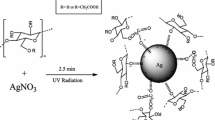
Abstract
Merely silver nanoparticles (AgNPs) were synthesized as a colloidal solution without containing reducing or stabilizing agents using a totally green, one-pot, quite simple method. The unique advantage of this method is the use of a removable reducing agent to produce merely AgNPs. The reducing features and insolubility property of cellulose fibers make them the preferred potential removable reducing agents. Three different cellulosic fibers with different degrees of polymerization, namely viscose, lyocell and cotton fibers, were used. The best results for preparation of AgNPs was obtained by using viscose, followed by cotton then lastly lyocell fibers. When using viscose, the highest surface plasmon resonance peak for AgNPs and small particle size (mean = 9.5 nm) were obtained after 15 min. The carboxyl content of cellulose fibers was increased after treatment with AgNO3, indicating the conversion of reducing groups of cellulose to carboxylic groups by the reduction of Ag+ to Ag0. Results showed that 30 % of AgNPs were aggregated and precipitated after storage for 2 months. The prepared AgNPs were more convenient to use in the medical and biomedical fields as the pure solution does not contain any other chemicals of reducing or stabilizing agents.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The unique properties of metallic silver nanoparticles (AgNPs) make them ideal for numerous applications, technologies and incorporation into a wide array of consumer products. This is probably due to the high surface area to volume ratio and variation of their size and shapes. AgNPs are incorporated into apparel, footwear and wound dressings for their antibacterial properties, in which AgNPs play a critical role in the suppression and killing of various pathogenic microorganisms (Abdel-Mohsen et al. 2012; Emam et al. 2013, 2014; El-Rafie et al. 2014; Zahran et al. 2014c). AgNPs are utilized in biomedical applications (Larguinho and Baptista 2012) and enhance the optical properties of materials (Battie et al. 2011). AgNPs as a metallic state are used in conductive inks and integrated into composites to enhance thermal and electrical conductivity (Hsi-Wen Tien et al. 2011; Alshehri et al. 2012). Because of their surface plasmon resonance, AgNPs are used in the coloration of fibers and fabrics (Emam et al. 2014; Bin et al. 2011, 2013; Big et al. 2012; Watson 2009).
As a result of the huge number of applications for AgNPs, thousands of publications have studied different procedures for the preparation of AgNPs. Most of the synthetic methods for AgNPs reported to date rely heavily on the use of organic solvents and toxic reducing agents such as hydrazine (Sakai et al. 2006), N-dimethylformamide (Pastoriza-sontos and Liz-Marzan 2002) and sodium borohydride (Van Hyning et al. 2001). All these chemicals are highly reactive and pose potential environmental and biological risks. With the increasing interest in minimization/elimination of waste and adoption of sustainable processes, the development of green chemistry approaches is desirable. Increasing the awareness of green chemistry and other biological processes has evoked interest in developing an ecofriendly approach to the synthesis of nanoparticles.
Instead of organic solvents and hazardous reducing agents, dendrimers and hyperbranched polymers have been used as templates to synthesize AgNPs with small size (Castonguay and Kakkar 2010; Richter et al. 2009; Scott et al. 2005). The unique chemical and physical properties, biodegradability and biocompatibility of these polymers with their potential applications in drug and gene delivery are making them very suitable for preparation of AgNPs for applications in the medical field (Gao and Yan 2004; Menjoge et al. 2010). However, their high price and difficulty of the preparation steps have retarded their utilization in the synthesis of metal nanoparticles and make them not commercially viable.
Like dendrimers and hyperbranced polymers, polysaccharide materials are biocompatible and biodegradable. The sustainability, low cost and availability of large-scale commercial production of polysaccharides have given them high priority in the field of metal nanoparticle preparation. Recently, many reports have been published on the preparation of AgNPs by simple techniques using polysaccharide materials such as carboxymethyl cellulose (Hebeish et al. 2010), chitosan (Abdel-Mohsen et al. 2012), cellulose (Emam et al. 2013, 2014), schizophyllan (Abdel-Mohsen et al. 2014), starch (El-Rafie et al. 2014), alginate (Zahran et al. 2014a, c) and pectin (Zahran et al. 2014b).
