Abstract
Bio-synthesis of nanostructures is an ever green approach; therefore, the current work attempts to use Arabic gum as bio-synthesizer for manufacturing Ag–Au bimetallic nanocomposite, using seed-mediated growth technique. The relevance of the as-used natural gums for manufacturing of Ag–Au nanocomposite in quite simple, energy saving and cost effective methodology, has been successfully approved. Arabic gum acted concurrently as a reducer for metal ions to nano-sized structures and crystal growth modifier of the so-generated nanocomposite. Monitoring of interchanging in size distribution of the as-produced bimetallic nanocomposite by the effect of the gum concentration, concentration of nanometals precursors, their addition sequencing in reaction liquor, and reaction temperature, was illustrated and well characterized. UV–Visible spectra showed that the characteristic surface plasmon resonance peak for Ag–Au bimetallic nanocomposite was observed at 480–495 nm and XRD patterns confirmed the preparation of bimetallic nanostructures. According to zetasizer analyses and TEM micrographs, small sized (3.1 nm) Ag–Au bimetallic nanocomposite with narrow size distribution (1–7 nm) were obtained. The all prepared bimetallic nanocomposite showed good stability with polydispersity index ranged in 0.203–0.530. The mechanism of nanostructures’ generation was suggested and approved using FT-IR, 1HNMR and 13CNMR spectra. The prepared Ag–Au bimetallic nanocomposites showed excellent antibacterial activity against both of gram + ve and gram − ve strains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past decade, the green chemistry was mainly aimed to avoid the needing for hazardous components in designing, developing, and implementing of chemical processes and products [50, 51]. Therefore, recent researches were reported for obeying the green chemistry concepts in synthesis numerous of nanomaterials using environment-friendly materials instead of toxic chemicals [2, 3, 20, 21, 23, 28, 30, 61,62,63]. Additionally, the use of plant extracts for synthesis of nanomaterials is described to be competitive with any of the other conventional methods. However, nanostructures synthesized by exploiting the different plant extracts were found to be characterized by appealing properties, but unfortunately, their applications are usually hindered by poor colloidal stability upon storage for a long time. This disadvantage can be overcome by dispersing such nanostructures in biopolymer solution of natural gums [66].
Arabic gum is one of the natural, highly branched, neutral or slightly acidic arabinogalactan polysaccharides, with a high molecular weight, and is mainly obtained from the stems and branches of Arabic Senegal tree. Arabic macromolecule basically consists of three main fractions: the major fraction is a highly branched polymer of a β-(1–3)-galactose backbone with inter-branch of arabinose and rhamnose, to be terminated with glucuronic acid. The second one (nearly equal to 10 wt% of the total) is mainly composed of arabinogalactan–protein complex [GAGP (Gum Arabic-Glycoprotein)], in which arabinogalactan chains are covalently linked to a protein chain through serine and hydroxyproline groups [38, 40].
Also, the functional group (–OH) present in arabinose and rhamnose, and –COOH of glucuronic acids play a crucial role in the functionality of such type of gum, as it’s the main generator of nanostructures, and subsequently, the proteinaceous core with amino acids acts in stabilizing the as-produced nanostructures [66]. Therefore, it has been widely applied as a generator for various metal nanomaterials, such as carbon nanotubes [5], nanogold [34], nanosilver [39, 60], nanopalladium [15, 56], nanoplatinum [55], oxide nanoparticles [65]. Additionally, it showed a super capability in the production of quantum dot nano colloids [48], and as a crystal growth modifier for metal carbonates [57]. Different gums as biopolymers have been applied in synthesis of only monometallic nanostructures, such like, Acacia gum [15, 23, 34, 39], xanthan gum [28] and guar gum [1]. However, according to our knowledge, there is no reports were published for studying the employment of Arabic gum in synthesis of bimetallic nanostructures.
Synthesis of bimetallic nanostructures is considered in order to exploit unique properties for each of the nanometals for different purposes [37, 45]. Manufacturing of bimetallic Au–AgNPs was especially studied in some of the recent reports due to its surface plasmon resonance (SPR) band which is mainly observed in between 410 and 520 nm [8, 12]. Ag–Au nanostructures are advantageous by combining the physical and chemical reactivity of both nanometals. Additionally, size-controlled/bimetallic nanostructures were investigated to be capable of acting as auto-fluorescent [53, 67] and biosensors rather than individual nanometals [32]. Hence, synthesis of small-sized Ag–Au bimetallic nanostructures of a great challenge due to their precise composition and high surface activity.
The most recent researches considered with synthesis of bimetallic Ag–Au nanostructures represented various methodologies including; wet chemical synthesis by co-reduction of Au and Ag salts with citrate [46], NaBH4 [49] or starch and glucose [52], reduction with hydrazine in water–oil emulsions [10], sol–gel processes [14] or UV irradiation [29]. However, most such reported procedures could be ascribed as complex, expensive and required the employment of different strong ligands as stabilizers for nanostructures [64]. One of the as-mentioned methods is the co-reduction process, which is expressed as the most favorable method compared to the others, for the manufacturing of Au–Ag nanostructures. Such a method is basically relied on using reducers and capping agents [45]. Moreover, it has been reported that; in such method, controlling of the experimental conditions such as concentration, variation in time, pH, pressure, and temperature could result in the manufacturing of nanostructures, variable in morphological growth, size, chemical reactivity and biological properties [9, 16, 35, 47]. Unlike the simultaneous or co-reduction method, seed-mediated growth technique successfully produces bimetallic nanostructures in predetermined core–shell or alloyed particles [58]. Seed-mediated growth for synthesizing of bimetallic nanostructures relies upon reduction one of the metal component over the other pre-nucleated metal component, referred to as the seed.
