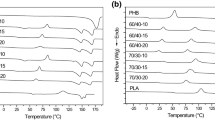
Thermogravimetric analysis was conducted to evaluate the influence of SOR and PEG plasticizers on the thermal stability of PVA/CA films. Figure 6a, b shows the derivative thermograms (DTG curves) of the PVA, CA, un-plasticized PVA/CA and plasticized PVA/CA films. Besides, TGA data are listed in Table 3. As represented in Fig. 6, pristine PVA and all PVA/CA films showed three degradation steps while pristine CA showed two degradation steps. For pristine PVA and CA films, the first weight loss took place around 113 °C and 138 °C respectively, due to the evaporation of residual water (~ 4–6% of weight loss). Furthermore, all PVA/CA films exhibited water loss around 110–127 °C. According to the listed data in Table 2, the second weight loss of pristine PVA started at 335 °C (~ 50% of weight loss), followed by a third smaller weight loss step. These are assignable to acetate group elimination and main chain decomposition of PVA [31, 36]. For the pristine CA film, the main degradation step (Tonset-2) occurred at 259 °C corresponds to the degradation of the casein backbone [20]. Un-plasticized PVA/CA film (PLAST-0) exhibited three degradation steps similar to PVA that exhibited decomposition temperature values between PVA and CA. On the other hand, the residual weight of the PLAST-0 film was higher than both PVA and CA at 500 °C. This may be due to the chemical interactions between the PVA and CA. With the addition of PEG to the structure, it was seen that the Tonset-2 value of PLAST-0 decreased by 2 to 7 °C and also residual weights of the films containing PEG were lower than PLAST-0. When the results of films containing SOR are analyzed, it is seen that there is a decrease in degradation temperatures and residual weights. These results are directly related to the plasticizing effects of PEG and SOR. It is clear that plasticizers affect the polymer-protein intermolecular interactions and decrease the thermal stability of the films [36, 43]. On the other hand, in Fig. 6b, the peak intensities of the DTG curves of PEG plasticized films are generally lower than PLAST-0. This indicates to decreasing weight loss rate. In films containing SOR (Fig. 6a), the opposite result is seen. The peak intensities of SOR-plasticized films are generally higher than PLAST-0. This result indicates increasing in weight loss rate with plasticization. This may be due to the SOR degraded at this temperature range (Tmax-1 = 369 °C) and increase weight loss. Also, according to Table 3, the thermal stability of pristine PEG is higher than the pristine SOR. As a result, plasticizers a little decreased the thermal stability of the PVA/CA film, but they still have good thermal stability.
Differential scanning calorimetry is another method to determine the thermal properties of polymer materials. The DSC curves of un-plasticized and plasticized PVA/CA films were given in Fig. 7a, b. When Fig. 7 is analyzed, it is seen that there are some endothermic transitions in the DSC curves. For all films, a clear glass transition temperature could not be observed. This may be due to the hydrophilic nature of the films, amorphous chains surrounded by the crystalline phase and the presence of moisture [44]. The first endothermic transition at around 100–110 °C may be attributed to the moisture evaporation for all films. The other endothermic peak is melting temperatures (Tm) of the films. The Tm value of PLAST-0 film is 180 °C which decreased with the addition of plasticizers. The similar effect of plasticizer was reported by Tian et. al [36] and Cazon et. al [29]. The addition of plasticizers increases the segmental mobility of the PVA/CA matrix and decrease ordered association of chains. Thus, crystallinity and Tm of the matrix decline [36]. Figure 7a and b compared; it is seen that SOR decreased Tm more than PEG. This may be due to the excessive plasticizing efficiency of the SOR. This situation was also seen in mechanical property results.
Conclusions
PEG and SOR plasticized PVA/CA films were successfully prepared by the solution casting method and all films exhibited excellent film-forming characteristics. The effects of plasticizer type and concentration on the structural, mechanical, thermal, barrier properties of the films were evaluated. This work demonstrated that PVA/CA/PEG and PVA/CA/SOR form compatible blends via hydrogen bonding interactions. The PEG and SOR containing films are more flexible than un-plasticized film. Generally, increasing the content of plasticizers increased flexibility and decreased tensile strength. It is also seen that SOR provided more flexibility than PEG. All films exhibited hydrophilic surface property, but the water contact angle value increased with the addition of plasticizer. The water vapour permeability value of un-plasticized film decreased by adding 25% PEG and increased with the addition 25% SOR. Also, the thermal stability and melting temperature of the un-plasticized PVA/CA film slightly decreased by plasticization. It was observed that this reduction was higher when SOR was added. Overall, SOR as plasticizer more effective for PVA/CA film than PEG. Although the films containing SOR are more flexible, its high water vapour permeability is its disadvantage. On the other hand, films prepared with both plasticizers still showed acceptable properties compared to many biobased protein-polymer blend films. Also, their properties are close to conventional films used as food packaging materials and bioplastic areas.
