Abstract
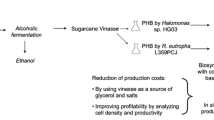
Vinasse, a recalcitrant waste of the ethanol industry was employed for the production of polyhydroxyalkanoate (PHA) by the extremely halophilic archaeon, Haloarcula marismortui in shake flasks. The PHA was recovered by osmotic lysis of the cells and subsequent purification by sodium hypochlorite and organic solvents. Through UV–vis spectroscopy, differential scanning calorimetry, Fourier transform infrared, and nuclear magnetic resonance spectroscopy, the PHA was found to have characteristics very similar to that of the standard polyhydroxybutyrate (PHB) from Sigma. Inhibitory effect of polyphenols contained in vinasse was assessed by a quick and reliable cup-plate agar-diffusion method. Raw vinasse (10%) was utilized leading to accumulation of 23% PHA (of cell dry weight) and following an efficacious pre-treatment process through adsorption on activated carbon, 100% pre-treated vinasse could be utilized leading to 30% accumulation of PHB by H. marismortui. Maximum specific growth rate, specific production rate, and volumetric productivity attained using 10% raw vinasse were comparable to that obtained using a previously reported nutrient deficient medium (NDM), while the values with 100% pre-treated vinasse were higher than that determined using NDM medium. This is the first report of polyhydroxybutyrate production by a halophilic microorganism utilizing vinasse.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many microorganisms overproduce polyesters if they have access to a surplus of carbon source. Polyhydroxyalkanoates (PHA) are linear polyesters produced by bacterial fermentation from renewable natural sources such as sugars and lipids and genetic modification of green plants (Flieger et al. 2003). Halophilic microorganisms are an important source of biopolyesters and hold promise for providing an economically competitive industrial scale production process (Quillaguamán et al. 2010). Haloarchaeal strains employed as PHA producers have many advantages. As there are very few microorganisms that are able to survive and grow at high salinities, the risks of microbial contamination can be reduced. Haloarchaea can be easily lysed in distilled water; therefore, the use of large quantities of organic solvents can be avoided and time for PHA preparation can be saved. Cheap carbon sources can be used to synthesize PHAs by haloarchaea and so the cost of PHA production will be lowered (Han et al. 2007; 2010). In this regard, renewable resources like extruded starch, extruded rice bran, and hydrolyzed whey were utilized for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) production by Haloferax mediterranei (Quillaguamán et al. 2010), while wheat bran and potato waste were tested for poly-3-hydroxybutyrate (PHB) production by Halomonas boliviensis (Van-Thuoc et al. 2008).
The alcohol industry uses molasses to produce ethanol through yeast fermentation. The residue left after removal of the ethanol from the fermentation broth is known as vinasse that consists of water and non-volatile components from the fermentation broth. Vinasse is produced at a rate 10 to 15 times greater than the ethanol itself, and its disposal is a major environmental problem worldwide. There are several biological methods (both aerobic and anaerobic) available for the utilization of vinasse. Vinasse contains a number of phenolic compounds (more than 10) and their polymers. These compounds are known to be difficult to degrade biologically and possess antimicrobial and phytotoxic properties. Prior removal of these compounds should accelerate biological treatment of the waste (García García et al. 1997). Literature reports that adsorption processes on activated carbons (ACs) or other adsorbent materials are good alternatives for removing polyphenolic and dark-colored compounds from vinasse (Caqueret et al. 2011). ACs possess perfect adsorption ability for relatively low-molecular-mass organic compounds such as phenols. They can be manufactured in such a way that a highly fractal material is obtained. There are two most common physical forms in which AC is used, i.e., powder-like AC and granular (Dąbrowski et al. 2005).
In the present work, we have attempted to utilize vinasse for the production of PHA by the extremely halophilic archaeon, Haloarcula marismortui. Recently, the operon required for PHA synthesis by this archaebacterium, in this case PHB, was characterized and the expression profile of the genes analyzed (Han et al. 2007). To the best of our knowledge, PHA production in halophilic microorganisms utilizing vinasse is being endeavored for the first time.
