Abstract
Condensed tannins, extractable from tree bark have been assessed as functional additives to provide a protective role to acrylic-based coating resins. In addition to retaining high antioxidant capacity, the UV absorption properties of native and chemically modified tannins were found to be variously impacted by pH and degree of esterification or etherification. When added to acrylic-based coatings, these tannins were found to contribute colour to a white-base, but only small perceptive differences were found for clear coated wood using typical additive loadings of 0.1–0.4%. Integration of tannins in native or modified form to do not inhibit the cure of acrylic coatings or found to leach from cured coating films. Accelerated weathering was used to evaluate the photo-stability of tannin-modified acrylic and styrene-acrylic coatings. Native and modified tannins with maleate or methylcarboxylate groups retaining high antioxidant activity were associated with significantly greater coating longevity and performance than use of a synthetic photostabiliser. Moreover, esterified condensed tannins with a high degree of substitution also outperformed synthetic additives indicating the inherent UV absorption potential of these materials also contributed this efficacy within the acrylic and styrene-acrylic coating systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A drive for increased environmental sustainability of paint and coating systems has seen a number of innovations within the industry [1]. These include efforts to establish renewable, bio-based materials incorporated directly into synthetic polymer backbones or their use as polymer building blocks. Some examples include native and modified starches, proteins and vegetable oils integrated into coating systems [2, 3]. In addition to generating coating polymers which incorporate renewables, the use of bio-based additives to coatings and paints is also of interest [4, 5]. Functional additives primarily introduce performance and longevity to paints and coatings [6]. Generally, these are commonly petrochemical-derived synthetics and include UV stabilisers, antioxidants, plasticizers, wetting agents and coalescence aids. To improve sustainability options and renewables use in paints and coatings, there is also a need for parallel developments for renewable functional additives which provide equivalent performance and service life as their synthetic counterparts in coatings.
Condensed tannins are polyphenolic compounds found in the leaf, stem and bark of plants and trees and have been trialled as functional additives in various plastics [7–9]. Extractable into water, condensed tannins have varying uses in the pharma, nutraceutical, adhesives and leather tanning industries [10]. Typically, it is the inherent properties of tannins including protein complexation and inhibition, high antioxidant capacity and chemical reactivity which have been utilised in these applications. The polyhydroxyl patterns present in the flavonoid unit of condensed tannins (Fig. 1) are responsible for the attributes of these polyphenolics [10, 11]. Moreover, the chemistries of these polyphenolics also provide UV absorption and antioxidant protection roles within plants and trees [12]. This protective role has been applied in petrochemical- and bio-plastics where condensed tannins in modified form conferred a photo-oxidative stabilisation role, contributing to plastic longevity [8, 9].
Given the promising protective role demonstrated by condensed tannins in plastics [8, 9], tannins have been evaluated as antioxidant and UV stabiliser functional additives into acrylic and styrene-acrylic coating formulations [13]. In the current paper, condensed tannins extracted from pine bark have been assessed for their compatibility, emulsion stability and aid performance of acrylic-based coating formulations. Tannins, in both native and chemically modified form have been surveyed to provide a photo-oxidative protection role to the polymer coating and promote coating longevity. Additionally, the tannins were assessed for any photo-stabilisation and contribution to colour retention of weathered wood substrates.
Methods and Materials
The base coating resins were an acrylic coating resin Setaqua 6704 and a styrene-acrylic copolymer resin Viscopol 6191 which were both sourced from Nuplex Ltd (New Zealand). Each coating formulation was obtained as the base resin coating without post-polymerisation additives and used as received. Chimasorb™ 90 was the commercial additive and also obtained from Nuplex. Catechin was from Aldrich and used as received. The native tannin (HWTan) was an extract obtained from the hot water extraction of radiata pine bark [14]. From this HWT extract the tannin esters and ethers were prepared according to published procedures to give tannin acetate (TanAc), tannin laurate, tannin maleate (TanMal), tannin maleate acetate (TanMalAc) and sodium tannin methylcarboxylate (TanMC) described elsewhere [8, 15–18].