The current study aims to represent quite simple, energy saving, timeless, solventless and cost-effective strategy for the manufacturing of Ag–Au bimetallic nanocomposite by employing Arabic gum. The as-used gum was employed as bio-synthesizer and crystal growth modifier of the required bimetallic nanocomposite via seed-mediated growth technique. The superior role of the as-mentioned gum in manufacturing Ag–Au bimetallic nanocomposite was confirmed through the instrumental analyses of the so-manufactured nanocomposite using the UV–Visible spectrophotometer, TEM, Zetasizer, XRD, FTIR, and NMR mapping data. Size distribution of the so-produced nanocomposite was estimated from TEM micrographs and Zetasizer data. XRD, FTIR and NMR data were represented for confirming the redox reaction between gum macromolecules and metals precursors for manufacturing the as-mentioned nanostructures. Based on the data obtained, the preparation mechanism of Ag–Au bimetallic nanocomposite was suggested and presented.
Experimental
Materials and Chemicals
Silver nitrate (AgNO3, 99.5%, from Panreac, Barcelona—Spain), Gold chloride (AuCl3, 99%, from, Bombay—India), Sodium hydroxide (99%, from Merck, Darmstadt—Germany), and Arabic gum (El-Nasser Company for Pharmaceuticals and Chemicals, Egypt) were all used without any further purification.
Procedure
Definite weights of Arabic gum (1 and 2 g) were dissolved in one liter of distilled water. To 50 mL of gum solution, 0.5 mL of metal salts solution (0.1 M) was added drop-wisely under magnetic stirring. Sodium hydroxide (100 mmol/L) as strong alkali was separately added to the reaction liquor. The addition sequencing of alkali and metal salts were interchanged in order to investigate its effect on the nanocluster growth (keeping in mind that the total volume of the reactants liquor is 50 mL). The reaction was kept under continuous stirring for 30 min at room temperature (RT) or at 70 °C. Table 1 includes the preparation conditions of Ag–Au bimetallic nanostructure and the addition sequencing of materials. The progression of the reaction was monitored by UV–Visible absorption; aliquots from the reaction bulk were withdrawn at given time intervals and have been evaluated.
After preparation of bimetallic nanocomposite as a colloidal solution, Ag–Au bimetallic nanocomposite in the powder form was obtained by transferee the colloidal solutions to a petri dish, followed by drying at 100°Cover night. The obtained powder was used in the analyses of X-ray diffraction, Fourier transformation infrared and nuclear magnetic resonance.
Analysis and Characterization
UV–Visible Spectra
Ag–Au bimetallic nanomaterials colloidal solutions were manifested by an intense absorption peak due to the surface plasmon resonance (SPR). Thus the UV–visible absorption spectra were used for giving information of successive generation of nanostructures in dispersed form. The UV–Visible absorption spectra of nano-structural colloids were measured in the wavelength range of 250–750 nm using a multi-channel spectrophotometer (T80 UV/VIS, d = 10 mm, PG Instruments Ltd, Japan).
Transmission Electron Microscopic (TEM)
Topographical features and size distribution of the so-synthesized Ag–Au bimetallic nanocomposite were monitored by anticipating of a JEOL-JEM-1200 (High-Resolution Transmission Electron Microscope from Japan). The colloidal solutions were carefully dropped on a 400 copper grid coated by carbon film and then evaporated in air at room temperature before conducted in the microscope. The diameter and distribution size of the prepared bimetallic nanocomposite were calculated by 4 pi analysis software using TEM photos. The average diameter of the nano-objects was determined at least from 50 particles.
Zetasizer
The average size, size distribution and polydispersity index of the synthesized Ag–Au bimetallic nanocomposite were all measured by using Zetasizer (Malvern Zetasizer Nano ZS, from Malvern Instruments Ltd—UK). The instrument was attached with a He-Ne laser lamp (0.4 mW) at the wavelength of 633 nm. Measurements were performed at 25 °C in an insulated chamber using dynamic light scattering technique.
X-ray Diffraction (XRD)
Both of the pristine gum and bimetallic nanocomposite were analyzed by powder X-ray diffraction using X’Pert MPD diffractometer system from Philips, at room temperature. Diffraction patterns were detected in the diffraction angle (2θ) range of 3.5–50° using monochromatized (CuKα X-radiation at 40 kV, 50 mA, and λ = 1.5406 Å) with a step size of 0.03° and scanning rate of 1 s.
Fourier Transformation Infrared (FTIR)
Infrared spectra were examined for pristine gum and bimetallic nanocomposite using infrared Spectrometer (Jasco FT/IR 6100) conducted to the detector of deuterated triglycine sulfate (TGS). The spectra were collected in the range of 4000–400 cm−1 using transmission mode (T%), resolution of 4 cm−1 with 1 cm−1 interval scanning and scanning speed of 2 mm/s.
Nuclear Magnetic Resonance Spectroscopy (NMR)
The spectra of NMR (1H NMR&13C NMR) for the bimetallic nanocomposite were recorded on Bruker Avance 300 spectrometer (300 MHz for 1H NMR and 75 MHz for 13C NMR). Solutions were prepared by adding 7 mg of powder samples in 500 µLof d6-DMSO.