Materials and methods
Pre-treatment of vinasse
The vinasse (that produces ethanol from sugarcane molasses) used in this study was obtained from IFB Agro Industries, Noorpur, India. In order to carry out all the experiments with the same waste, appropriate amounts of vinasse, distributed in 250 mL plastic bottles, were stored at −18°C. Activated carbon (AC) supplied by Merck (India), Mumbai in concentrations 1.0, 2.5, and 5.0 g were added to 100 mL of raw vinasse at different pH values (2.0, 4.0, 6.0, and 8.0) to determine the optimal condition of the pre-treatment process. The pH was adjusted to tested values by addition of concentrated HCl/NaOH. AC (50 g) was mixed with distilled water in a porcelain container and heated in a muffle furnace at 500°C for 1 h. Required amounts (1.0, 2.5, and 5.0 g) of this AC was added to the raw vinasse and was kept agitated (160 rpm) in rotary shaker for 1 h at 25°C. The AC was then removed by centrifugation at 7,000 rpm for 15 min (Eppendorf model 5810R, rotor F-34-6-38). The supernatant was filtered through Whatman No. 1 filter paper, neutralized with NaOH/HCl as required, and the filtrate characterized as mentioned in the sub-section “Analytical methods”.
Microorganism and growth conditions
H. marismortui MTCC 1596 (obtained from MTCC, Chandigarh, India) was used in this study. For PHA accumulation, the archaebacterium was first cultivated in 50 mL of the growth medium as suggested by MTCC, composed of (grams per liter) NaCl 200.0; MgSO4·7H2O 37.0; KCl 0.5; CaCl2·2H2O 0.7; MnCl2·4H2O 0.5; yeast extract 5.0; pH to 7.0 in a 250-mL Erlenmeyer flask for 5 days at 37°C in a rotary shaker. One millilitre of the culture was transferred into a nutrient deficient medium (NDM, Han et al. 2007) composed of (grams per liter) NaCl 200.0; MgSO4·7H2O 20.0; KCl 2.0; sodium glutamate 1.0; KH2PO4 0.0375; FeSO4·7H2O 0.05; MnCl2·4H2O 0.00036, yeast extract 1.0, pH 7.2 supplemented with 2% glucose and incubated at 37°C for 5 days in a rotary shaker. For cultivations with vinasse, all components of the NDM (except glucose) having the same concentration as described by Han et al. (2007) were added to different concentrations (percent, v/v), 1% to 10%, 15%, 20%, 25%, 50%, 75%, and 100% of raw or pre-treated vinasse. The pH was finally adjusted to 7.2. The conductivity of the 10% raw vinasse containing medium and 100% pre-treated vinasse were determined by a conductivity meter (Milwaukee Instruments, USA), and the values multiplied by 600 to obtain salinity as percentage.
Evaluation of inhibitory effect of vinasse
The inhibitory effect of raw vinasse and dilutions thereof as well as pre-treated vinasse and dilutions therefrom were evaluated by the cup-plate agar diffusion method. Petri plates were prepared containing the growth medium and swabbed with culture of H. marismortui containing 108 CFU/mL. The test samples (100 μL) were added to the cups and the plates incubated for 5 days at 37°C, following which, the diameter of the zones of inhibition were recorded.
Determination of cell dry weight
One milliliter of the liquid culture obtained at the end of the process was centrifuged at 5,000 rpm (Eppendorf model 5810R, rotor F-34-6-38) for 10 min. The salt-free cell dry weight (CDW) of the pellet was determined by drying at 105°C to constant mass in an oven and subtracting the mass of the ash remaining after heating at 400°C for 4 h in a muffle furnace (Garcia Lillo and Rodriguez-Valera 1990).
Detection of PHA
Aqueous solution of Nile Blue A (Sigma, 1%) was mildly heated and filtered before use (Ostle and Holt 1982). Heat fixed smear of bacterial cells were stained with Nile Blue A solution at 55°C in a water bath for 10 min. After staining, the slides were washed with 20% brine water followed by 8% aqueous acetic acid for 1 min, washed again, blotted dry, and covered with a glass cover slip. The samples were examined in a Leica DMLB fluorescence microscope following excitation at 460 nm. Escherichia coli MTCC 739 which is not known as a PHA producer was used as a negative control. The test was performed at the end of the cultivation where the cell concentration in the different samples: NDM, 10% raw vinasse containing medium, and 100% pre-treated vinasse were within a maximum range of 30 ± 2% as judged by the measurements of the cell dry weights (Table 3).
