Abstract
Two natural plasticizers derived from cardanol (CD), cardanol acetate (CA) and epoxidized cardanol acetate (ECA), were synthesized and characterized by 1H NMR and 13C NMR. The plasticizing effects of the obtained plasticizers on semi-rigid polyvinylchloride (PVC) formulations were also investigated. Two commercial phthalate ester plasticizers, dioctyl terephthalate (DOTP) and diisononyl phthalate (DINP), were used as controls. Mechanical and thermal properties, compatibility, thermal stability, microstructure, and workability were assessed by dynamic mechanical analysis (DMA), mechanical analysis, thermogravimetric analysis (TGA), scanning electron microscopy (SEM), and dynamic stability analysis, respectively. Results indicated that the natural plasticizer ECA had overallsuperior flexibility, compatibility, thermal stability, and workability comparable to both controls. The obtained CA and ECA have lower volatility resistance and similar extraction and exudation resistance than that of DOTP and DINP. The CA was further blended with DOTP in soft PVC films. Results of DMA, TGA and mechanicalanalysis indicated that CA can serve as a secondary plasticizer to improve the related properties of soft PVC formulations. These CD derived plasticizers show promise as an alternative to fully or partially replace petroleum-based plasticizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyvinyl chloride (PVC) has been widely used in many applications, such as packaging, construction, toys, furniture, household, automotives, electrical, and electronics, etc. [1,2,3,4,5]. However, PVC is brittle, rigid and with low thermal stability, it has to be blended with plasticizers to improve its mechanical properties, processibility, and thermal stability to meet product quality and specification requirements [6, 7]. Currently, there are more than 100 plasticizers which are of commercial importance, among which phthalates are the most widely used plasticizers [8]. Recently, it was reported that phthalate plasticizers have demonstrated toxicity in animals and risk of adverse health effects in humans [9,10,11,12,13].
In order to solve this problem, researchers in academia and industry have looked for alternative plasticizers to replace phthalate plasticizers. Currently, there is increasing interest in the use of natural-based plasticizers characterized by low toxicity to substitute conventional petroleum-based plasticizers [6, 14,15,16]. Natural plasticizers from vegetable origin, such as modified or epoxidized vegetable oil, epoxidized fatty acid methyl ester, glycerin acetates, etc., are new alternatives [17, 18]. Numerous raw materials have been used, such as soybean, corn, sunflower, palm, flaxseed, etc. [19].
One of the most commonly used renewableraw materials [20], cardanol (CD), and its derivatives, have major applications in developing new eco-friendly materials [21,22,23,24,25]. In recent years, CD has been used as a plasticizer in the polymer and rubber industries. It was reported that CD and phosphorylated CD have shown significant plasticizing effects [2, 26]. Although not much research has been reported, some CD derivatives have also beenused as efficient plasticizers for PVC [27, 28]. Esterification and epoxidation have been used to modify CD in order to improve the miscibility of CD derived plasticizers [27]. However, in the epoxidation process in Ref. [27], chloroperbenzoic acid was mainly used. Chloroperbenzoic acid is expensive and will significantly increase costs. In addition, evaluation of plasticizers on the mechanical, thermal stability and workability characterization was not addressed.
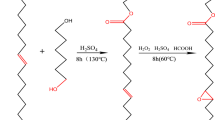
In this work, cardanol acetate (CA) was obtained by esterification and epoxidized cardanol acetate (ECA) was obtained by further epoxidation using H2O2. The synthesis route of CA and ECA were shown in Scheme 1. An environmentally friendly, low-cost processing method was developed in producing ECA. The mechanical and thermal characterizations, thermal stability and workability properties of the CD derivatives for semi-rigid PVC were evaluated. Furthermore, the plasticizing effects of both CA and DOTP or DINP in soft PVC are reported.