References
Plastic Europe (2019) Plastics-the Facts 2019. Report Available in: https://www.plasticseurope.org/en/resources/market-data
European Bioplastics (2018) Bioplastics Market Development. Berlin, Available in: https://www.europeanbioplastics.org/news/publications/#MarketData
Picchio ML, Ronco LI, Passeggi MCG et al (2018) Poly(n-butyl acrylate)–casein nanocomposites as promising candidates for packaging films. J Polym Environ 26:2579–2587
Audic J-L, Chaufer B (2012) Properties of biodegradable poly(butylene adipate-co-terephtalate) and sodium caseinate blends. J Appl Polym Sci 125:E459–E467
Arvanitoyannis IS, Tserkezou P (2008) Waste treatment management: treatment methodologies of foods of animal origin. In: Arvanitoyannis IS (ed) Waste management for the food industries, 1st edn. Elsevier, USA, pp 801–844
Ryder K, Ali MA, Carne A, Billakanti J (2017) The potential use of dairy by-products for the production of nonfood biomaterials. Crit Rev Environ Sci Technol 47:621–642
Siroěić AP, Krehula LK, Kataněić Z, Hrnjak-Murgić Z (2016) Characterization of casein fractions–comparison of commercial casein and casein extracted from cow’s milk. Chem Biochem Eng Q J 30:501–509
Chen H, Wang J, Cheng Y et al (2019) Application of protein-based films and coatings for food packaging: a review. Polymers (Basel) 11:2039
Calva-Estrada SJ, Jiménez-Fernández M, Lugo-Cervantes E (2019) Protein-based films: advances in the development of biomaterials applicable to food packaging. Food Eng Rev 11:78–92
Chen H (2002) Formation and properties of casein films and coatings. In: Gennadios A (ed) Protein-based films and coatings, 1st edn. CRC Press, USA, pp 181–209
Biranje S, Madiwale P (2019) Porous electrospun casein / PVA nanofibrous mat for its potential application as wound dressing material. J Porous Mater 26:29–40
Aloui H, Baraket K, Sendon R et al (2019) Development and characterization of novel composite glycerol-plasticized films based on sodium caseinate and lipid fraction of tomato pomace by-product. Int J Biol Macromol 139:128–138
Bonnaillie LM, Zhang H, Akkurt S et al (2014) Casein films: the effects of formulation, environmental conditions and the addition of citric pectin on the structure and mechanical properties. Polymers (Basel) 6:2018–2036
Audic J-L, Chaufer B (2005) Influence of plasticizers and crosslinking on the properties of biodegradable films made from sodium caseinate. Eur Polym J 41:1934–1942
Colak BY, Gouanve F, Degraeve P et al (2015) Study of the influences of film processing conditions and glycerol amount on the water sorption and gas barrier properties of novel sodium caseinate films. J Memb Sci 478:1–11
Siew DCW, Heilmann C, Easteal AJ, Cooney RP (1999) Solution and film properties of sodium caseinate/glycerol and sodium caseinate/polyethylene glycol edible coating systems. J Agric Food Chem 47:3432–3440
Chick J, Ustunol Z (2006) Mechanical and barrier properties of lactic acid and rennet precipitated casein-based edible films. J Food Sci 63:1024–1027
Chevalier E, Assezat G, Prochazka F, Oulahal N (2018) Development and characterization of a novel edible extruded sheet based on different casein sources and influence of the glycerol concentration. Food Hydrocoll 75:182–191
Lin HC, Wang BJ, Weng YM (2020) Development and characterization of sodium caseinate edible films cross-linked with genipin. Lwt Food Sci Technol 118:108813
Bajpai SK, Bajpai M, Shah FF (2016) Alginate dialdehyde (AD)-crosslinked casein films: Synthesis, characterization and water absorption behavior. Des Monomers Polym 19:406–419
Rai S, Poonia A (2019) Formulation and characterization of edible films from pea starch and casein. J Pharmacogn Phytochem 8:317–321
Durmaz BU, Aytac A (2019) Poly (vinyl alcohol) and casein films: the effects of glycerol amount on the properties of films. Res Eng Struct Mater 5:155–165
Durmaz BU, Aytac A (2019) Development and characterization of poly(vinyl alcohol) and casein blend films. Polym Int 68:1140–1145