Compared to the previous studies cited above, the current work presents a novel approach to synthesizing a merely AgNP colloidal solution using different cellulosic fibers. The novelty of manufacturing of an AgNP colloidal solution is that it does not contain reducing or stabilizing agents and involves a quite simple one-pot process. The cellulose fibers act as removable reducing agents. The prepared AgNPs were characterized using UV-Vis absorption spectra and transmission electron microscopy (TEM). Aging with time up to 120 days was tested. The reduction process was monitored by measuring the carboxyl contents for cellulose fibers before and after the reduction reaction.
Experimental
Materials and chemicals
Regenerated cellulosic fibers, namely lyocell staple fibers (CLY, TENCEL®) and viscose fibers (CV, Lenzing, Viscose®) of linear density 1.3 dtex and length 38 mm, respectively, were both kindly provided by Lenzing AG (Lenzing, Austria). The regenerated cellulose fibers did not contain any spin finish and were used without further treatment. In addition, a cotton fiber (CO, Giza 85) with different degrees of polymerization (DP) (960 and 1,850) was kindly provided by the Cotton Research Institute (Giza, Egypt).
Silver nitrate (99.5 %, from Panreac, Barcelona, Spain) and sodium hydroxide (99 %) were all used without further purification.
Procedure
Silver nanoparticles (AgNPs) were prepared using different cellulosic fibers (viscose, lyocell and cotton) using a simple technique described as follows: a known weight of cellulosic materials was immersed in 100 ml of 0.01 N NaOH with stirring, and then the reaction temperature was raised to 70 ± 3 °C. Then 1 mmol/l of silver nitrate solution was added dropwise to the reaction mixture with continuous stirring. After 15 min, cellulosic materials were taken out, and the reaction mixture was kept under continuous stirring for an additional 15 min. The progression of the reaction was controlled by detecting the change in the color of the solution. Thus, the absorption spectra were measured for reaction solution at different time intervals from the addition of silver nitrate. For all solutions, ten dilutions were carried out before the measurements. The removed cellulosic materials were rinsed by tap water for neutralization and then dried at 75 ± 5 °C prior to further characterization.
Measurements
Absorbance of solutions
According to the surface plasmon resonance (SPR) of AgNPs, AgNP colloidal solutions display an absorption peak. Thus, a multichannel spectrophotometer (T80 UV-Vis, d = 10 mm, PG Instruments Ltd., Japan) was used to measure the extinction of AgNP colloidal solutions. The measurement was performed in the wavelength range of 250–600 nm by using a 2-nm interval and 5-s scan speed.
Transmission electron microscope (TEM)
For more characterization of the prepared AgNPs, two drops of the supernatant colloidal solutions were placed on a 400-mesh copper grid coated by an amorphous carbon film. Then the solvent was evaporated in air at room temperature, and the grid was placed on the microscope equipment. The morphology was characterized by means of a JEOL-JEM-1230 Transmission Electron Microscope (Japan) with an electron beam from Oxford Instruments. The diameter and size distribution of AgNPs were calculated by 4pi analysis software using TEM photos.
Moisture content
The moisture content of all cellulose fibers was measured as follows: a 1-g fiber sample was weighed accurately up to four-digit numbers and then dried at 105 °C for ca. 4 h. The dried samples were reweighed up to the fixing weight, and then the moisture contents were calculated according to Eq. 1. The obtained moisture contents were 12.44, 12.64 and 10 % for CLY, CV and cotton, respectively.
where MC is the moisture content (%), W 1 = the initial condition weight (g), and W 2 = the weight of oven-dried fiber (g).
Carboxyl content
Carboxylic group contents of cellulose fibers before and after treatments were measured using the methylene blue method (Klemm et al. 1998; Emam et al. 2013, 2014). The method can be described briefly as follows: solutions of 300 mg/l aqueous methylene blue (A), borate buffer solution with pH = 8.5 (B) and 0.1 M HCl (C) were prepared. Then 25 ml of both solutions A and B was added to ca. 0.17 g of cellulose fiber (considering MC) in a 50-ml bottle, then shaken at room temperature. After 20 h, a 2.5-ml solution mixture was transferred to a 50-ml measuring flask, and 5 ml of solution C was added. Then the volume was completed to 50 ml by distilled water. The absorbance of solutions was measured using a multichannel spectrophotometer (T80 UV-Vis, d = 10 mm, PG Instruments Ltd., Japan) at a wavelength of 664.5 nm (λ max of methylene blue). The carboxyl content was calculated using Eq. 2.
where COOH is the carboxyl content (mmol/g), [MB]I = the concentration of methylene blue in the blank (sample without fiber) (mg/l), [MB]F = concentration of methylene blue in the samples (in the presence of fibers) (mg/l), W = weight of the fiber samples (g), and MC is the moisture content (%).