Isolation and characterization of PHA
Broth containing H. marismortui cells was centrifuged at 5,000 rpm (Eppendorf model 5810R, rotor F-34-6-38) for 10 min. The pellet was suspended in distilled water for 48 h. The lyzed suspension was centrifuged at 5,000 rpm for 10 min and the pellet washed 3 to 4 times with distilled water. A white-colored substance was obtained that was dried until constant mass. The white dust was dissolved in 25 mL sodium hypochlorite, and the mixture was incubated at 30°C for 2 h. The mixture was centrifuged at 5,000 rpm for 10 min, washed with distilled water, acetone, and methanol. The pellet was dissolved in 10 mL of boiling chloroform, which was subsequently evaporated. The undissolved material was removed by filtration (Fernandez-Castillo et al. 1986 and Tamboli et al. 2010). All organic solvents were recovered by distillation and recycled in subsequent batches.
The thermal property of the PHA was examined by differential scanning calorimetry (DSC) using a Mettler Toledo DSC 1 instrument with Stare SW 9.20 software. The calorimeter had one sample cell and one reference cell. Samples were exposed to a temperature profile over 0°C to 350°C, at a heating rate of 10 K/min. KBr pellets were prepared using PHA from H. marismortui and standard PHB (Sigma). Fourier transform infra red (FTIR) spectra were recorded in a PerkinElmer Spectrum BX spectrometer in the spectral range 4,000–700 cm−1. 1H NMR spectroscopy was done in a Bruker DPx-300 (300 MHz) spectrometer. Sample and standard PHB from Sigma (10 mg/mL) were dissolved in 1 mL CDCl3 by heating at 60°C. Tetramethylsilane was used as the internal standard.
Determination of PHB content
The PHB was assayed by the method of Law and Slepecky (1961). The final product obtained as described in the previous sub-section was hydrolyzed and dehydrogenated with concentrated sulfuric acid to obtain crotonic acid, which can be quantified by its A235. The standard curve was obtained by using PHB (Sigma). The UV–vis spectra of the treated residue of standard PHB and our isolated product were acquired with a PerkinElmer Lambda 25 spectrophotometer.
Analytical methods
The pH, BOD5, COD, turbidity, phosphate, and total Kjeldahl nitrogen content of vinasse were measured in accordance with the procedures given in Standard Methods for the Examination of Water and Wastewater (American Public Health Association 1998). The Folin–Ciocalteu method was used to estimate the amount of total polyphenolic compounds in the vinasse in terms of equivalent gallic acid (Nawaz et al. 2006). Total carbohydrate was measured by hydrolyzing the polysaccharides to simple sugars followed by addition of anthrone reagent (Hedge and Hofreiter 1962), while total organic carbon was determined following Walkley and Black (1934).
Results and discussion
Pre-treatment of vinasse
A comparison of the efficacies of the vinasse pre-treatment methods is provided in Table 1. Maximum reductions in total polyphenol content as well as the other parameters have been obtained following pre-treatment at pH 2.0 with 5.0 g activated carbon per 100 mL raw vinasse. The pH value of the solution influences the mechanisms of adsorption and interactions between adsorbate and adsorbent. The pH value modifies not only the nature of the acidic organic functions of the molecules but also the protonation of the activated carbon sites. Similar to the observations of Caqueret et al. (2008), more efficient adsorption of polyphenolics in vinasse occurred at acidic pH. According to Qi et al. (2004), the decrease in adsorption capacity in the basic pH range can be due to a deprotonation of first, the activated carbon acidic functional groups and second, of the carboxylated molecules present in the solution, like phenolic acids. At higher pH values, the electrostatic interactions are maximal and involve forces of repulsion between the dissociated molecules and the activated carbon surface functional groups. These repulsive forces may prevent the specific interactions of the molecules with the active sites on the carbon surface, especially the hydrogen bondings.