Biological Activity
Biological efficiency of the produced Ag–Au bimetallic nanocomposite were quantitatively examined versus selected gram positive and gram negative bacterial strains, by using the ASTM standard test method according to the dynamic flask test method the [4]. In this experiment, two different bacterial strains (Escherichia coli ATCC-25922 as gram − ve bacteria and Staphylococcus aureus ATCC 47077 as gram + ve bacteria) were used. In brief, microbial suspensions of 2.4–3.0 × 108 CFU/mL from the selected bacterial strains were prepared in buffered KH2PO4 solution with pH 7.2. An exact volume (0.5 mL) was added in microbial solution (20 mL) at 25 °C, and then was vigorously shaken. A 1 mL from the microbial suspension was taken, dropped to nutrient agar and then incubated at 37 and 30 °C, respectively. After incubation for different time (2, 4, 24 h), colonies of bacteria were counted and the biological activities of composites were estimated by using Eq. 1.
where R% is the reduction in survived microbial colonies, A is the number of microbial colonies alive on the agar plate, and B is the number of microbial colonies on the agar plate from control experiments.
Results and Discussion
For bimetallic nanostructure, silver and gold were in-grown simultaneously, and a complete bimetallic nanostructure is supposed to be synthesized as gold and silver are in the same group of the periodic table. Moreover, they have the same structures and similar atomic radius which might be resulted in full miscibility within well-dispersed alloyed nanostructures. Therefore, the natural biopolymer of Arabic gum was herein employed as bio-synthesizer in synthesizing of Ag–Au bimetallic nanocomposite via using seed-mediated growth technique. Sodium hydroxide as strong alkali was used in order to increase the reducibility of gum macromolecules and regulating the nucleation and crystal growth of the as-produced bimetallic nanostructures.
UV–Visible Spectroscopic Analyses
The detection of UV–Visible spectra could give an information about the successive growth of bimetallic nanostructures. Mixing for the solutions of the two prepared nanostructure is detected in the UV–Visible spectrum as two separated SPR bands. However, when the two nanoparticulate metals interact to produce bimetallic nanostructure, the as-mentioned bands have not existed and a single characteristic band is observably detected [29].
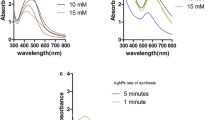
The preliminary detection of Ag–Au nanocomposite was carried out by visual observation of the color changes for the reaction liquors. These changes were correlated to the excitation of surface plasmon resonance (SPR) for the bimetallic nano objects [29]. Typically, UV–Visible absorption is used to investigate SPR for Au and Ag nano colloids. For employing of Arabic gum in the manufacturing process, the influences of gum concentration, metal salts concentrations, additional sequencing of metal salts and alkali, reaction duration and temperature on the synthesis of bimetallic nanocomposite were investigated by an illustration of absorption spectra for the as-prepared nano-colloidal solutions (Fig. 1). According to pieces of literature; significant SPR bands are mainly detected near 520–560 nm for Au [2, 3, 12, 20] and at 400–420 nm for Ag [8, 21, 23, 28, 30], when such referred nanometals are separately prepared in colloidal form. However, the spectral data represented in Fig. 1 showed that a single SPR peak is observed in the range of 480–495 nm which is characteristic for Ag–Au bimetallic nanocomposite [29]. This foundation gives a confirmation about the successive clustering of alloyed Ag–Au bimetallic nanocomposite by using Arabic gum as bio-synthesizer.
Additionally, by referring to the plotted data; the concentration of gum and addition sequencing for both of metal salts and alkali, were significantly reflected in shifting and broadening of the as-mentioned band. The increment of gum concentration from 1 g/L to 2 g/L resulted in considerable band broadening. The addition of silver salt firstly reflected in red shifting and sharpening of the so-detected SPR band. By the addition of sodium hydroxide after salts, a blue shift and more broadened SPR peak were observed. While sharper and red shifted SPR peak was obtained by raising the reaction temperature to 70 °C. These findings could be interpreted as follows; (i) increment of Arabic gum concentration resulted raising of the viscosity of the as-prepared reaction solution, which might result in steric effects to produce much enlarged and agglomerated nanostructures; (ii) addition of silver salt firstly, results in nucleation of silver nanostructure, which is predicted to act as active surface area for starting the growth of gold nanocluster, for generating higher size controlled nanostructures, (iii) more of band broadening is observably detected when gold salt is added firstly, attributing to the differences in the standard reduction potentials between Ag and Au metals, which may sign for the production of the nanocomposite differently in size and shape [29]; (iv) addition of alkali before metal salts is supposed to activate the as-used gum polymeric chains to be more powerful nano-synthesizer, for generating more sized regulated and highly stabilized nano-particulate structures, and, (v) raising the reaction temperature to 70 °C is supposed to acts in providing high thermal energy for accelerating the nucleation of more size-regulated bimetallic nanocomposite [2, 3, 17, 20, 23].
Zetasizer Analysis
Zetasizer recorded data for size distribution and the polydispersity index of bimetallic nanostructures, which were synthesized by employing Arabic gum are presented in Fig. 2. The figured out analyses were detected for selected samples (S2, S3 and S7), in order to clarify the effect of reaction conditions on the particle size of the produced nanostructures. The data revealed that Arabic gum was successfully exploited as concurrent bio-synthesizer and cluster growth modifier of bimetallic nanostructures. The addition of alkali after metal salts resulted in much-enlarged nanocomposite to be increased from 3.154 nm (S2) to 4.036, 6.612 and 12.70 nm (S3). While the increment of Arabic gum concentration reflected in the production of greatly enlarged nanocomposite in two separated peaks with mean sizes of 22.79 and 62.06 nm (S7). These foundations are in accordance with that of UV–Visible spectral data.