Results and discussion
A common method to prevent AgNP aggregation is applying ‘stabilizing’ or ‘dispersing’ agents, but these agents function by forming a layer surrounding the particles, leading to interference with their antimicrobial activity. The high surface energy of nanoparticles makes it very difficult to completely remove reagent residues from their surface, resulting in a toxic effect on medical applications (Krutyakov et al. 2008; Tankhiwale and Bajpai 2009). For the same reason, nanoparticles obtained by methods considered environmentally friendly (Castonguay and Kakkar 2010; Richter et al. 2009; Scott et al. 2005; Hebeish et al. 2010; Abdel-Mohsen et al. 2012) have not been viable for biomedical applications.
The present work focuses on developing a simple and effective, one-pot, totally green approach to the rapid synthesis of merely AgNPs with well-defined size using different cellulosic fibers as removable reducing agents for silver ions, without using any capping agents.
It is well known that AgNP colloidal solutions have color according to their SPR absorption. The SPR bands were affected by the shape and size of the AgNPs (Abdel-Mohsen et al. 2012; Bin et al. 2011; Deivaraj et al. 2005; Emam et al. 2013, 2014; Gopinath et al. 2012; Hebeish et al. 2010; Sadhan et al. 2012; Zahran et al. 2014a, b). Thus, the absorbance spectrum measurement is a good indication of the preparation of AgNPs. In the current work, three different cellulosic fibers, namely viscose, lyocell and cotton, were used to prepare AgNPs. The preparation process was initially followed by measuring the UV-Vis absorbance spectra. Then the electron microscope was used to observe the AgNPs and to detect their shape and size.
Cellulose was used as a reducer for Ag+, but the prepared Ag0 was incorporated in situ inside the cellulose matrix (Emam et al. 2013, 2014). In the current work, cellulose fibers were used to prepare a merely AgNP colloidal solution without using any stabilizing or reducing agents. The preparation process was planned to be performed at short immersion times to reduce the sorption of Ag+ and deposition of Ag0 on the cellulose fibers because of the high affinity of cellulose fibers.
Viscose
Figure 1 shows the UV-Vis spectra, TEM and size distribution for the AgNPs prepared using viscose fibers. Regardless of the reaction duration, an absorption band was detected at 262 nm by using 50 °C temperature (Fig. 1a), which is attributed to silver ions (Hebeish et al. 2010; Emam et al. 2014). This reflects that AgNPs were not formed at 50 °C. By raising the temperature of the reaction medium to 70 °C, an absorbance peak at 406 nm appeared after 15 min reaction time (Fig. 1b). As reported in the literature, this peak is SPR for spherical AgNPs (Harekrishna et al. 2009; Hebeish et al. 2010). After removing fibers from the reaction medium, the reaction preceded further for an additional 15 min, but no change in the absorption was observed. Contrary to 50 °C, at 70 °C, the peak of silver ions was not detected, confirming that there was no Ag+ in the reaction medium. It can be concluded that the reduction of Ag+ to Ag0 by cellulose fibers needs mild heating (70 °C) to proceed. When the concentration of AgNO3 increased two or five times, keeping the weight of cellulose fibers the same (10 g/l), the SPR peak of AgNPs was not observed (Fig. 1c). AgNPs were not formed because of the presence of an insufficient amount of cellulose fibers in the reaction medium to reduce Ag+ ions.
Preparation of AgNPs using viscose fibers. a UV-Vis absorbance of colloidal solutions using 1 g/l viscose and 1 mmol/l AgNO3 at 50 °C. b UV-Vis absorbance of colloidal solutions using 1 g/l viscose and 1 mmol/l AgNO3 at 70 °C. c UV-Vis absorbance of colloidal solutions using 1 g/l viscose and different concentrations of AgNO3 at 70 °C. d TEM photo for AgNPs prepared at 70 °C for 15 min using 1 mmol/l AgNO3. e Size distribution of AgNPs in the corresponding TEM photo
Considering the UV-Vis absorbance values of the same concentration prepared from AgNP colloidal solution in the literature (Hebeish et al. 2010; Zahran et al. 2014a, b), a similar absorbance value and intensity were obtained using viscose fibers after only 15 min. This achieved our desired goal of minimizing the sorped amount of Ag+ and diminishing the deposited Ag0 on the cellulose.