Characteristics of vinasse
Recently the composition of vinasses has been published by Parnaudeau et al. (2008). Sugarcane vinasse contains significant amounts of sucrose as well as oxalate, lactate, acetate, and malate, and pyruvate which are ready metabolites to be fed to the tricarboxylic acid cycle. Although a substantial fraction of its organic content is potentially biodegradable, the presence of polyphenolic compounds can be toxic to and/or inhibit the producer microorganism (Martín Santos et al. 2003). Thus, pre-treatment for removal of polyphenolic compounds was necessary. Caqueret et al. (2008) noted that it was difficult to remove the polyphenolic compounds by oxidation with classic biological treatments, and an adsorption process could be a good alternative to remove these compounds from agro-industrial residues. This method is also cheaper compared to other recent approaches such as ozonation and hence was used in this study. We observed concomitant reduction of organic carbon content with enhanced removal of polyphenolic compounds (Table 2). The lower organic carbon concentration, that was half of the raw value, brought about a decrease in the BOD5 value. Thus, the pre-treatment process lowered the pollution potential of the vinasse. Although decrease in the carbohydrate content occurred by about twofold, the amount was still high enough to support growth and PHA production by H. marismortui.
Inhibitory effect of vinasse on H. marismortui
It is evident from Fig. 1a that 10% raw vinasse concentrations (percent raw content in water, v/v) has no antagonistic effect on the growth of the producer archaebacterium. Higher concentrations of the raw vinasse (percent raw content) 25, 50, 75, and 100 inhibit the growth of H. marismortui in increasing extents (Fig. 1b–e). However, the pre-treated vinasse allowed the growth of the producer microorganism at the same concentrations (percent pre-treated content, v/v) 25, 50, 75, and 100 that inhibited the growth in the raw (untreated) form (Fig. 1f–i). Growth of H. marismortui apparently seemed to improve with the increase in vinasse concentration. The enhanced color intensity of the plates was due to the increasing concentrations of melanoidins (which impart brown color to the vinasse) diffusing out of the hole and not owing to the growth of the bacterium. The brown color masked the characteristic light pink color of H. marismortui. As the composition of vinasses differ from industry to industry, the described agar diffusion cup-plate method can be applied as a quick and reliable method to judge the vinasse strength that may be appropriate for the cultivation of H. marismortui. As observed by us, the concentration of vinasse that did not inhibit growth of the archaebacterium in the cup-plate method was exactly the same concentration that allowed growth and PHA accumulation by the producer organism in the shake flask. Thus, the pre-treatment process proved to be effective as 100% pre-treated vinasse could be utilized. Rhodopseudomonas palustris, an anaerobic photosynthetic bacterium, was cultivated in a medium containing vinasse obtained from Brazilian distillery by Peres et al. (1986). Authors observed no bacterial growth at COD values higher than 10,000 mg/L. Nitrogen-fixing bacterial strains Azospirillum brasiliense, Bacillus polymyxa, and Azotobacter chroococcum could utilize 6%, 8%, 10%, and 15% vinasse obtained from an Egyptian distillery (Omar et al. 2002). Fungi such as Fusarium oxysporum, Sclerotinia sclerotiorum, Pythium aphanidermatum, and Phytophthora parasitica were inhibited by 15% vinasse obtained from Spanish breweries (Santos et al. 2008).
Detection of PHA granules in H. marismortui cells grown in vinasse
Fluorescence microscopy at excitation wavelength of 460 nm revealed numerous brightly fluorescent orange granules within the cells grown on both raw vinasse (10%) as well as pre-treated vinasse (100%) as evident in Fig. 2, thus confirming the production of PHA.