The homogeneity in the dispersion of bimetallic nano colloid can be estimated by the polydispersity index (PdI). The as-prepared Ag–Au bimetallic nanocomposite as the colloidal solution may have some of the uniformity when their shape and size are regular. Therefore, regardless of the reaction conditions of the preparation process, the so-obtained bimetallic nanocomposite showed good stability, as the value of PdI was ranged in 0.203–0.530. The best stability of solution recorded primarily by detecting the PdI value of 0.3 [2, 3] which is observed for S2.
TEM Micrographs
For further confirmation, the topographical features of the synthesized bimetallic nanocomposite were presented by TEM micrographs (Fig. 3) for selected samples (S1, S2 and S3) according to zetasizer data. The represented data in Fig. 3 confirmed that all the as-tested samples contained well-dispersed Ag–Au bimetallic nanostructures. TEM data further affirmed that Arabic gum is an excellent concurrent nano-synthesizer and crystal growth modifier for preparation of size-regulated, stable and spherical shaped bimetallic Ag–Au nanostructures. From Fig. 3b, raising reaction temperature reflected in well dispersed and smaller sized bimetallic nanocomposite to be diminished from 6.5 nm (size distribution; 3–12 nm) for S1 to 3.1 nm (size distribution; 1–7 nm) for sample S2. The identical average size of bimetallic nanocomposite (S2) detected by zetasizer and TEM micrograph reflects the homogeneity of the prepared sample and the accuracy of measurements. However, production of heterogeneous-sized bimetallic nanocomposite was obtained by addition of alkali lastly (S3) with average sizes of 6.6 and 14.9 nm (size distribution; 4–20 nm). All of these observations are in accordance with that illustrated for UV–Visible spectral and zetasizer data.
XRD Patterns
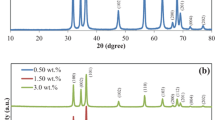
Arabic gum and Ag–Au bimetallic nanocomposite (S2) were characterized by XRD analyses and results are represented in Fig. 4. The pristine gum was detected with two intense diffraction peaks at 2θ = 7.7° and 18.5° which are signing for the amorphous structure of Arabic gum [31]. In case of Ag–Au bimetallic nanocomposite, the crystalline structure of gum was fully distorted and the as-mentioned diffraction peaks disappeared owing to the redox reaction between gum macromolecules and metal salts.
Additionally, numerous of intense diffraction peaks were observed at 2θ = 26.3°, 27.8°, 30.3°, 31.9°, 33.4°, 34.6°, 35.4°, 38.1°, 40.1°, 41.7°, 44.6°, 45.7, 46.7°, and 48.5°, 53.7°, 54.8°, 56.7° for bimetallic nanocomposite. These diffraction peaks are corresponding to Ag and Au nanostructure. According to diffraction data of international center, diffraction patterns at 38.1° and 44.6° are referring to (111) and (200) of face-centered crystalline (FCC) structures for silver and gold (JCPDS data number 04-0783 card and 4-0784 card) [2, 18, 20, 33, 36, 41, 68]. These diffraction analyses further confirmed the data of absorbance spectra, zetasizer, and micrographs for successful preparation of Ag–Au bimetallic nanocomposite by using Arabic gum macromolecules.
FTIR
The suggestion of the reaction mechanism for the redox reaction between metal ions and gum macromolecular chains during the manufacturing of Ag–Au bimetallic nanocomposite could be achieved through, the detection of the change in the molecular structure of gum backbone, which in turn could be formerly showed in the data of infrared spectra. The FTIR spectra of native Arabic gum and Arabic gum after its employment as bimetallic nano-synthesizer were shown in Fig. 5. From the represented spectra, it could be described that a broad absorption band located at 3291 cm−1, is a characteristic band of the glycosidic ring and referring to the stretching vibration of O–H. The absorption peak at 2889 cm−1, is attributed to the C–H stretching vibration, while, the COO-asymmetric stretching is detected at 1586 cm−1. The detected peak at 1182–907 cm−1 is a fingerprint of carbohydrates, which assigned to in-plane bending of C–H (wagging of CH=CH and wagging of CH2) [13, 44, 54].
The FTIR spectrum of Ag–Au bimetallic nanocomposite clarified that (i) the observable band at 3291 cm−1 is showed to be greatly broadened with smaller intense, (ii) the absorption peak at 2889 cm−1 is completely disappeared, (iii) the intensities of absorption bands at 1586 cm−1 and at 1182–907 cm−1 are observably decreased, (iv) additionally, two new sharpened bands at 1419 cm−1 and 876 cm−1 are detected, referring to C=O stretching of carbonyl groups and, stretching mode of carbonate ion (COO-M), respectively [54]. Therefore, the spectral data approved the successive exploitation of carboxylate and hydroxyl groups of Arabic gum to strongly interact with metal ions and assisted in crystal growth of Ag–Au bimetallic nanocomposite [54].
NMR Spectral Analyses
The identification of the chemical structure for a certain gum, an extensive number of analytical tests needs to be performed; one such referred analyses is NMR spectral data. Hence, NMR analyses were also carried out in order to approve the interchanging in the chemical formula of gum macromolecules by its employment in the production of bimetallic nanostructures. Figure 6 shows 1H and 13CNMR spectra of Acacia gum before and after of production of bimetallic nanostructures.