The prepared AgNP colloidal solution using viscose was examined under the transmission electron microscope (Fig. 1d). Spherical particles in the nano dimension were seen with almost even size. This result is in agreement with the result of UV-Vis spectroscopy. Although no reducing or stabilizing agent was still in the reaction medium, the aggregation and agglomeration were not obviously detected under the microscope, but could not be avoided by time. The distribution of particle size was measured using the microscopic photos and software program, and the data are shown in Fig. 1e. The size distribution of the prepared AgNPs was recorded to be in a wide range of 0–30 nm. The majority of Ag nanoparticles (ca. 70 %) were located in the range of 0–10 nm, while only ca. 30 % of AgNPs in the sample were in the domain of 10–30 nm. Based on the size distribution results, the mean size was calculated to be 9.5 nm.
Lyocell
Figure 2 represents the UV-Vis spectra, TEM and size distribution of the AgNPs prepared using lyocell fibers. The absorbance was measured after 15 min. Absorbance spectra show that 10 g/l of lyocell is not enough to perform the reduction process, as the SPR peak for AgNPs did not appear (Fig. 2a). By increasing the amount of lyocell fibers in the reaction medium to 20 g/l, the SPR peak for AgNPs at 412 nm started to appear and became sharper with higher intensity by using 30 g/l lyocell fibers. However, the SPR peak for AgNPs was broader with lower intensity compared to that of viscose fibers using 10 g/l. This observation could be explained by the nature of the regenerated cellulosic fibers for both viscose and lyocell. It is known that lyocell fibers are produced by dissolving cellulose pulp in N-methyl morpholine N-oxide (NMMO), while CS2 was used for preparation of viscose fibers. Thus, NMMO as an oxidant could be supposed to retard the reduction process of Ag+, and the production of AgNPs by using a higher amount of lyocell supports this argument.
Preparation of AgNPs using lyocell fibers. a UV-Vis absorbance of colloidal solutions using different concentrations of lyocell fibers and 1 mmol/l AgNO3 at 70 °C after 15 min. b TEM photo of AgNPs prepared using 3 g/l lyocell and 1 mmol/l AgNO3 at 70 °C after 15 min reaction time. c Size distribution of AgNPs in the corresponding TEM photo
The TEM photos show that spherical AgNPs were found, which is consistent with the UV-Vis absorbance spectra results (Fig. 2b). The prepared particles were shown to be reasonably homogeneous in size. Compared to AgNPs produced by viscose fibers, aggregations and agglomerations of particles were viewed in microscopic images. Size distribution was measured to be 0–30 nm. The majority of particles (65 %) were shown to be located in 0–10 nm. From the data of the size average shown for both lyocell and viscose fibers, enlarged AgNP clusters were formed using lyocell (the average size of AgNPs was 12.4 nm higher than that in case of using viscose fibers), which confirms that more aggregations of particles resulted from using lyocell fibers.
Cotton
The UV-Vis spectra, TEM and size distribution of AgNPs synthesized by cotton fibers are exhibited in Fig. 3. The absorbance was measured after 15 min using 10 g/l cotton fibers. Two cotton fibers with different DPs of 960 and 1,850 were used. Characteristic broadening of the SPR band (at 414 nm) was observed for fibers with a lower DP. The sharpness and intensity of the peak were increased by increasing the DP to 1,850. Regardless of the DP, both the sharpness and intensity were very low compared to those produced when using viscose fibers, but they are almost similar to those found by using lyocell fibers.