Isolation and characterization of PHA
The white mass obtained from H. marismortui cells grown in medium containing 10% raw vinasse as well as in 100% pre-treated vinasse as described in the “Materials and methods” section was chemically characterized. As apparent from Supplementary Fig. 1, the λ max value of 235 nm corresponded to crotonic acid derived from hydrolysis and dehydrogenation of polyhydroxybutyrate. The major thermal phase transition observed for H. marismortui PHA was at 257°C and that of standard PHB was at 276°C. This difference may be attributed to the variation in molecular packing of the samples (Supplementary Fig. 2a and 2b). The FTIR spectrum of the PHA obtained from H. marismortui was compared with that of standard PHB from Sigma (Supplementary Fig. 3). FTIR analysis of the isolated polymer revealed absorption bands at 1,724 cm−1, corresponding to the ester carbonyl group (Shrivastav et al. 2010). 1H NMR spectrum of the sample was also compared with the standard spectrum of PHB from Sigma (Supplementary Fig. 4a and 4b). Both spectra showed identical proton signals: the double doublet of diastereotopic –CH2 proton having the delta value between 2.43 and 2.64 of the sample and 2.46 and 2.64 of the standard. Similarly, a multiplet of the chiral at C3 showed multiplet between 5.22 and 5.28 for the sample and 5.23 and 5.30 for the standard. The methyl protons gave multiple signals between 1.25 and 1.32 for three protons of the sample, while the signals appeared between 1.27 and 1.32 for the standard. The broad peak at 1.60 was assigned to water and the peak close to 0.90 was not assigned to any protons in the polymeric backbones. Based on the characterization of the PHA produced by H. marismortui through UV–vis spectroscopy, FTIR, DSC, and NMR analyses and subsequent comparison with the standard PHB (Sigma), it was deduced that the PHA obtained from H. marismortui by utilizing vinasse had characteristics very similar to that of the standard PHB (Sigma).
The major step of the separation process is the extraction of PHA granules. The extraction step of the described procedure consumed no energy and chemicals compared to other recovery methods such as solvent and supercritical fluid extraction, digestion by surfactants, sodium hypochlorite, and enzymes as well as mechanical disruption of cell walls by high-pressure homogenization and ultrasonication (Jacquel et al. 2008). Although spontaneous liberation of PHA by osmotic lysis of haloarchaea is a simple and appealing alternative for extraction, our results showed that subsequent purification steps were essential to obtain a high-purity product.
Production of PHB using raw and pre-treated vinasse
There was a significant lowering of all the measured wastewater parameters of vinasse at the end of the process carried out with 10% raw vinasse containing medium. Moderate to low (15–40%) reduction of the measured wastewater characteristics were noted at the end of the process performed with 100% pre-treated vinasse (Table 2). Table 3 shows the bioprocess parameters determined for growth and PHB production by H. marismortui using raw and pre-treated vinasse at the end of the process. The PHB content of H. marismortui, when cultured in minimal medium with 2% glucose as the excessive carbon source, reached its maximum at 21% (of CDW) after 180 h of fermentation and then began to decrease slowly as reported by Han et al. (2007). In this investigation, replacement of glucose in their medium by raw or treated vinasse allowed production of PHB and an improvement by 7% (of CDW) when 100% pre-treated vinasse was used. Significant amounts of carbohydrates in the vinasse used in our study provided the necessary carbon source for the production of PHB.
The lag phases of growth, as seen in Table 3, were prolonged. It is commonly known that lag depends on the actual environmental conditions. Several authors report on the reasonable effect of the pre-culturing conditions on the subsequent lag duration. Lag is also affected by the magnitude and the rate of the change between the past and present environment. Actively growing cells adjust more rapidly to changes as compared to cells during the lag or the stationary phase. The lag time is extended when the inoculum is severely stressed by starvation and the inoculum size is very small. This inoculum size effect can be explained by an increase in the variability of the individual cells' lag time when cells are damaged by stress. The inoculum size effect is more pronounced near the growth/no growth interface, because not all cells adapt to inimical conditions (Swinnen et al. 2004).
Generally, the lag phase in H. marismortui is 35 h in a nutrient-rich medium as observed in two independent studies, the first on the construction of a H. marismortui strain with two of its three chromosomal rRNA operons deleted (Tu et al. 2005) and second, on the regulation of acetate and acetyl-CoA converting enzymes during growth of H. marismortui on acetate and/or glucose (Bräsen and Schönheit 2004). In the present investigation, change from nutrient rich medium to nutrient deficient medium resulted in lengthening of the lag phase (96 h) during cultivation in the NDM and with 10% raw vinasse. The lag phase was even longer (144 h) when H. marismortui was grown in 100% pre-treated vinasse. This growth medium is not alike the natural environment for this strain isolated from the hypersaline water of the Dead Sea (Oren et al. 1990). It was possible that some other component(s) present in excess (phosphate, nitrogen, and carbohydrate) or non-optimal salinity created unfavorable environmental conditions to which H. marismortui needed to adapt. The optimal salinity for growth of H. marismortui is 25% (López-López et al. 2007), while the salinity of 100% pre-treated vinasse medium was 19.8 ± 0.5% (330 ± 08 mS/cm multiplied by 600). This phenomenon is generally observed in this class of highly specialized microorganisms and as a specific example, Haloferax volcanii, a moderate halophile required a lag period and synthesis of specific, high-salt-related proteins to adapt to hypersaline conditions (Franzetti et al. 2002). Again, Tu et al. (2005) noted that there was a lot of variability in both the doubling time and the lag time of H. marismortui cultures grown under apparently identical conditions. Lengthening of the lag phase due to simultaneous removal of growth factors through the adsorption process could also be a possible explanation. Therefore, it is difficult to give an explanation for the extended lag phase in the present study which could be speculated due to a number of reasons.