Biswas A, Cheng HN, Evangelista R et al (2020) Evaluation of composite films containing poly(vinyl alcohol) and cotton gin trash. J Polym Environ 28:1998–2007
Lara BRB, Araújo ACMA, Dias MV et al (2019) Morphological, mechanical and physical properties of new whey protein isolate/ polyvinyl alcohol blends for food flexible packaging. Food Packag Shelf Life 19:16–23
Wu W, Tian H, Xiang A (2012) Influence of polyol plasticizers on the properties of polyvinyl alcohol films fabricated by melt processing. J Polym Environ 20:63–69
Iqbal DN, Tariq M, Khan SM et al (2020) Synthesis and characterization of chitosan and guar gum based ternary blends with polyvinyl alcohol. Int J Biol Macromol 143:546–554
Monjazeb Marvdashti L, Koocheki A, Yavarmanesh M (2017) Alyssum homolocarpum seed gum-polyvinyl alcohol biodegradable composite film: Physicochemical, mechanical, thermal and barrier properties. Carbohydr Polym 155:280–293
Cazón P, Vázquez M, Velazquez G (2018) Cellulose-glycerol-polyvinyl alcohol composite films for food packaging: Evaluation of water adsorption, mechanical properties, light-barrier properties and transparency. Carbohydr Polym 195:432–443
Wu H, Xiao D, Lu J et al (2020) Preparation and properties of biocomposite films based on poly(vinyl alcohol) incorporated with eggshell powder as a biological Filler. J Polym Environ 28:2020–2028
Ma Q, Du L, Yang Y, Wang L (2016) rheology of fi lm-forming solutions and physical properties of tara gum fi lm reinforced with polyvinyl alcohol ( PVA ). Food Hydrocoll 63:677–684
Talens P, Krochta JM (2006) Plasticizing effects of beeswax and carnauba wax on tensile and water vapor permeability properties of whey protein films. J Food Sci 70:E239–E243
Gurgel M, Vieira A, Altenhofen M et al (2011) Natural-based plasticizers and biopolymer films : a review. Eur Polym J 47:254–263
Cao L, Liu W, Wang L (2018) Developing a green and edible film from cassia gum: the effects of glycerol and sorbitol. J Clean Prod 175:276–282
Razavi SMA, Mohammad Amini A, Zahedi Y (2015) Characterisation of a new biodegradable edible film based on sage seed gum: influence of plasticiser type and concentration. Food Hydrocoll 43:290–298
Tian H, Liu D, Yao Y et al (2017) Effect of sorbitol plasticizer on the structure and properties of melt processed polyvinyl alcohol films. J Food Sci. https://doi.org/10.1111/1750-3841.13950
Sun Y, Liu Z, Zhang L et al (2020) Effects of plasticizer type and concentration on rheological, physico-mechanical and structural properties of chitosan/zein film. Int J Biol Macromol 143:334–340
Huntrakul K, Harnkarnsujarit N (2020) Effects of plasticizers on water sorption and aging stability of whey protein/carboxy methyl cellulose films. J Food Eng 272:109809
TeckKim Y, Min B, WonKim K (2013) General characteristics of packaging materials for food system. In: Han JH (ed) Innovations in food packaging, 2nd edn. Elsevier, Amsterdam, pp 13–35
Sothornvit R, Krochta JM (2001) Plasticizer effect on mechanical properties of β-lactoglobulin films. J Food Eng 50:149–155
Cao N, Yang X, Fu Y (2009) Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocoll 23:729–735
Tapia-Blácido DR, do Amaral Sobral PJ, Menegalli FC, (2011) Optimization of amaranth flour films plasticized with glycerol and sorbitol by multi-response analysis. LWT-Food Sci Technol 44:1731–1738
Barreto PLM, Pires ATN, Soldi V (2003) Thermal degradation of edible films based on milk proteins and gelatin in inert atmosphere. Polym Degrad Stab 79:147–152
Aydin AA, Ilberg V (2016) Effect of different polyol-based plasticizers on thermal properties of polyvinyl alcohol: starch blends. Carbohydr Polym 136:441–448
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ucpinar Durmaz, B., Aytac, A. Effects of Polyol-Based Plasticizer Types and Concentration on the Properties of Polyvinyl Alcohol and Casein Blend Films. J Polym Environ 29, 313–322 (2021). https://doi.org/10.1007/s10924-020-01881-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01881-x