Figure 6a, b represent 1HNMR spectral data for blank gum and Ag–Au bimetallic nanocomposite, respectively. The crowded signals in the 1HNMR spectrum between 3 and 8 ppm are typical for polysaccharides and consequently reflect the presence of similar sugar residues, for each of blank and the nano-synthesizer gum. Signals that detected at 3.3–3.8 ppm are expressed to the presence of –O–CH2. Additionally, the non-anomeric protons (H2–H6) are assigned between 3.3 and 4.7 ppm. For the bank gum sample, the high intense band at 4.4–4.70 ppm is related to the presence of H2O. In the anomeric region (4.8–5.19 ppm), the detected peaks are significant for pristine acacia gum [43]. While for Ag–Au bimetallic nanocomposite, a prominent peak at 8.48 ppm corresponding to ketones and aldehydes (flavanones, terpenoid aldehydes) is detected, which confirms the redox reaction between silver and gold metal ions and the alcoholic groups of the employed gum macromolecular chains.
From 13CNMR spectra (Fig. 6c, d), both of Arabic gum and Ag–Au bimetallic nanocomposite, the signals due to the mono-saccharides non-anomeric carbons C2–C5, and anomeric carbons were detected at 60–85 ppm [42] and 90–110 ppm [11], respectively. The peak at 181 ppm reflects the presence of the amide group of protein portion in gum.
For Ag–Au bimetallic nanocomposite spectra, the peak arouses around 175 ppm is attributed to uronic acid typical of the C6 signal. Additionally, a significant peak at 23.37 ppm is basically belonging to the carbon of methylene group of rhamnose [11]. While, the peaks at 171, 160 and 96 ppm were appeared for the unsaturated compounds, reducing sugars and carbonyl groups, respectively [54]. These foundations give more confirmation about the successive redox reaction between the metal ions and the reducing building units of gum macromolecules.
The Suggested Reaction Mechanism
According to the previously illustrated data, the pristine Arabic gum as a natural biopolymer was successively employed nano-synthesizer for clustering of well dispersed-Ag–Au bimetallic nanostructures. The reaction mechanism between metal ions and the as-used gum was suggested and designed in Fig. 7. Referring to the designed diagram, Gum macromolecules were concurrently employed as nanoparticles’ synthesizer and crystal growth modifier, where, it could be ascribed as a template for bimetallic nanostructures’ clustering. In accordance with works of literature [2, 3, 19,20,21,22,23, 25,26,27, 30]; in alkaline media, alcoholic groups were well known to be de-protonated, and the ionic gum macromolecules were assumed to be covalently coordinated with to Ag or Au ions for further reduction and stabilization. Additionally, raising the reaction temperature in alkaline medium resulted in enhancement of the reactivity of gum macromolecules and consequently the redox reaction with metal ions is supposed to be more accessible. The reducible groups of gum building blocks were assumed to reduce Ag+ 1 and Au+ 3 to Ag0 and Au0, respectively in nano-sized dimension. While, such groups are oxidized to carbonyl (aldehydic and/or ketonic groups), and subsequently employed with carbonate groups to assist in coordination and crystal regulation of the as-produced bimetallic nanocomposite [21, 22, 24]. However, the effect of reaction sequencing could be illustrated as follows:
-
I.
When Ag salt was first added to reaction liquor followed by Au salt:
-
Ag ions are assumed to be formerly reduced by the action of reducible gum macromolecules.
-
When Au salt was then added, the positive potential of the redox reaction resulted in a spontaneous exchanging of Ag by Au. But, due to the difference in atomic valency, one Au atom is supposed to replace three of Ag atoms. This assumption could be attributed to the ionization potential and electron affinity values of Au atoms which is well known to be higher than that of the Ag atoms [59]. Additionally, the higher electro-negativity of Au atom compared to that of Ag results in effective charge transfer from Ag0 to Au+ 3.
-
Subsequently, the generated AuNPs were supposed to be as a seed for the successive growth of AgNPs. Therefore, it could be summarized that the consequent seed- mediated growth of Au and Ag nanostructure by employing of gum functional groups in addition to the inter-dispersion of Au and Ag, results in the generation and growth Ag–Au bimetallic nanocomposite.
-
II.
In case of adding Au salt to reaction liquor firstly followed by Ag salt:
-
In case of adding Au salt to the reaction liquor, gold ions were supposed to be reduced to Au0 by the alcoholic groups of gum nano-synthesizer.
-
Subsequent addition of Ag salt to the as-prepared reaction liquor suggested to be reflected in co-reduction silver ions along with gold ions, and nanosilver generated particles are supposed to be successfully grown and clustered on the surface of gold seeds. Where Au0 nanosized particles could be ascribed to play as seeds or active sites for immediate growth of Ag0 nano-objects. With raising the reaction temperature, bimetallic nanocomposite will grow gradually in a more regulated and smaller sized form to be well dispersed and stabilized by the as-employed polymer macromolecular chains [2, 3, 30].
Antibacterial Action
The biological activity of the so-produced Ag–Au bimetallic nanocomposites was studied for three selected samples (S2, S3 and S7) considering different particle sizes (3.1, 6.6/14.9 and 22.1). Two different bacterial strains (E. coli as gram − ve and S. aureus as gram + ve) were chosen in this test. The biological properties data were tabulated in Table 2. Arabic gum wasn’t exhibited any antibacterial properties, while, all bimetallic nanocomposites showed antibacterial action against the selected bacterial strains. From the tabulated data it could be pointed out that; (i) the reduction percentage in the number of the survived microbes was increased by increment of the incubation time, (ii) the reduction in grame + ve bacteria was rationally higher than that of grame − ve bacteria, (iii) at lower incubation time, small size bimetallic nanocomposite showed much higher bacterial reduction compared to the bigger size and the bacterial reduction reached 83.2–87% after only 6 h incubation for bimetallic nanocomposite with size of 3.1 nm and (iv) the difference in bacterial reduction between the tested samples was insignificant after 24 h incubation time and the reduction percentage in bacteria was ranged in 95.3–100. It could be summarized that, the antibacterial activities of the produced bimetallic nanocomposites are indirectly proportional to the particle size at lower incubation time. However, longer incubation time, resulted in nearly full reduction in the growth of the bacterial cells for all tested samples. Compared to the individual nanoparticles (Ag and Au) and Ag–Au bimetallic reported in literature [3, 6, 7; MubarakAli et al. 2011, 7], the results of antibacterial efficacy for Ag–Au bimetallic nanocomposite were better which may be attributed to the bi-action of the two NPs (Ag and Au) and the smaller size of nano composite, respectively.