Han et al. (2007) noted that there was a minimal lag phase when H. marismortui was cultivated in a bioreactor. Bioreactors allow the creation and manipulation of growth conditions more suited to the microorganism being cultured. Due to the low solubility of oxygen in salt-saturated brines, oxygen may easily become a limiting factor for development of members of the Halobacteriaceae (Oren 2002). In the study of Han et al. (2007), it could be possible that the aeration allowed high dissolved oxygen concentration in the medium, and consequently, the cells entered the exponential phase without delay.
The initial organic carbon concentration of 10% raw vinasse was 1,285 ± 19 mg/L while that of 100% pre-treated vinasse was 5,869 ± 22 mg/L (Table 2). Although nearly all of the organic carbon was utilized in the cultivations with 10% raw vinasse, 3,621 ± 29 mg/L of organic carbon remained unutilized in cultures with 100% pre-treated vinasse. This may be due to some limiting factors. Non-optimal salinity may be limiting to the growth of H. marismortui. The optimal salinity for growth of H. marismortui is 25% (López-López et al. 2007); while the salinity of 100% pre-treated vinasse medium was 19.8 ± 0.5%. The salinities of NDM and 10% raw vinasse containing medium were 23.5 ± 0.5%.
The maximum values of cell dry mass, PHB content, specific growth rates, specific production rates, as well as volumetric productivity were higher in cultures with pre-treated vinasse in contrast to other two conditions. The yield coefficient values varied according to the initial organic carbon contents. Overall process performance using 10% raw vinasse was comparable using NDM medium (Han et al. 2007), while performance with 100% pre-treated vinasse was better than using the NDM medium.
Although the family Halobacteriaceae includes 30 genera, currently, only a few haloarchaeal strains belonging to the genera Haloferax, Haloarcula, Haloquadratum, Haloterrigena, Halorhabdus, Halobiforma, and Halopiger are found to accumulate short-chain-length PHAs, such as PHB and PHBV (Han et al. 2010). H. mediterranei is so far the best PHA producer of the family Halobacteriaceae (Quillaguamán et al. 2010). In this study, raw vinasse (10%) was utilized leading to accumulation of 23% PHB (of cell dry weight) and following pre-treatment through adsorption on activated carbon, 100% pre-treated vinasse could be utilized leading to 30% accumulation of PHB by H. marismortui. Although the values may seem to be low compared to H. mediterranei, we believe there is much scope of improvement through optimization techniques. Optimization can be carried out by varying the concentrations of the components of the NDM (except glucose) added to the 10% raw or 100% pre-treated vinasse. Further improvement may be done by investigating fed-batch, semi-batch, and continuous processes. Therefore, given the limited number of halophilic microorganisms producing PHAs, this study should open up the possibility of exploring other halophilic archaebacteria for utilization of vinasse for the production of PHAs.
Conclusions
The economic feasibility of PHB fermentation is comparable with petrochemical processes when cheap or waste substrates are used and unconventional microorganisms applied (Flieger et al. 2003). This study demonstrated the successful utilization of a recalcitrant waste for PHB production by a halophilic microorganism at the shake-flask level. The production of PHB from vinasse may be profitable in terms of the economic efficiency of the entire process as the production can be easily integrated into an ethanol manufacturing unit. There would be costs saving related to purchase and transportation of raw material, purchase and operation of equipment, as well as financial requirements for personnel in addition to the advantages of a no energy and chemical requiring initial PHB recovery method and utilization of a waste carbon source. The product obtained was of the same quality as that produced by Sigma.