Experimental
Materials
CD (stabilized, 88.5%) was purchased from Meidong Biological Material Co., Ltd., Shanghai, China, and used after distillation at 240–250 °C under 3–5 Torr. Acetic anhydride, toluene, potassium carbonate, sodium bicarbonate, anhydrous sodium sulfate, formic acid, and p-Toluenesulfonic acid were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China and used as received. Hydrogen peroxide (50%) was provided by ARKEMA Hydrogen Peroxide Co., Ltd., Shanghai, China. PVC (DG-1000 K) was purchased from the Tianjin Dagu Chemical Co., Ltd., Binhai, Tianjin, China. Calcium stearate and zinc stearate were supplied by Changzhou JiaRenWo Chemical Co., Ltd., Changzhou, China. Dioctyl terephthalate (DOTP) (97%) and diisononyl phthalate (DINP) (99%) were obtained from Aladdin Reagents Co., Ltd., Shanghai, China and used as received.
Preparation of CA and ECA
To a 50 ml flask equipped with a mechanical stirrer, reflux condenser and thermometer were charged 10 g of CD and0.75 g of potassium carbonate. The mixture was stirred and slowly heated to 60 °C and then 4.1 g acetic anhydride was added. The final mixture was kept for 3 h. Next, the mixture was washed with 2% NaHCO3 and distilled water, respectively. Finally, the CA was dried by anhydrous sodium sulfate and filtered.
In a three-necked, round bottom flask equipped with a magnetic stirrer, thermometer sensor, and reflux condenser, was added 5 g CA,0.36 g formic acid, 10 ml toluene, and 0.15 g p-Toluenesulfonic acid, then this mixture was heated to 50 °C. About 2 g H2O2 was added dropwise to the mixtureafter 30 min and stirred for several hours. The final mixture was heated to 65 °C and kept for 3 h. After the reaction was complete, the crude product was filtered and washed with 2% NaHCO3 and distilled water, respectively. Finally, the organic phase was dried by vacuum distillation. The ECA had an epoxy value of 4.21% and acid value of 0.51 mg/g.
Preparation of plasticized PVC test specimens
A series of plasticized PVC test specimens with different plasticizers were prepared. The compositions of different formulas are shown in Table 1. First, PVC powder, plasticizer, and thermal stabilizers were mixed using a mechanical mixer at room temperature for 5 min. Second, the mixture was compounded into a homogeneous mixture at 165 °C for 3 min by a HAAKE torque rheometer (Thermo Fisher Scientific Inc., Pittsburgh, PA, USA). Finally, the test specimens were made using a HAAKE MiniJet II micro injection molding machine (Thermo Fisher Scientific, Pittsburgh, PA, USA). The mold for tensile test samples is based on ISO527–2-5A with the dimensions of 75 × 12.5 × 2 mm. The mold for dynamic mechanical analysis (DMA) samples is based on ISO180/179 with the dimensions of 80 × 10 × 4 mm. The specimens were carefully removed from the mold and examined for tensile test and DMA.
1H and 13C NMR analysis
1H and 13C NMR spectra of the compounds in deuterated chloroform (CDCl3) were recorded with a Bruker ARX 300 spectrometer (Bruker, Rheinstetten, Germany) at room temperature.
Dynamic mechanical analysis (DMA)
DMA was conducted by using a DMA Q800 (TA Instruments Co., New Castle, DE, USA) in a dual cantilever mode with an oscillating frequency of 1 Hz. The temperature was swept from −60 to 80°C at a heating rate of 3 °C/min. For each sample, duplicated tests were performed in order to ensure the reproducibility of data.
Tensile properties
Tensile properties were measured using a SANS7 CMT-4303 universal testing machine (Shenzhen Xinsansi Jiliang Instrument Co., Shenzhen, China) according to ISO 527–2: 1993. The tests were conducted at a crosshead speed of 10 mm/min. All samples were conditioned at 50% humidty and 23 °C for 2 days prior to tensile testing. Five replicates were prepared for each sample to obtain an average value.
Thermogravimetric analysis (TGA)
TGA was carried out on a thermogravimetric analyzer (Netzsch Co., Selb, Germany). Each sample was scanned from ambient temperature to 600 °C under a nitrogen atmosphere at a heating rate of 10 °C/min.
Scanning electron microscopy (SEM)
SEM examinations of the stretch-fractured samples were conducted on an S-3400 N Scanning Electron Microscope (HITACHI, Ltd., Tokyo, Japan). The surface of the fractured samples after completion of the tensile tests was coated with a gold film prior to SEM observation.