Conclusion
Herein, the represented study has successfully worked on the introduction of an efficient, environmentally friendly «green» methodology for the production of Ag–Au bimetallic nanocomposite using Arabic gum. Gum macromolecules were approved to be capable of playing the dual role of nanostructures’ generator and crystal growth modifier, via seed growth method. The preparation of nanocomposite was detected by measurements of UV–Vis absorbance spectra and confirmed by TEM micrographs, zetasizer analyses, and XRD patterns. The obtained results showed that the particle size of the as-synthesized Ag–Au bimetallic nanocomposite can be interchanged by the effect of gum concentration, the reaction temperature and sequencing for the addition of materials. Additionally, FT-IR, 1HNMR, 13CNMR spectra and XRD data were all represented for interpreting the reaction mechanism of the redox reaction, supposed to take a place between metal ions and gum building units. According to the detected data, the metal ions were supposed to be reduced to metallic form through employing the reducible groups of the as-employed gum macromolecules, which in turn were oxidized to carbonyl groups (aldehydic and/or ketonic). Regardless to their size, the prepared Ag–Au bimetallic nanocomposites showed excellent antibacterial activity against both of gram + ve and gram − ve strains and 95.3–100% of bacterial strains were diminished after incubation for 24 h. Eventually, this study presents an eco-friend, simple, energy saving and cost-effective strategy for production of Ag–Au bimetallic nanocomposite. Moreover, such technique can be widely-scaled applicable for production of other bimetallic nanostructures, which is predicted to be of high important for extensive applications.
References
Abdel-Halim E, El-Rafie M, Al-Deyab SS (2011) Polyacrylamide/guar gum graft copolymer for preparation of silver nanoparticles. Carbohydr Polym 85(3):692–697
Ahmed HB, Abdel-Mohsen A, Emam HE (2016) Green-assisted tool for nanogold synthesis based on alginate as a biological macromolecule. Rsc Adv 6(78):73974–73985
Ahmed HB, Zahran M, Emam HE (2016) Heatless synthesis of well dispersible Au nanoparticles using pectin biopolymer. Int J Biol Macromol 91:208–219
ASTM (2001) E2149-10: standard test method for determining the antimicrobial activity of immobilized antimicrobial agents under dynamic contact conditions. ASTM, West Conshohocken, PA
Bandyopadhyaya R, Nativ-Roth E, Regev O, Yerushalmi-Rozen R (2002) Stabilization of individual carbon nanotubes in aqueous solutions. Nano Lett 2(1):25–28
Banerjee M, Sharma S, Chattopadhyay A, Ghosh SS (2011) Enhanced antibacterial activity of bimetallic gold-silver core–shell nanoparticles at low silver concentration. Nanoscale 3(12):5120–5125
Bankura K, Maity D, Mollick MMR, Mondal D, Bhowmick B, Roy I, Midya T, Sarkar J, Rana D, Acharya K (2014) Antibacterial activity of Ag–Au alloy NPs and chemical sensor property of Au NPs synthesized by dextran. Carbohydr Polym 107:151–157
Bright RM, Musick MD, Natan MJ (1998) Preparation and characterization of Ag colloid monolayers. Langmuir 14(20):5695–5701
Carnes CL, Klabunde KJ (2000) Synthesis, isolation, and chemical reactivity studies of nanocrystalline zinc oxide. Langmuir 16(8):3764–3772
Chen D-H, Chen C-J (2002) Formation and characterization of Au–Ag bimetallic nanoparticles in water-in-oil microemulsions. J Mater Chem 12(5):1557–1562
Cui SW (2005) Food carbohydrates: chemistry, physical properties, and applications. CRC Press, Boca Raton
Daniel M-C, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104(1):293–346
Daoub RM, Elmubarak AH, Misran M, Hassan EA, Osman ME (2016) Characterization and functional properties of some natural Acacia gums. J Saudi Soc Agric Sci. https://doi.org/10.1016/j.jssas.2016.05.002
Devarajan S, Bera P, Sampath S (2005) Bimetallic nanoparticles: a single step synthesis, stabilization, and characterization of Au–Ag, Au–Pd, and Au–Pt in sol–gel derived silicates. J Colloid Interface Sci 290(1):117–129
Devi DK, Pratap SV, Haritha R, Sivudu KS, Radhika P, Sreedhar B (2011) Gum acacia as a facile reducing, stabilizing, and templating agent for palladium nanoparticles. J Appl Polym Sci 121(3):1765–1773
Devi GS, Subrahmanyam VB, Gadkari S, Gupta S (2006) NH3 gas sensing properties of nanocrystalline ZnO based thick films. Anal Chim Acta 568(1–2):41–46
El-Rafie M, Ahmed HB, Zahran M (2014) Facile precursor for synthesis of silver nanoparticles using alkali treated maize starch. Int Sch Res Not. https://doi.org/10.1155/2014/702396