Antioxidant Assay
The 2,2′-azinobis(3-ethylbenzothiazoline-6-sulphonic acid) radical cation (ABTS⋅+) decolorisation assay was a modified method described by Gülçin et al. [19]. A 7 mM solution of ABTS was prepared in distilled water. The ABTS⋅+ radical was then generated by mixing 5 ml of this solution with 88 μl of 140 mM K2S2O8. The ABTS⋅+ solution was diluted with either 1:1 ethanol:water (hydrophilic samples) or 8:2 acetone:water (lipophilic samples) to achieve an absorbance of 0.70 ± 0.02 at 734 nm. Typically, this required 1 ml of ABTS⋅+ solution to 80–100 ml of solvent. Trolox standards were prepared to give final concentrations (once added to the ABTS⋅+ solution) in the range of 2.5–20 μM. Approximately 5 mg of Trolox was dissolved in 10 ml 1:1 ethanol:water or 8:2 acetone:water. This stock solution was then serially diluted to give five concentrations ranging from 31.3 to 250 μg/ml and used to construct a concentration response curve (calibration curve) for Trolox. Similarly, the various tannin samples were prepared in 1:1 ethanol:water (hydrophilic samples) or 8:2 acetone:water (lipophilic samples) to concentrations of approximately 62.5–125 μg/ml. To six quartz cuvettes was added 4 ml of ABTS⋅+ working solution and the cuvettes were placed in a multicell reader within a Cary 300 Bio UV–Vis spectrophotometer using an absorbance at 734 nm. The cuvettes were incubated at 30 °C and the time zero (0 min) absorbance readings were recorded. Eighty μl of a standard or sample solution was added to each cuvette sequentially and the solutions manually mixed then measured precisely after 6 min. The percentage inhibition of the absorbance of the ABTS⋅+ was calculated using Eq. 1 and determined from the Trolox calibration curve. The TEAC (Trolox equivalence antioxidant capacity) value for each antioxidant was calculated using Eq. 2.
where: A0 = absorbance at 0 min.
A6 = absorbance at 6 min
where: CT = concentration of Trolox corresponding to the measured inhibition.
CA = concentration of the antioxidant.
Modification of Coatings
For each modified coating, either the commercial additive or a tannin-based additive was typically added at 0.2% loading w/w on wet emulsion. The commercial additive was added as an aqueous slurry by first adding this material to a minimum amount of water. For modified tannins, each was initially dissolved in a small amount of polyethylene glycol (PEG 400) as ca. 20% solutions. These PEG solutions were then added to the base resin which was stirred sufficiently to allow addition without coagulating the coating formulation. For the HWT and TanMalAc additives, each was also added at a rate of 0.4% to the styrene–acrylate copolymer resin with the 0.2% and 0.1% samples obtained by dilution with further coating formulation.
Coating Application and Evaluation
The preparation of coated substrates, accelerated weathering and coating assessments were conducted according to the AS/NZS1580 Standard. The wood substrate was untreated radiata pine clears (280 × 60 × 10 mm) and was sanded (150 grit) prior to coating application. Coated wood substrates were also prepared by pre-coating an exterior grade white acrylic coating onto these substrates. The base resin and modified coatings were applied directly to the wood at an overall application rate of ca. 50–75 g/m2. Two coats were applied per specimen (both sides) and achieved by initially applying the first coat with a second coat applied at least 2 h later. The coated specimens were then end-sealed with the exterior white acrylic coating to limit moisture and water ingress.
Colour Assessments
The colour of coated samples was measured using a Konica Minolta Chroma CR-400 colourmeter. For weathered samples, each exposure area (50 × 80 mm, 2 per specimen) had a total of 5 measurements comprising the four corners and centre of this area (total of 10 per specimen and 20 measurements per sample). Measurements were referenced to the CIE L*a*b* colour spectrum. Changes in sample colour were converted to ∆L, ∆a, ∆b and ∆E values.
Modified Coating Leaching
Each coating sample was applied to at least three UV cuvettes (two coats of each sample were applied as above). After attaining full cure, a UV absorption profile was measured for each cuvette. One set of cuvettes was immersed in water for 24 h. After soaking the curvettes were removed, allowed to dry and re-measured comparing the original and soaked absorption profiles at key wavelengths to determine any loss in UV absorption. In addition to soaking samples, a reference (control) set of cuvettes was also measured before and after soaking. The before and after soaking UV absorptions were compared by normalising to the non-soaked reference samples.
Coating Accelerated Weathering
Samples were exposed to artificial weathering conditions using a QUV accelerated weather tester made by Q Panel Lab Products. Protocols for AS/NZS1580 483.1 were followed where both UV (8 h) and condensation (4 h) cycles were undertaken at 45 °C for up to 4000 h. UV exposure was achieved through the use of UVA-340 lamps at an irradiance of 0.89 W/m². Two specimens were used per sample, with specimens each having two exposure areas (50 × 80 mm). Specimens were rotated every 250 h to ensure even exposure to QUV conditions. At this time specimens were photographed, colour measurements and coating evaluations undertaken.