Preparation of AgNPs using cotton fibers. a UV-Vis absorbance of colloidal solutions using 1 g/l cotton with two different DPs and 1 mmol/l AgNO3 at 70 °C after 15 min reaction time. b TEM photo of AgNPs prepared using 1 g/l cotton with DP = 960 and 1 mmol/l AgNO3 at 70 °C after 15 min reaction time. c Size distribution of AgNPs prepared with cotton fibers with DP = 960. d TEM photo of AgNPs prepared using 1 g/l cotton with DP = 1,850 and 1 mmol/l AgNO3 at 70 °C after 15 min reaction time. e Size distribution of AgNPs prepared with cotton fibers with DP = 1,850
The microscope photos show that spherical-shaped AgNPs were produced by using cotton fibers, affirming the outcomes of absorbance spectra. The particles were attached firmly together with homogeneity of acceptable size when using fibers with DP 960. By increasing the DP to 1,850, the particles become closer to each other, forming a structure that looks like chains. Compared with viscose fibers, AgNPs formed by cotton fibers with DP of 960 showed some aggregations such as in the case of using lyocell fibers (Fig. 3b). The aggregations appeared clearly by using cotton fibers with a DP of 1,850 (Fig. 3d). Size distribution results confirmed the data recorded for the absorbance and TEM image. The particle size was placed between 0–35 and 0–50 nm by using cotton fibers with a DP of 960 and 1,850, respectively. By using DP = 960, a large number of particles was found to be in the range of 5–10 nm with ca. 50 % of all particles with a calculated mean size = 11.6 nm. On the other hand, around 60 % of all AgNPs were in the range of 10–20 nm, giving 19 nm as an average size. These observations explained by the aggregation of Ag particles resulted from using cotton fibers with a high DP value.
Aging
The aging was studied in order to observe the effect of storage on the prepared AgNPs in the absence of both reducing and stabilizing agents. The AgNPs prepared by viscose fibers were stored at room temperature for 4 months. The absorbance and TEM were both measured after 2 and 4 months, and the results are shown in Fig. 4. The absorbance peak characteristic of AgNPs was still observed after the storing process, but the intensity of the peak was observed to decrease by storing (Fig. 4a). After 2 and 4 months, the absorbance was recorded as 30 and 83 % lower than that for the fresh sample, respectively. The decrement in absorbance values was denoted because the concentration of AgNPs in the colloidal solution decreased with time. This was related to the precipitation process of the dispersed particles, which was a result of the absence of the dispersing agent (stabilizer). The electron microscopic photos of the stored AgNPs point to three observations: (1) The Ag particles became closer to each other and attached together after storage. (2) Some aggregations and agglomerations appeared after 2 months of storage, and they became heavy and more obvious after 4 months of storage. Hence, the TEM photos (Fig. 4b, d) support the indication of the UV-Vis spectra. (3) Calculations of particle size suggest that the size distribution range was 0–40 nm after 2 months of storage, which was wider compared to the fresh sample. The majority of particles were in the size range of 10–20, with 16 nm as the average size of particles, which is larger than that for the fresh sample. After storing for 4 months, the size of Ag particles grew to a mean size = 19 nm; 40 and 30 % of all particles were in the range of 10–20 and 20–30 nm, respectively. These calculations verify the aggregation process observed in the TEM photos.
Effect of aging on the AgNPs prepared using viscose fibers. a Comparison of the UV-Vis absorbance of AgNP colloidal solutions prepared immediately and after aging for 2 and 4 months. b TEM photo of the prepared AgNPs after aging for 2 months. c Size distribution of AgNPs after aging for 2 months. D TEM photo of the prepared AgNPs after aging for 4 months. e Size distribution of AgNPs after aging for 4 months
Mechanism and carboxyl content
The reduction of silver by cellulose fibers was initially detected by the change of the solution color to yellow and was later confirmed by electron microscopic observation. Based on these results, a schematic diagram of the formation of AgNPs by the action of cellulose fibers is suggested in Fig. 5. The proposed mechanism for the reduction of silver ions (Ag+) to atomic silver (Ag0) can be explained as follows: the reduction power of cellulose, including the reducing end group (hemiacetal) and alcoholic groups (e.g., CH2OH), was activated in alkaline medium. The reducing groups of cellulose reduced Ag+ to Ag0 in the nanosize dimension as yellow color of the solution was observed. It is known that the silver exhibits a tendency to auto-catalytic reduction, i.e., Ag0 acts as a center for further reduction of the Ag+ (Rabilloud et al. 1994; Harada and Katagiri 2010). The heating and light could play a role in the catalysis of the reduction process (Cai et al. 2008; Ifuku et al. 2009; Kotelnikova et al. 2003; Ju and Tallahassee 2010; Khanna and Subbarao 2003). After formation of the first Ag0 nuclei, two or more Ag0 cascades coalesced to form dimer, trimer and higher order Ag0 clusters, known as AgNP clusters (Janata 2003). The aggregation of Ag0 clusters into higher clusters occurred as the nucleation in the solution increased. The possibility of precipitate formation is increased according to the use of no stabilizing agent, as shown in the section on the effect of aging.