Dynamic stability analysis
Dynamic stability analysis was performed using a HAAKEtorque rheometer (Thermo Fisher Scientific Inc., Pittsburgh, PA, USA) according to ASTM D 2538–02. The PVC/plasticizer compounds were tested at 180 °C with a rotor speed of 30 rpm for 60 min. The dynamic thermal stabilizing time is defined as the time when the torque on the rotor starts to change abruptly [29].
Migration tests
Extraction tests were based on ASTMD 1239–98. The PVC specimens were immersed in distill water at 23 ± 1 °C and 50 ± 5% relative humidity. After 24 h, the extracted PVC specimens were rinsed with flowing water and then wiped up. Afterward, all of the specimens were dried in a convection oven at 30 °C for 24 h and reweighed. The weight losses before and after the dipping were measured. Three specimens were tested to obtain an average value.
Exudation of the plasticizer was evaluated by placing a PVC specimen between two pieces of filter paper. These systems were then placed in a convection oven at 60 °C for 48 h. Then, the weight increment of the filter papers was calculated. Three specimens were tested to obtain an average value.
Volatility tests were determined by ISO 176:2005, the activated carbon method. The specimen was placed on the bottom of a metal container and about 120 cm3 of activated carbon was spread over this specimen and then the lid was put on the container. The container was placed in the convection oven at a temperature of 70 °C ± 1 °C. After 24 h, the container was removed from the oven and cooled at room temperature in a desiccator. The specimens were brushed and reweighed. The weight losses were measured before and after the heating. Three specimens were tested to obtain an average value.
Results and discussion
Characterization of CA and ECA
The NMR technique is employed to investigate the structure of the obtained plasticizers. Fig. 1 and Fig. 2 displayed the 1H NMR and 13C NMR of CD, CA, and ECA, respectively. The chemical shift assignments were also labeled. In Fig. 1, the characteristic peaks at 6.7–7.3 ppm were attributed to the proton on the benzene ring. The peaks at 1.3–2.6 ppm were assigned to the hydrogen of the –CH2– on the side chain of CD. The peak at 2.8 ppm was according to the –CH2– beside the double bonds. The peak at 0.93 ppm was assigned to the hydrogen of the –CH3– groups. When we compared the spectrum of CD (Fig. 1a) and CA (Fig. 1b), the peaks at 5.3 ppm corresponding to the proton of –OH– almost disappeared, which indicated that the phenolic hydroxyl group had been converted. Additionally, the new peak at 2.3 ppm showed the existence of an ester group in CA [30]. Furthermore, the peaks at 2.9–3.2 ppm were attributed to the hydrogen of the –CH–O–CH– groups, which have appeared on the 1H NMR spectrum of ECA (Fig. 1c), indicating the occurrence of epoxidation. The chemical shifts of the hydrogen at 1.2–1.8 ppm also displayed the existence of epoxy groups in ECA. Figure 2 showed that the peaks at 20.7 and 169.2 ppm due to the ester, carbonyl carbon, have appeared in the 13C NMR spectra of CA. The chemical shift of the benzene carbon from 155.2 to 150.5 indicates the phenolic hydroxyl converted into an ester when CD progressed into CA. The peak at 128.4–130.2 ppm due to the olefinic carbons observed for CD and CA have disappeared in the 13C NMR spectra of ECA. The signal assigned as carbons beside the epoxy groups appeared at 56.9 ppm [31].