It is true that the process cost will be enhanced by the long lag phase of H. marismortui, but there is a definite opportunity of reducing the lag period in the future by providing enhanced aeration, application of a concentrated inoculum and using inoculum cells from the exponential phase to start the process. Regarding the second limitation, the unutilized residual organic carbon in the process with 100% pre-treated vinasse can be recycled in the next batch or a continuous process may be prospected. Further cultivations will be carried out in bioreactors where these two problems will be addressed.
References
American Public Health Association (1998) Standard methods for the examination of water and wastewater, 20th edn. APHA, Washington
Bräsen C, Schönheit P (2004) Regulation of acetate and acetyl-CoA converting enzymes during growth on acetate and/or glucose in the halophilic archaeon Haloarcula marismortui. FEMS Microbiol Lett 241:21–26
Caqueret V, Bostyn S, Cagnon B, Fauduet H (2008) Purification of sugar beet vinasse—adsorption of polyphenolic and dark colored compounds on different commercial activated carbons. Bioresour Technol 99:5814–5821
Caqueret V, Cagnon B, Bostyn S, Fauduet H (2011) Removal of dark coloured and polyphenolic compounds of sugar beet vinasse by adsorption onto activated carbons: application to a crosscurrent adsorption process. Can J Chem Eng 9999:1–9
Dąbrowski A, Podkościelny P, Hubicki Z, Barczak M (2005) Adsorption of phenolic compounds by activated carbon—a critical review. Chemosphere 58:1049–1070
Fernandez-Castillo R, Rodriguez-Valera F, Gonzalez-Ramos J, Ruiz-Berraquero F (1986) Accumulation of poly (β-hydroxybutyrate) by Halobacteria. Appl Environ Microbiol 51:214–216
Flieger M, Kantorova M, Prell A, Řezanka T, Votruba J (2003) Biodegradable plastics from renewable sources. Folia Microbiol 48:27–44
Franzetti B, Schoehn G, Garcia D, Ruigrok RWH, Zaccai G (2002) Characterization of the proteasome from the extremely halophilic archaeon Haloarcula marismortui. Archaea 1:53–61
García García I, Bonilla Venceslada JL, Jiménez Peña PR, Ramos Gómez E (1997) Biodegradation of phenol compounds in vinasse using Aspergillus terreus and Geotrichum candidum. Water Res 31:2005–2011
Garcia Lillo J, Rodriguez-Valera F (1990) Effects of culture conditions on poly(β-hydroxybutyrate acid) production by Haloferax mediterranei. Appl Environ Microbiol 56:517–2521
Han J, Lu Q, Zhou L, Zhou J, Xiang H (2007) Molecular characterization of the phaECHm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui. Appl Environ Microbiol 73:6058–6065
Han J, Hou J, Liu H, Cai S, Feng B, Zhou J, Xiang H (2010) Wide distribution among halophilic archaea of a novel polyhydroxyalkanoate synthase subtype with homology to bacterial type III synthases. Appl Environ Microbiol 76:7811–7819
Hedge JE, Hofreiter BT (1962) Estimation of carbohydrates. In: Whistler RL, BeMiller JN (eds) Carbohydrate chemistry. Academic Press, New York, pp 17–22
Jacquel N, Lo C-W, Wei Y-H, Wu H-S, Wang SS (2008) Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem Eng J 39:15–27
Law JH, Slepecky RA (1961) Assay of poly-β-hydroxybutyric acid. J Bacteriol 82:33–36
López-López A, Benlloch S, Bonfá M, Rodríguez-Valera F, Mira A (2007) Intragenomic 16S rDNA divergence in Haloarcula marismortui is an adaptation to different temperatures. J Mol Evol 65:687–696
Martín Santos MA, Fernández Bocanegra JL, Martín Martín A, García García I (2003) Ozonation of vinasse in acid and alkaline media. J Chem Technol Biotechnol 78:1121–1127
Nawaz H, Shi J, Mittal GS, Kakuda Y (2006) Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep Purif Technol 48:176–181