The redox reaction between Ag+ and cellulose included a reduction reaction for Ag+ and oxidation reaction for cellulose. As Ag+ turns to Ag0, simultaneously cellulose (alcoholic and aldehydic groups) changes to an oxidized form (carboxylic groups). Thus, increasing the carboxylic content of cellulose fibers means that the redox reaction between cellulose fibers and Ag ions is running, thus implying an increase in the affinity for production of AgNPs. Hence, the carboxylic content (COOH) of all different cellulose fibers before and after the reaction with AgNO3 is an interesting parameter to measure.
For viscose fibers, the COOH content was 16.75 mmol/kg before the reaction was initiated and became 18.77 and 28.44 mmol/kg at the end of the reaction at 50 and 70 °C, respectively. This result is in harmony with the UV-Vis absorbance (Fig. 1a, b), as no peak was recorded for AgNPs at 50 °C, which reflects that there is no redox reaction at that temperature as the COOH content did not increase significantly. The COOH content of lyocell fibers did not obviously grow after the reaction with AgNO3 by using 10 and 20 g/l fibers as COOH increased from 18.7 to 21.5 and 21.7 mmol/kg, respectively. By increments of the concentration of lyocell fibers to 30 g/l, the COOH content increased to 24.9 mmol/kg, which explains the successful formation of AgNPs; this is in accord with the data of the UV-Vis absorbance and TEM photos (Fig. 2). The previously mentioned data could be related to the presence of traces of NMMO, which retards the redox reaction by using a lower concentration of lyocell fibers. However, by raising the concentration of lyocell fibers to 30 g/l, the effect of NMMO is diminished compared to the amount of reducing groups in cellulose; subsequently, the redox reaction predominated, and AgNPs were formed.
When using cotton fibers with different DPs (960 and 1,850), the COOH content increased from 17.8 to 24.0 mmol/kg for cotton fibers with DP 960, while it increased from 8.5 to 14.0 mmol/kg for cotton fibers with a DP of 1,850. Although the COOH contents grew by similar amounts, cotton fibers with a DP of 1,850 gave better results of UV-Vis absorbance, TEM and particle size for the formed AgNPs. Thus, the DP factor could be affected by the redox reaction. The effect of DP of different cellulose fibers ranging from 300 for viscose, 600 for lyocell to 960 and 1,850 for cotton on the reduction process of silver to AgNPs from the preceding results will be discussed. By using carboxymethyl cellulose (CMC) as a reducing agent for silver, the intensity of the SPR of AgNPs is proportional to the DP (Hebeish et al. 2010). This agrees with cotton fibers as it is a natural fiber for which increasing the DP drives an increase in the alcoholic groups (CH2OH). However, this does not work when using regenerated cellulose fibers (viscose and lyocell) regenerated from wood pulp. Viscose with a lower DP gave the highest intensity of SPR and the smallest size of AgNPs. To the complete contrary, lyocell with a moderate DP showed the lowest SPR intensity even using a three times higher amount of lyocell compared to viscose and cotton. Thus, both the regeneration process and source of cellulose play effective roles in the reduction process of silver; hence, it cannot be compared between different DPs from different cellulose fibers because of their different sources and treatments. However, from all data shown, it could be confirmed that viscose fibers are the better removable cellulose fibers used to produce merely small-sized and well-dispersed AgNPs (Table 1).
Conclusions
The current study presented a new method to prepare merely AgNP colloidal solution using cellulose fibers as a removable reducing agent. Three different cellulose fibers with different DPs based on viscose, lyocell and cotton were used in this study. The prepared AgNP colloidal solutions were tested by using UV-Vis spectroscopy, TEM micrographs and particle size measurement. The carboxyl content of cellulose fibers was measured before and after the preparation process. Results displayed that viscose is the best fiber for preparation of merely AgNPs with the maximum concentration and smallest particle size. The effect of storing was tested for up to 4 months to check the stability of the prepared AgNP colloidal solution with time. The method utilized in the present work introduced a simple green method to prepare merely AgNP colloidal solution without a complicated system or intermediate steps. This work will open the way for researchers to access new methods to create a stable merely AgNP colloidal solution that may be more suitable for biomedical applications.