Elumalai EK, Kayalvizhi K, Silvan S (2014) Coconut water assisted green synthesis of silver nanoparticles. J Pharm Bioallied Sci 6(4):241
Emam HE, Ahmed HB (2016) Polysaccharides templates for assembly of nanosilver. Carbohydr Polym 135:300–307
Emam HE, Ahmed HB (2018) Carboxymethyl cellulose macromolecules as generator of anisotropic nanogold for catalytic performance. Int J Biol Macromol 111:999–1009
Emam HE, El-Bisi M (2014) Merely Ag nanoparticles using different cellulose fibers as removable reductant. Cellulose 21(6):4219–4230
Emam HE, El-Hawary NS, Ahmed HB (2017) Green technology for durable finishing of viscose fibers via self-formation of AuNPs. Int J Biol Macromol 96:697–705
Emam HE, El-Rafie M, Ahmed HB, Zahran M (2015) Room temperature synthesis of metallic nanosilver using acacia to impart durable biocidal effect on cotton fabrics. Fibers Polym 16(8):1676–1687
Emam HE, El-Zawahry MM, Ahmed HB (2017) One-pot fabrication of AgNPs, AuNPs and Ag–Au nano-alloy using cellulosic solid support for catalytic reduction application. Carbohydr Polym 166:1–13
Emam HE, Mowafi S, Mashaly HM, Rehan M (2014) Production of antibacterial colored viscose fibers using in situ prepared spherical Ag nanoparticles. Carbohydr Polym 110:148–155
Emam HE, Saleh N, Nagy KS, Zahran M (2015) Functionalization of medical cotton by direct incorporation of silver nanoparticles. Int J Biol Macromol 78:249–256
Emam HE, Saleh N, Nagy KS, Zahran M (2016) Instantly AgNPs deposition through facile solventless technique for poly-functional cotton fabrics. Int J Biol Macromol 84:308–318
Emam HE, Zahran MK (2015) Ag0 nanoparticles containing cotton fabric: synthesis, characterization, color data and antibacterial action. Int J Biol Macromol 75:106–114
Gonzalez CM, Liu Y, Scaiano J (2009) Photochemical strategies for the facile synthesis of gold–silver alloy and core–shell bimetallic nanoparticles. J Phys Chem C 113(27):11861–11867
Hebeish A, El-Rafie M, Abdel-Mohdy F, Abdel-Halim E, Emam HE (2010) Carboxymethyl cellulose for green synthesis and stabilization of silver nanoparticles. Carbohydr Polym 82(3):933–941
Hindi S, Albureikan MO, Al-Ghamdi AA, Alhummiany H, Ansari MS (2017) Synthesis, characterization and biodegradation of gum Arabic-based bioplastic membranes. Nanosci Nanotechnol 4(2):32–42
Huang J, Vongehr S, Tang S, Lu H, Shen J, Meng X (2009) Ag dendrite-based Au/Ag bimetallic nanostructures with strongly enhanced catalytic activity. Langmuir 25(19):11890–11896
Jiang G, Wang L, Chen W (2007) Studies on the preparation and characterization of gold nanoparticles protected by dendrons. Mater Lett 61(1):278–283
Kattumuri V, Katti K, Bhaskaran S, Boote EJ, Casteel SW, Fent GM, Robertson DJ, Chandrasekhar M, Kannan R, Katti KV (2007) Gum Arabic as a phytochemical construct for the stabilization of gold nanoparticles: in vivo pharmacokinetics and X-ray-contrast-imaging studies. Small 3(2):333–341
Lee J, Easteal A, Pal U, Bhattacharyya D (2009) Evolution of ZnO nanostructures in sol–gel synthesis. Curr Appl Phys 9(4):792–796
Litvin VA, Minaev BF (2014) The size-controllable, one-step synthesis and characterization of gold nanoparticles protected by synthetic humic substances. Mater Chem Phys 144(1–2):168–178
Liu X, Wang A, Wang X, Mou C-Y, Zhang T (2008) Au–Cu alloy nanoparticles confined in SBA-15 as a highly efficient catalyst for CO oxidation. Chem Commun 27:3187–3189
López-Franco YL, Gooycolea FM, Lizardi-Mendoza J (2014) Gum of Prosopis/Acacia species. In: Ramawat K, Mérillon JM (eds) Polysaccharides. Springer, Cham. https://doi.org/10.1007/978-3-319-03751-6_14-1
Mohan YM, Raju KM, Sambasivudu K, Singh S, Sreedhar B (2007) Preparation of acacia-stabilized silver nanoparticles: a green approach. J Appl Polym Sci 106(5):3375–3381
Montenegro MA, Boiero ML, Valle L, Borsarelli CD (2012) Gum Arabic: more than an edible emulsifier. In: Verbeek C (ed) Products and applications of biopolymers. InTech. https://doi.org/10.5772/33783
Mott D, Thuy NT, Aoki Y, Maenosono S (2010) Aqueous synthesis and characterization of Ag and Ag–Au nanoparticles: addressing challenges in size, monodispersity and structure. Philos Trans R Soc Lond A 368(1927):4275–4292
Nep EI, Conway BR (2010) Characterization of grewia gum, a potential pharmaceutical excipient. J Excip Food Chem 1(1):30–40
Nie S-P, Wang C, Cui SW, Wang Q, Xie M-Y, Phillips GO (2013) The core carbohydrate structure of Acacia seyal var. seyal (Gum Arabic). Food Hydrocolloids 32(2):221–227