Coating Evaluations
Each coating sample was evaluated using AS/NZS1580 481 criteria. This included appraisal of the general appearance and gloss of the exposed coating as well as several specific evaluations including: checking and cracking; blistering; flaking or chalking; and colour change. In each case, evaluations were undertaken comparing any changes to the reference (original) specimen of the same sample which had not been exposed to any light/weathering.
Results and Discussion
Antioxidant & UV Absorption Data
Initially, native tannins were chemically modified to induce compatibility with acrylic polymers given tannin compatibility has been shown to be an important factor for tannin miscibility within polyolefin and polyester plastics [7]. Being common, simple modification approaches, esterification and etherification were both used to introduce hydrophobicity to the water soluble tannins [15]. Acetylation, maleation and alkylcarboxylation were employed to give varying degrees of flavonoid substitution (Fig. 1). Targeting both partial- and full-esterification (Table 1) also recognised a need for the mixed compatibility of the tannins in aqueous acrylic latexes and on coating application to allow coalescence, crosslinking and full cure of the coating. These types of tannin modifications were found to variously impact the tannin UV absorption profile due to differing conjugation and inductive effects arising from the introduced functionalities (–OC(O)CH3, Ac; –O(O)C=C–C(O)OH, Mal; –OCH2C(O)OH, Carb). Shown in Fig. 2 are representative UV absorption spectra of modified tannin derivatives revealing absorption profiles dominating the UVB region (280–320 nm) and extending to >350 nm. Each modified tannin additive exhibited a peak maxima at 270–290 nm which shifted with chemical modification and degree of substitution. Alkylcarboxylation which introduced both ether and carboxylic linkages (TanCarb) was found to broaden the 275 nm peak profile. Esterification, via acetic or maleic anhydride treatments (TanAc, TanMal), could be tailored to give a differing degree of substitution (DS) ranging from 1 to >5 ester groups per flavonoid unit (Fig. 1; Table 1). A low degree of ester substitution (DS = 2) had the effect of broadening the 275 nm maxima, whereas full esterification (DS > 5) narrowed this peak indicative of the influence of hydroxyl substitution (Fig. 1) on the UV absorption. Moreover, an alkaline pH, typical of acrylic polymer coatings [20], also had the effect of broadening the profile of the 275 nm peak due to phenolate formation and resonance effects [12].
Tannin chemical modification also had the effect of reducing the antioxidant activity of the modified tannins (Table 1). The antioxidant capacity of native tannin (>4500 TEAC equivalents) reduces by almost half with a DS of 1. Greater substitution further reduces the antioxidant capacity where full acetylation (DS > 5) gave ABTS values <200 TEAC equivalents. Substitution of tannin hydroxyls with laurate [17] or maleate groups (TanMal) also lowered antioxidant capacity as did full esterification of the mixed ester tannin maleate acetate (TanMalAc). This antioxidant capacity of modified tannins was also found to have solvent dependency which may provide insights to anticipated activities when incorporated within the acrylic polymer coating compared to aqueous solution.
On comparing tannins in native and modified form, hydroxyl substitution did not significantly impact UVB absorption characteristics (Fig. 2), but had the effect of reducing antioxidant capacity to that found for commercial additives (Table 1). As an initial evaluation of their UV absorption potential in aqueous coating formulations, the tannin materials were assessed in a styrene-acrylic latex formulation (Fig. 3). At typical additive loadings (<0.5% w/w), native tannin contributed to an increase in the UV absorption profile of the coating formulation, being similar to a commercial additive (Supplementary Material). Similarly, addition of modified tannins also revealed significant increases in UV absorption extending to the UVA region. While dependent on the tannin concentration, this increase in UV absorption within the coating formulation indicates potential to provide a photo-stabilisation role to coatings.
The contrasting colour imparted to modified styrene-acrylic coatings by 0.1–0.4% (w/w) tannin materials in the aqueous coating formulation (top) and after application onto a wood substrate (bottom). Left or right, base resin (a), 0.2% commercial (b), 0.4% HWTan (c), 0.2% HWTan (d), 0.1% HWTan (e), 0.4% TanMalAc (f), 0.2% TanMalAc (g), 0.1% TanMalAc (h), 0.2% TanMal (j), 0.2% HWTan (k), 0.1% HWTan (l), 0.2% TanCarb (m)
Tannin Integration and Stability of Acrylic Coatings
Tannin in native or modified form could be readily added to a range of acrylic and styrene–acrylic copolymer paint and clear coating formulations. The tannin materials did not induce emulsion instability when combined without coagulation [13]. However, the condensed tannins introduce colour to both clear coat (Fig. 3) and white base acrylic coatings, particularly at concentrations of 1% (w/w coating polymer, Table 2). This darkening was comparable to colour intensities observed in plastic systems [7]. In characterising colour contributions using the CIE L*a*b* colour index, 1% native tannin gave an ∆L* value of 10 with this perceptive difference significantly reduced at lower additive content within a white base coating. Chemical modification further reduces the introduced colour with full esterification contributing ∆L* values of ≤2 at 1% concentration, perhaps reflective of the actual tannin content (Table 1). Moreover, at typical additive loadings (<0.5%) any introduced colour by native and modified tannins was considered low.