DMA, tensile properties and hardness character
Figure 3 displays changes in E’ and the tan δ values with the temperature of the PVC films plasticized with different plasticizers. The glass transition temperature (T g ) is determined from the peak of the tan δ curve. As compared to the films plasticized with other plasticizers, the ECA/PVC exhibited the sharpest transitions in the E’ value and the tan δ peak. Furthermore, ECA/PVC displayed a maximum decrease of 3.74 °C in the T g compared with those of DOTP/PVC and DINP/PVC. According to the free volume theory of plasticization [32, 33], the increase in the peak size and decrease in the peak temperature (T g ) of tan δ value reflects good compatibilityand strong plasticization of the ECA plasticizer with PVC resin. This strong plasticizing efficiency can be attributed to it having a relatively lower molecular weight and more epoxy polar groups than the phthalates investigated, having higher ability to lubricate by incorporating itself among the polymer chains, and reducing PVC-PVC interactions by replacingpart of the plasticizer-PVC interactions [34] (Fig. 4). It is also observed that the largest decrease of the E’ value of the film plasticized with ECA as a plasticizer is in agreement with the conclusion that the E’ value is negatively correlated with the density of the polymer crosslink when above the T g [35, 36]. For CA, the increase in the T g suggests weaker plasticizing efficiency and compatibility with PVC as compared with two other commercial phthalates. This might be due to the unsaturated double-bonds on the side chain of CA. The changes in tensile strength, elongation at breakand elastic modulus of the obtained plasticized PVC films are shown in Fig. 5. It was observed that the elongation at break increases in the order: DOTP/PVC, DINP/PVC, CA/PVC and ECA/PVC, indicating the increase of flexibility and toughness for all the films. In addition, the tensile strength and elastic modulus were decreased in the same trend. These results are consistent with the DMA results, indicating that ECA has a significant effect on the flexibility properties and exhibits the best toughness [37]. In other words, less CA and ECA are required to reach the same flexibility compared tothose of DOTP and DINP.
Thermal stability
Figure 7 shows the TGA results for PVC films with different plasticizers heated in nitrogen at 10 °C/min. From the characteristic temperatures in TGA curves (Table 2), it was observed that the degradation of all the films consisted of three weight loss steps. The weight loss step around 220–340 °C is fast, due to dechlorination of PVC and a few chlorinated hydrocarbons [38] when the temperature reached~340–420 °C, the weight was almost stable, which may be associated with the formation of aromatic compounds by the cyclization of conjugated polyene sequences [39, 40]. The third mass loss step is above 420 °C, corresponding to the degradation and decomposition of the complex structures resulting from aromatization [40, 41]. It can be seen clearly that ECA/PVC has a much higher thermal stability compared to the other three films. This result may be due mainly to the epoxy groups of ECA which can scavenge for HCl and delay the degradation events [42,43,44].
Microstructure
The plasticizing effects on fractured surfaces of the films obtained from the mechanical tests were also determined by SEM as shown in Fig. 6a and b which corresponded to PVC films plasticized by DOTP and DINP, respectively. Both samples have strong roughness and cracking, are responsible for the higher tensile strength and the lower value of elongation at break. However, with the addition of CA and ECA, the appearances of the micrographics are different. For CA/PVC (Fig. 6c), oriented shallow ridges can be seen in the fractured surface, indicating a high plastic deformation that lasted before break [45, 46]. Furthermore, as shown in Fig. 6d, the fractured surface of ECA/PVC is smooth and homogeneous with the formation of some directed and shallow ridges, and the elongation at break reaches the maximum value of 155.84%. The results are in agreement with the tensile properties previously investigated (Fig. 7).
Workability
Meanwhile, to investigate the effects of plasticizers on the workability of PVC, the dynamic stability property of obtained samples was tested by a HAAKE torque rheometer (Thermo Fisher Scientific Inc., Pittsburgh, PA, USA). Figure 8 shows the results of the dynamic stability testingof PVC samples at 180 °C. The parameters of dynamic stability time and balance torque were calculated in Table 3. It can be seen that the values of dynamic stability time follow the order: DINP/PVC < DOTP/PVC < CA/PVC < ESO/PVC and ECA/PVC. Compared with DOTP/PVC and DINP/PVC, the CA/PVC has a maximum increase of 2.2 min in dynamic stability time, and a maximum decrease of 1.2 Nm in balance torque. Furthermore, the ECA/PVC has the highest dynamic stability time above 30 min, as well as the lowest balance torque of 5.1 Nm. This probably relates to the high content of the functional ester and epoxy groups and the long side chain in CA and ECA,which will reducethe melt viscosity during processingand extend the processing time [34]. Therefore, it can be concluded that CA and ECA improve the workability of PVC. On the other hand, these results showed good agreement with the results of TGA, suggesting that both CD derived plasticizers can increase the thermal stability of PVC.
Migration properties
The weight losses of the PVC samples by extraction, exudation and volatility are shown in Fig. 9. It could be seen that the four samples presented similar extraction and exudation loss. The weight loss of DOTP/ PVC and DINP/ PVC were slightly higher than the other two samples. Furthermore, the volatility loss decreased in the following order: CA/ PVC, ECA/ PVC, DINP/ PVC, and DOTP/PVC. It was suggested that the volatilization loss was largely depended on the molecular weight of the plasticizer [47], so the CA had the highest weight loss.