Omar MNA, Mostafa AT, Ahmed AS (2002) Concentrated vinasse as a novel diazotrophs growth medium (biovinasse inoculant) and soil conditioner to improve faba bean yield under dripping irrigation system. Symposium No. 3, Paper No. 137, 17th WCSS, 14–21 August, Thailand
Oren A (2002) Halophilic microorganisms and their environments. Kluwer Academic Publishers, Dordrecht, p 136
Oren A, Ginzburg M, Ginzburg BZ, Hochstein LI, Volcani BE (1990) Haloarcula marismortui (Volcani) sp. nov., nom. rev., an extremely halophilic bacterium from the Dead Sea. Int J Syst Bacteriol 40:209–210
Ostle AG, Holt JG (1982) Nile blue A as a fluorescent stain for poly-β-hydroxybutyrate. Appl Environ Microbiol 44:238–241
Parnaudeau V, Condom N, Oliver R, Cazevieille P, Recous S (2008) Vinasse organic matter quality and mineralization potential, as influenced by raw material, fermentation and concentration processes. Bioresour Technol 99:1553–1562
Peres CS, Figueiredo MG, Vitoratto E, Perege L Jr (1986) Growth of anaerobic photosynthetic bacteria in vinasse medium. Rev Microbiol 17:1–9
Qi J, Li Z, Guo Y, Xu H (2004) Adsorption of phenolic compounds on micro- and mesoporous rice husk-based active carbons. Mater Chem Phys 87:96–101
Quillaguamán J, Guzmán H, Van-Thuoc D, Hatti-Kaul R (2010) Synthesis and production of polyhydroxyalkanoates by halophiles: current potential and future prospects. Appl Microbiol Biotechnol 85:1687–1696
Santos M, Diánez F, de Cara M, Tello JC (2008) Possibilities of the use of vinasses in the control of fungi phytopathogens. Bioresour Technol 99:9040–9043
Shrivastav A, Mishra SK, Mishra S (2010) Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int J Biol Macromol 46:255–260
Swinnen IAM, Bernaerts K, Dens EJJ, Geeraerd AH, Van Impe JF (2004) Predictive modelling of the microbial lag phase: a review. Int J Food Microbiol 94:137–159
Tamboli DP, Kagalkar AN, Jadhav MU, Jadhav JP, Govindwar SP (2010) Production of polyhydroxyhexadecanoic acid by using waste biomass of Sphingobacterium sp. ATM generated after degradation of textile dye Direct Red 5B. Bioresour Technol 101:2421–2427
Tu D, Blaha G, Moore PB, Steitz TA (2005) Gene replacement in Haloarcula marismortui: construction of a strain with two of its three chromosomal rRNA operons deleted. Extremophiles 9:427–435
Van-Thuoc D, Quillaguamán J, Mamo G, Mattiasson B (2008) Utilization of agricultural residues for poly(3-hydroxybutyrate) production by Halomonas boliviensis LC1. J Appl Microbiol 104:420–428
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Acknowledgements
Authors wish to thank Mr. Swapan K Bayen, Mr. Santanu Ghose, and Mr. Anindya Roy of IFB Agro Industries for the gift of the vinasse. Assistance from Mr. Biswarup Saha during fluorescence microscopy is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
UV–vis scanning spectroscopy following hydrolysis and dehydrogenation of standard PHB from Sigma (red line) and PHA from H. marismortui (blue line) (JPEG 88 kb)
Supplementary Figure 2
DSC thermograms of a PHA from H. marismortui (JPEG 74 kb)
Supplementary Figure 2
DSC thermograms of b standard PHB from Sigma (JPEG 71 kb)
Supplementary Figure 3
FTIR spectra of PHA from H. marismortui (lower trace), compared with standard PHB from Sigma (upper trace) (JPEG 265 kb)
Supplementary Figure 4
1H NMR spectra of a PHA from H. marismortui (JPEG 204 kb)
Supplementary Figure 4
1H NMR spectra of b standard PHB from Sigma (JPEG 134 kb)
Rights and permissions
About this article
Cite this article
Pramanik, A., Mitra, A., Arumugam, M. et al. Utilization of vinasse for the production of polyhydroxybutyrate by Haloarcula marismortui . Folia Microbiol 57, 71–79 (2012). https://doi.org/10.1007/s12223-011-0092-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-011-0092-3