Despite contributing to highly coloured liquid formulations (Fig. 3), applying modified coating formulations to wood surfaces resulted in the coated surfaces retaining a measurably similar colour as the unmodified coatings (Fig. 4). For example, addition of the esterified tannins TanAc, TanMal and TanMalAc gave similar L*a*b* colour values as the original, unmodified base resin (Fig. 3). Using L* values, the commercial UV stabiliser additive or flavonoid monomer catechin (Fig. 1) also gave similar L* values (ca. 80) as these samples, consistent with side by side visual comparisons of samples. Only with native tannin or the methylcarboxylate derivative TanCarb was there a perceptive darkening of the wood surface on coating application. For these samples, L* values of ca. 75 (∆L* = 5) were determined with similar changes observed in both the a* (red-green) and b* (yellow-blue) colour components. The increase in these ∆a* and ∆b* values (3–6) was consistent with the red–yellow colours introduced by the inherently brown-coloured tannin derivatives.
There is a misconception within the surface coatings community that natural tannins within the wood stain the coated surface or contribute to inhibition of coating cure [21]. To assess cure inhibition and suitability of modified coatings for extended weathering, styrene-acrylic coatings were soaked in water evaluating any coating dissolution or tannin leaching. Water immersion revealed styrene-acrylic samples become opaque, but do not delaminate. On redrying, generally there was retention of UV absorption profiles and relative intensity (Fig. 2) with only the base, unmodified resin (A) having decreased relative UV absorption (Fig. 5). Modified coatings retain similar UV absorption and, with tannin contributing to UV absorption, this suggests the tannin components do not leach from these coatings. Moreover, anecdotally, the presence of tannin at 0.1–0.4% content may not inhibit coating coalescence and cure, but instead promote crosslinking of polymeric components. In assessing under-cured coating films (<7 days), water soaking revealed the base resin was prone to delamination and dissolution indicating a relatively slower cure of this unmodified coating. In comparison, tannin-modified coatings did not delaminate, suggestive of a relatively greater degree of cure within this timeframe and worthy of further work to establish any tannin induced crosslinking or potential integration within the cured coating films.
Potential for Wood Surface Photo-stabilisation and Colour Retention
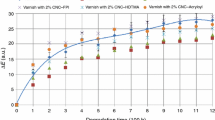
The potential of tannins to provide antioxidant and UV stabilising efficacy was assessed under accelerated weathering and photo-oxidative aging of modified coating polymers applied over wood. Wood clear-coated with modified acrylic and styrene-acrylic coating systems was exposed to UV light and condensation cycling to compare any efficacy of each tannin with commercial additives and the flavonoid monomer catechin. While visually evident, CIE L* profiles confirm accelerated weathering led to the relative darkening of wood samples on exposure (Figs. 6, 7). Wood darkening is not unexpected [22], with this particularly evident within 500–750 h of exposure in which samples were observed to rapidly darken. For the acrylic coating which contained a range of modified tannins at 0.2% content, the unmodified base resin, commercial additive- and catechin-modified samples were observed to have relatively greater ∆L* values, or proportionate darkening. After 750 h ∆L* values of 18–20 were achieved for these samples compared to tannin-containing samples (∆L* = 13–16). On extended exposure (>1000 h), the rate of darkening was observed to slow with relatively smaller increases in ∆L* values through to 2500 h. For the remainder of the exposure period L* measurements revealed all acrylic-based samples remained relatively stable in colour up to 4000 h (Fig. 6). In considering the extent of ∆L* values at 4000 h, the base resin and commercial samples showed the greatest change in L* values (19–21). Across the 0.2% tannin-containing samples, TanAc, TanMal and TanAcMal all exhibit similar ∆L* values (ca. 18) above 1000 h. The lowest ∆L* values (14) were associated with the native tannin and the tannin methylcarboxylate (TanCarb) additives. While this was visually evident with these samples having a relatively similar colour as other tannin-containing samples (Supplementary Material), the initial darker appearance of both native tannin and TanCarb coatings (Fig. 2) has contributed to these observed lower ∆L* values. A visual perception that samples were beginning to whiten/lighten (Fig. 3) was due to coating chalking, particularly above 3000 h (Table 3). In addition to the contrasting darkening of samples, the colour changes in the a* (red–green) and b* (yellow–blue) spectral components were also observed (Fig. 7). Wood yellowing is expected on artificial weathering [22] and observed as a general trend of b* toward yellow (positive values) during the exposure period. As found for L* values, there was a relatively large change in b* values after initial exposure. On longer exposure there appeared a stable rate of sample yellowing.