The usage of CA as a second plasticizer in soft PVC
To explore the effects of CA as an assistant plasticizer on the properties of soft PVC, films plasticized with DOTP were analyzed. The composition of the different soft PVC films is shown in Table 4. As shown in Fig. 10 and Table 4, the E’ value was lower with the higher CA content, and the T g was decreased in the same trend. This result indicated that the partial substitution of DOTP with CA will improve the plasticizing efficiency of the soft PVC films [33].
TGA was performed to evaluate the thermal stability of soft PVC films with different CA contents. TGA and differential thermogravimetry (DTG) curves are shown in Fig. 11. The onset temperature of degradation (T o ) and temperature of maximum rate of mass loss (T max ) were noted in Table 4. It can be seen that the value of the T o is higher in the case of F1, compared to the film plasticized with pure DOTP (F0), and the T max is in the same trend. This result might be due to the lower molecular weight of CA and the relatively higher content ofthe benzene rings which improve the thermal stability of this complex plasticizer [48]. However, the F2-F4 films have lower thermal degradation temperatures than F0, suggesting the thermal stability decreased with the increased content of CA, when the usage of CA is above 20% of the total content of the plasticizer. Tensile properties of soft PVC samples with CA as a secondary plasticizer were also performed in Table 5. Compared to the film plasticized with pure DOTP, partial substitution of DOTP with CA causes a remarkable increase in elongation and a general decrease of tensile strength and modulus. It also suggests that the softness and flexibility properties of the PVC films can be improved with CA as a secondary plasticizer.
Conclusions
In this work, two natural plasticizers, CA and ECA, were successfully synthesized and characterized by 1H NMR and 13C NMR. The plasticizing effects of CA and ECA on these mirigid PVC blend were investigated. By the incorporation of ECA into PVC, the thermo-mechanical properties, Tg, tensile strength, and E’ of plasticized PVC film decreases, while the films elongation at break, thermal stability and workability were improved. This could indicate that ECA has a higher plasticizing effect than commercial phthalate plasticizers. The ECA has slighter lower volatility resistance and similar extraction and exudation resistance than that of DOTP and DINP. For CA, the thermo-mechanical property is weaker than phthalates when used as a primary plasticizer in a semi-rigid PVC formulation. However, when CA was used as a secondary plasticizer, combined with DOTP, the soft PVC film showed improved mechanical and thermal properties, compared to those obtained with pure DOTP. The CA can partially replace DOTP in soft PVC formulations to reduce costs and to become a more eco-friendly product. These natural plasticizers are very promising in the application of the PVC matrix as an alternative to fully or partially replace petroleum-based plasticizers. It also suggests that CD may represent a useful raw material to develop alternative plasticizers.
References
Braslau R, Schäffner F, Earla A (2013) Polymeric phthalates: potential nonmigratory macromolecular plasticizers. J. Polym. Sci. Pol. Chem 51:1175–1184
Alexander M, Thachil ET (2006) A comparative study of cardanol and aromatic oil as plasticizers for carbon-black-filled natural rubber. J Appl Polym Sci 102(5):4835–4841
Zhou Y, Yang N, Hu S (2013) Industrial metabolism of PVC in China: a dynamic material flow analysis. Resour. Conserv. Recy 73:33–40
Ali M, Hirai T (2012) Effect of plasticizer on the electric-field-induced adhesion of dielectric PVC gels. J Mater Sci 47:3777–3783
Vadukumpully S, Paul J, Mahanta N, Valiyaveettil S (2011) Flexible conductive graphene/poly (vinyl chloride) composite thin films with high mechanical strength and thermal stability. Carbon 49:198–205