References
Abdel-Mohsen AM, Aly AS, Hrdina R (2012) A novel method for the preparation of silver/chitosan-O-methoxy polyethylene glycol core shell nanoparticles. J Polym Environ 20:459–468
Abdel-Mohsen AM, Rasha MA, Moustafa MGF, Vojtova L, Uhrova L, Hassan AF, Salem SA, El-Shamy IE, Jancar J (2014) Preparation, characterization and cytotoxicity of schizophyllan/silver nanoparticle composite. Carbohydr Polym 102:238–245
Alshehri AH, Jakubowska M, Młożniak A, Horaczek M, Rudka D, Free C, Carey JD (2012) Enhanced electrical conductivity of silver nanoparticles for high frequency electronic applications. ACS Appl Mater Interfaces 4(12):7007–7010
Battie Y, Destouches N, Chassagneux F, Jamon D, Bois L, Moncoffre N, Toulhoat N (2011) Optical properties of silver nanoparticles thermally grown in a mesostructured hybrid silica film. Opt Mater Express 1(5):1019–1033
Big T, Mingwen Z, Xue-Liang H, Jing-Liang L, Lu S, Xun-Gai W (2012) Coloration of cotton fibers with anisotropic silver nanoparticles. Ind Eng Chem Res 51:12807–12813
Bin T, Jinfeng W, Shuping X, Tarannum A, Weiqing X, Lu S, Xun-Gai W (2011) Application of anisotropic silver nanoparticles: multifunctionalization of wool fabric. J Colloid Interface Sci 356:513–518
Bin T, Jing-Liang L, Xue-Liang H, Tarannum A, Lu S, Xun-Gai W (2013) Colorful and antibacterial silk fiber from anisotropic silver nanoparticles. Ind Eng Chem Res. doi:10.1021/ie3033872
Cai J, Kimura S, Wada M, Kuga S (2008) Nanoporous cellulose as metal nanoparticles support. Biomacromolecules 10(1):87–94
Castonguay A, Kakkar AK (2010) Dendrimer templated construction of silver nanoparticles. Adv Colloid Interface Sci 160:76–87
Deivaraj TC, Lala NL, Lee JY (2005) Solvent-induced shape evolution of PVP protected spherical silver nanoparticles into triangular nanoplates and nanorods. J Colloid Interface Sci 289:402–409
El-Rafie MH, Ahmed HB, Zahran MK (2014) Characterization of nanosilver coated cotton fabrics and evaluation of its antibacterial efficacy. Carbohydr Polym 107:174–181
Emam HE, Manian AP, Široká B, Duelli H, Redl B, Pipal A, Bechtold T (2013) Treatments to impart antimicrobial activity to clothing and household cellulosic-textiles e why “nano”-silver? J Clean Prod 39:17–23
Emam HE, Mowafi S, Mashaly HM, Rehan M (2014) Production of antibacterial colored viscose fibers using in situ prepared spherical Ag nanoparticles. Carbohydr Polym 110:148–155
Gao C, Yan D (2004) Hyper branched polymers: from synthesis to applications. Prog Polym Sci 29:183–275
Gopinath V, Mubarak AD, Priyadarshini S, Meera PN, Thajuddin N, Velusamy P (2012) Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloids Surf B 96:69–74
Harada M, Katagiri E (2010) Mechanism of silver particle formation during photoreduction using in situ time-resolved SAXS analysis. Langmuir 26(23):17896–17905
Harekrishna B, Dipak K, Bhui GP, Sahoo PS, Santanu P, Ajay M (2009) Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A: Physicochem Eng Asp 348:212–216
Hebeish AA, El-Rafie MH, Abdel-Mohdy FA, Abdel-Halim ES, Emam HE (2010) Carboxymethyl cellulose for green synthesis and stabilization of silver nanoparticles. Carbohydr Polym 82:933–941
Ifuku S, Tsuji M, Morimoto M, Saimoto H, Yano H (2009) Synthesis of silver nanoparticles templated by TEMPO-mediated oxidized bacterial cellulose nanofibers. Biomacromolecules 10(9):2714–2717
Janata E (2003) Structure of the trimer silver cluster Ag 23 . J Phys Chem B 107:7334–7336