Nie S-P, Wang C, Cui SW, Wang Q, Xie M-Y, Phillips GO (2013) A further amendment to the classical core structure of gum Arabic (Acacia senegal). Food Hydrocolloids 31(1):42–48
Oko DN, Zhang J, Garbarino S, Chaker M, Ma D, Tavares AC, Guay D (2014) Formic acid electro-oxidation at PtAu alloyed nanoparticles synthesized by pulsed laser ablation in liquids. J Power Sources 248:273–282
Pal A, Shah S, Kulkarni V, Murthy R, Devi S (2009) Template free synthesis of silver–gold alloy nanoparticles and cellular uptake of gold nanoparticles in Chinese Hamster Ovary cell. Mater Chem Phys 113(1):276–282
Pal U, Serrano JG, Santiago P, Xiong G, Ucer K, Williams R (2006) Synthesis and optical properties of ZnO nanostructures with different morphologies. Opt Mater 29(1):65–69
Park C-S, Lim K-H, Kwon D-W, Yoon T-H (2008) Biocompatible quantum dot nanocolloids stabilized by gum Arabic. Bull Korean Chem Soc 29(6):1277–1279
Petkov V, Prasai B, Ren Y, Shan S, Luo J, Joseph P, Zhong C-J (2014) Solving the nanostructure problem: exemplified on metallic alloy nanoparticles. Nanoscale 6(17):10048–10061
Poliakoff M, Anastas P (2001) A principled stance. Nature 413(6853):257
Poliakoff M, Fitzpatrick JM, Farren TR, Anastas PT (2002) Green chemistry: science and politics of change. Science 297(5582):807–810
Raveendran P, Fu J, Wallen SL (2006) A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem 8(1):34–38
Ristig S, Kozlova D, Meyer-Zaika W, Epple M (2014) An easy synthesis of autofluorescent alloyed silver–gold nanoparticles. J Mater Chem B 2(45):7887–7895
Sreedhar B, Devi DK, Neetha AS, Kumar VP, Chary K (2015) Green synthesis of gum-acacia assisted gold-hydroxyapatite nanostructures–characterization and catalytic activity. Mater Chem Phys 153:23–31
Sreedhar B, Devi DK, Yada D (2011) Selective hydrogenation of nitroarenes using gum acacia supported Pt colloid an effective reusable catalyst in aqueous medium. Catal Commun 12(11):1009–1014
Sreedhar B, Satyavani C, Keerthi Devi D, Rambabu C, Rao B, Saratchandra Babu M (2011) Bioinspired synthesis of morphologically controlled SrCO3 superstructures by natural gum acacia. Cryst Res Technol 46(5):485–492
Sreedhar B, Vani CS, Devi DK, Rao MB, Rambabu C (2012) Shape controlled synthesis of barium carbonate microclusters and nanocrystallites using natural polysachharide–gum Acacia. Am J Mater Sci 2(1):5–13
Toshima N, Yonezawa T (1998) Bimetallic nanoparticles—novel materials for chemical and physical applications. New J Chem 22(11):1179–1201
Tsai C-H, Chen S-Y, Song J-M, Haruta M, Kurata H (2015) Effect of ag templates on the formation of Au–Ag hollow/core-shell nanostructures. Nanoscale Res Lett 10(1):438
Velikov KP, Zegers GE, van Blaaderen A (2003) Synthesis and characterization of large colloidal silver particles. Langmuir 19(4):1384–1389
Vijayaraghavan K, Ashokkumar T (2017) Plant-mediated biosynthesis of metallic nanoparticles: a review of literature, factors affecting synthesis, characterization techniques and applications. J Environ Chem Eng 5:4866–4883
Vishnukumar P, Vivekanandhan S, Misra M, Mohanty A (2018) Recent advances and emerging opportunities in phytochemical synthesis of ZnO nanostructures. Mater Sci Semicond Process 80:143–161
Vishnukumar P, Vivekanandhan S, Muthuramkumar S (2017) Plant-mediated biogenic synthesis of palladium nanoparticles: recent trends and emerging opportunities. ChemBioEng Rev 4(1):18–36
Wang C, Yin H, Chan R, Peng S, Dai S, Sun S (2009) One-pot synthesis of oleylamine coated AuAg alloy NPs and their catalysis for CO oxidation. Chem Mater 21(3):433–435
Williams DN, Gold KA, Holoman TRP, Ehrman SH, Wilson OC (2006) Surface modification of magnetic nanoparticles using gum Arabic. J Nanopart Res 8(5):749–753
Xue D, Sethi R (2012) Viscoelastic gels of guar and xanthan gum mixtures provide long-term stabilization of iron micro-and nanoparticles. J Nanopart Res 14(11):1239
Yang L, Shang L, Nienhaus GU (2013) Mechanistic aspects of fluorescent gold nanocluster internalization by live HeLa cells. Nanoscale 5(4):1537–1543
Zhang P, Li J, Liu D, Qin Y, Guo Z-X, Zhu D (2004) Self-assembly of gold nanoparticles on fullerene nanospheres. Langmuir 20(4):1466–1472
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author wants to declare that no scientific or financial conflicts of interest exist.
Rights and permissions
About this article
Cite this article
Emam, H.E. Arabic Gum as Bio-Synthesizer for Ag–Au Bimetallic Nanocomposite Using Seed-Mediated Growth Technique and Its Biological Efficacy. J Polym Environ 27, 210–223 (2019). https://doi.org/10.1007/s10924-018-1331-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1331-3