Fenollar O, Garcia-Sanoguera D, Sanchez-Nacher L, Lopez J, Balart R (2010) Effect of the epoxidized linseed oil concentration as natural plasticizer in vinyl plastisols. J Mater Sci 45:4406–4413
Brown H, Stevens G (1978) Fracture of slightly plasticized polyvinyl chloride. J Mater Sci 13:2373–2379
Choi JS, Park WH (2004) Effect of biodegradable plasticizers on thermal and mechanical properties of poly (3-hydroxybutyrate). Polym Test 23:455–460
Koch HM, Bolt HM, Angerer J (2004) Di (2-ethylhexyl) phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol 78:123–130
Huber WW, Grasl-Kraupp B, Schulte-Hermann R (1996) Hepatocarcinogenic potential of di (2-ethylhexyl) phthalate in rodents and its implications on human risk. Crit Rev Toxicol 26:365–481
Sampson J, De Korte D (2011) DEHP-plasticised PVC: relevance to blood services. Transfus Med 21:73–83
Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, Roßkamp E, Schlüter C, Seifert B, Ullrich D (2004) DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Envi Heal 207:409–417
Hill SS, Shaw BR, Wu AH (2001) The clinical effects of plasticizers, antioxidants, and other contaminants in medical polyvinylchloride tubing during respiratory and non-respiratory exposure. Clin Chim Acta 304:1–8
Yang D, Peng X, Zhong L, Cao X, Chen W, Zhang X, Liu S, Sun R (2014) “Green” films from renewable resources: properties of epoxidized soybean oil plasticized ethyl cellulose films. Carbohydr Polym 103:198–206
Fenollar O, García D, Sánchez L, López J, Balart R (2009) Optimization of the curing conditions of PVC plastisols based on the use of an epoxidized fatty acid ester plasticizer. Eur Polym J 45:2674–2684
Taghizadeh A, Sarazin P, Favis BD (2013) High molecular weight plasticizers in thermoplastic starch/polyethylene blends. J Mater Sci 48:1799–1811
Vieira MGA, Silva MAD, Maçumoto ACG, Santos LOD, Beppu MM (2014) Synthesis and application of natural polymeric plasticizer obtained through polyesterification of rice fatty acid. Mater Res 17:386–391
Xie Z, Chen Y, Wang C, Liu Y, Chu F, Jin L (2014) Effects of bio-based plasticizers on mechanical and thermal properties of PVC/wood flour composites. Bio Resour 9:7389–7402
Coltro L, Pitta JB, Madaleno E (2013) Performance evaluation of new plasticizers for stretch PVC films. Polym Test 32:272–278
Rao B, Palanisamy A (2013) Synthesis of bio based low temperature curable liquid epoxy, benzoxazine monomer system from cardanol: thermal and viscoelastic properties. Eur Polym J 49:2365–2376
Sultania M, Rai J, Srivastava D (2012) Modeling and simulation of curing kinetics for the cardanol-based vinyl ester resin by means of non-isothermal DSC measurements. Mater Chem Phys 132:180–186
Barreto A, Rosa D, Fechine P, Mazzetto S (2011) Properties of sisal fibers treated by alkali solution and their application into cardanol-based biocomposites. Compos Part A Appl S 42:492–500
Sandrino B, Clemente CDS, Oliveira TMBF, Ribeiro FWP, Pavinatto FJ, Mazzetto SE, de Lima-Neto P, Correia AN, Pessoa CA, Wohnrath K (2013) Amphiphilic porphyrin-cardanol derivatives in Langmuir and Langmuir–Blodgett films applied for sensing. Colloid Surface A 425:68–75
Yadav R, Srivastava D (2009) Studies on the process variables of the condensation reaction of cardanol and formaldehyde by response surface methodology. Eur Polym J 45:946–952
Façanha MAR, Mazzetto SE, Beserra Carioca JO, De Barros GG (2007) Evaluation of antioxidant properties of a phosphorylated cardanol compound on mineral oils (NH10 and NH20). Fuel 86(15):2416–2421
Mohapatra S, Nando GB (2013) Chemical modification of natural rubber in the latex stage by grafting Cardanol, a waste from the cashew industry and a renewable resource. Ind Eng Chem Res 52:5951–5957
Greco A, Brunetti D, Renna G, Mele G, Maffezzoli A (2010) Plasticizer for poly (vinyl chloride) from cardanol as a renewable resource material. Polym Degrad Stab 95:2169–2174