Ju YK, Tallahassee FL (2010) Method for preparing an antimicrobial cotton of cellulose matrix having chemically and/or physically bonded silver and antimicrobial cotton prepared therefrom. United State Patent Application Publication, US 2010/0316693 A1
Khanna PK, Subbarao VS (2003) Nanosized silver powder via reduction of silver nitrate by sodium formaldehydesulfoxylate in acidic pH medium. Mater Lett 57(15):2242–2245
Klemm B, Philipp B, Heinze T, Heinze U, Wagenknecht W (1998) Comprehensive cellulose chemistry, 2nd edn. Wiley, Weinheim, p 236
Kotelnikova NE, Demidov VN, Wegener G, Windeisen E, Kotelnikov VP (2003) Silver cluster intercalation into the cellulose matrix. I: mechanisms of diffusion-reduction interaction of microcrystalline cellulose and silver ions. Cellul Chem Technol 37(3–4):225–238
Krutyakov YA, Kudrinskiy AA, Olenin AY, Lisichkin GV (2008) Synthesis and properties of silver nanoparticles: advances and prospects. Russ Chem Rev 77(3):233–257
Larguinho M, Baptista PV (2012) Gold and silver nanoparticles for clinical diagnostics—from genomics to proteomics. J Proteomics 75:2811–2823
Menjoge AR, Kannan RM, Tomalia DA (2010) Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today 15:171–185
Pastoriza-sontos I, Liz-Marzan LM (2002) Synthesis of silver nanoprisms in DMF. Langmuir 18:2888–2894
Rabilloud T, Vuillard L, Gilly C, Lawrence JJ (1994) Silver-staining of proteins in polyacrylamide gels: a general overview. Cell Mol Biol (Noisyle- Grand, France) 40(1):57–75
Richter TV, Schüler F, Thomann R, Mülhaupt R, Ludwigs S (2009) Nanocomposites of size-tunable ZnO-nanoparticles and amphiphilic hyper branched polymers. Macromol Rapid Commun 30:579–583
Sadhan S, Priyanka S, Santanu P, Gobinda PS, Ajay M (2012) Synthesis of silver nanodiscs and triangular nanoplates in PVP matrix: photophysical study and simulation of UV–Vis extinction spectra using DDA method. J Mol Liq 165:21–26
Sakai H, Kanada T, Shibata H, Ohkubo T, Abe M (2006) Preparation of highly dispersed core/shell-type titania nanocapsules containing a single Ag nanoparticle. J Am Chem Soc 128:4944–4945
Scott RWJ, Wilson OM, Crooks RM (2005) Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J Phys Chem B 109:692–704
Tankhiwale R, Bajpai SK (2009) Graft copolymerization onto cellulose-based filter paper and its further development as silver nanoparticles loaded antibacterial food-packaging material. Colloids Surf B 69(2):164–168
Tien H-W, Huang Y-L, Yang S-Y, Wang J-Y, Ma C-CM (2011) The production of graphene nanosheets decorated with silver nanoparticles for use in transparent, conductive films. Carbon 49:1550–1560
Van Hyning D, Klemperer W, Zukoski C (2001) Silver nanoparticle formation; predictions and verification of the aggregative growth model. Langmuir 17:3128–3135
Watson A (2009) Gold nanoparticles: a novel dye for synthetic fabrics. Master thesis, School of Chemical and Physical Sciences, Victoria University of Wellington
Zahran MK, Ahmed HB, El-Rafie MH (2014a) Alginate mediate for synthesis controllable sized AgNPs. Carbohydr Polym 111:10–17
Zahran MK, Ahmed HB, El-Rafie MH (2014b) Facile size-regulated synthesis of silver nanoparticles using pectin. Carbohydr Polym 111:971–978
Zahran MK, Ahmed HB, El-Rafie MH (2014c) Surface modification of cotton fabrics for antibacterial application by coating with AgNPs-alginate composite. Carbohydr Polym 108:145–152
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emam, H.E., El-Bisi, M.K. Merely Ag nanoparticles using different cellulose fibers as removable reductant. Cellulose 21, 4219–4230 (2014). https://doi.org/10.1007/s10570-014-0438-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0438-5