Neuse EW, Van Schalkwyk JD (1977) Cardanol derivatives as PVC plasticizers. II. Plasticizer evaluation. J Appl Polym Sci 21:3023–3033
Li M, Jiang J, Zhang J, Yang X, Zhang Y, Li S, Song J, Huang K, Xia J (2014) Preparation of a new liquid thermal stabilizer from rosin and fatty acid and study of the properties of the stabilized PVC. Polym Degrad Stab 109:129–136
Dorsey J, Dorsey G, Rutenberg A, Green L (1977) Determination of the epoxide equivalent weight of glycidyl ethers by proton magnetic resonance spectrometry. Anal Chem 49:1144–1145
Semsarzadeh M, Mehrabzadeh M, Arabshahi S (2002) Dynamic mechanical behavior of the dioctyl phthalate plasticized polyvinyl chloride–epoxidized soya bean oil. Eur Polym J 38:351–358
Ullah A, Wu J (2013) Feather fiber-based thermoplastics: effects of different plasticizers on material properties. Macromol Mater Eng 298:153–162
Sears JK, Darby JR (1982) The technology of plasticizers. Wiley, New York
Daniels PH (2009) A brief overview of theories of PVC plasticization and methods used to evaluate PVC-plasticizer interaction. J Vinyl Addit Techn 15:219–223
Gontard N, Ring S (1996) Edible wheat gluten film: influence of water content on glass transition temperature. J Agric Food Chem 44:3474–3478
Gupta V, Drzal L, Lee CC, Rich M (1985) The temperature-dependence of some mechanical properties of a cured epoxy resin system. Polym Eng Sci 25:812–823
Wypych G (2004) Handbook of plasticizers. Publishing, Chem. Tec
Da Silva MA, Vieira MGA, Maçumoto ACG, Beppu MM (2011) Polyvinylchloride (PVC) and natural rubber films plasticized with a natural polymeric plasticizer obtained through polyesterification of rice fatty acid. Polym Test 30(5):478–484
Soudais Y, Moga L, Blazek J, Lemort F (2007) Coupled DTA–TGA–FT-IR investigation of pyrolytic decomposition of EVA, PVC and cellulose. J Anal Appl Pyrolysis 78:46–57
Yao Q, Wilkie CA (2001) Thermal degradation of PVC in the presence of polystyrene. J Vinyl Addit Techn 7:26–36
Pitchaimari G, Sarma K, Varshney L, Vijayakumar C (2014) Influence of the reactive diluent on electron beam curable funtionalized N-(4-hydroxyl phenyl) maleimide derivatives–studies on thermal degradation kinetics using model free approach. Thermochim Acta 597:8–18
Ayrey G, Head B, Poller R (1974) The thermal dehydrochlorination and stabilization of poly (vinyl chloride). J Polym Sci Macr 8:1–49
Lerke I, Szymański W (1977) Radiation yield of hydrogen chloride in gamma-irradiated poly (vinyl chloride) stabilized by epoxy compounds. J Appl Polym Sci 21:2067–2075
Grossman RF (1993) Mixed metal vinyl stabilizer synergism. V: Dehydrochlorinations reactions of model compounds. J. Vinyl Addit. Techn. 15:25–28
Cadogan D, Howick C (1992) Plasticizers. In: Croschwitz JI, Howe-Grant M (eds) Kirk-Othmer encyclopedia of chemical technology. Wiley, New York
Garcia J, Marcilla A (1998) Rheological study of the influence of the plasticizer concentration in the gelation and fusion processes of PVC plastisols. Polymer 39:3507–3514
Marcilla A, García S, Garcia-Quesada J (2008) Migrability of PVC plasticizers. Polym Test 27:221–233
Li M, Zhang J, Huang K, Li S, Jiang J, Xia J (2014) Mixed calcium and zinc salts of dicarboxylic acids derived from rosin and dipentene: preparation and thermal stabilization for PVC. RSC Adv 4:63576–63585
Acknowledgements
The authors are grateful for the financial support from National “Twelfth Five-Year” Plan for Science & Technology Support (Grant number: 2015BAD15B08).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jie Chen is a visiting scholar at U.S. Department of Agriculture.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Chen, J., Liu, Z., Nie, X. et al. Plasticizers derived from cardanol: synthesis and plasticization properties for polyvinyl chloride(PVC). J Polym Res 25, 128 (2018). https://doi.org/10.1007/s10965-018-1524-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1524-4