Comparison of L* profiles for samples exposed to accelerated weathering over 4000 h of accelerated weathering. Where: Base resin (Black line), commercial (grey line), HWTan (red line), TanMal (violet line), TanMalAc (blue line), TanLau (yellow line), Catechin (yellow dashed line) and TanAc (yellow dotted line). (Color figure online)
Comparison of ∆a* (left) and ∆b * (right) profiles for samples exposed to accelerated weathering over 4000 h of accelerated weathering. Where: Base resin (Dark blue line), commercial (grey line), HWTan (red line), TanAc (blue line), TanMal (violet line), TanMalAc (blue line), TanLau (yellow line), Catechin (yellow dotted line) and TanAc (yellow dashed line). (Color figure online)
Wood coated with tannin-modified styrene-acrylic copolymer coatings was also exposed to accelerated weathering, but over a shorter timeframe (1500 h, Fig. 8). In this series, a focus was those modified tannins which contributed to greater coating performance in the acrylic system (Fig. 5) including native tannin, TanMal, TanMalAc and TanCarb with these also incorporated at differing additive contents. Shown in Fig. 8 are L* profiles which characterise the initial, rapid darkening of wood samples coated with styrene-acrylic coated samples (250 h). This rapid darkening was followed by a relatively gradual change through the remaining exposure period. As observed with the acrylic coating series (Fig. 6), styrene-acrylic samples without tannin show greater rates of darkening with the base resin and commercial additive exhibiting the largest ∆L* values over 1500 h. The tannin-containing samples have lower changes in ∆L* values of 5–8 compared to ca. 18 for the base resin and commercial additive. These ∆L* values were also reflected in overall ∆E colour changes of 11–20 compared with >26 for the reference samples (Supplementary Materials). The extent of this darkening was also dependent on the tannin additive loading with progressive decreases in either PBT or TanMalAc content (0.4–0.1%) leading to greater sample darkening. The 0.4% PBT and 0.4% TanMalAc samples retain the lightest colour which was achieved after 250 h. As an interesting point, the 0.4% PBT sample actually lightened in colour after 250 h, then maintained this lighter colour throughout the remaining exposure period. In contrast, the 0.1% TanMalAc sample showed a high rate of darkening, perhaps indicative of a minimum effective loading for a modified tannin. Addition of TanMal led to L* values which were relatively lighter than the base resin, which was also observed with the acrylic series (Fig. 5). For a* values, these initially show a relatively small change away from red, before becoming stable over the remainder of the exposure period (Fig. 9). Like L* values, there was also some dependency on tannin content where the 0.2% and 0.4% PBT samples also showed the least ∆a* change. As with the acrylic series, sample ∆b* values were initially large, before trending to yellow with longer exposure.
∆L* profiles showing increasing darkening of styrene-acrylic coated wood samples exposed to accelerated weathering Where: Base resin (black line), commercial (grey line), HWTan (red line),TanMal (violet line), TanMalAc (blue line), TanCarb (green line) and 0.4% (black dashed line), 0.2% (black line) and 0.1% (black dots). (Color figure online)
Comparison of ∆a* (left) and ∆b * (right) ∆L* profiles showing increasing darkening of styrene-acrylic coated wood samples exposed to accelerated weathering. Where: Base resin (black line), commercial (grey line), HWTan (red line), TanMal (violet line), TanMalAc (blue line), TanCarb (green line) and 0.4% (black dashed line), 0.2% (black line) and 0.1% (black dots). (Color figure online)
Photo-oxidative Degradation of Acrylic Coatings
The physical appearance of coatings exposed to accelerated weathering were assessed to gauge coating longevity and extent of oxidative degradation (Tables 3, 4). Not unexpectedly, the acrylic and acrylic-styrene resin series have contrasting periods of coating longevity. The modified styrene–acrylic copolymer systems began to fail before 1500 h, while the acrylic coatings only exhibit degradation approaching 4000 h (Supplementary Materials). During accelerated weathering, these acrylic coating samples generally remain in relatively good condition up to 2000 h. However, after 2500 h some samples show diminished gloss with chalking evident for the base resin, commercial and TanAc samples. On extended exposure the base resin and commercial had significant checking at 3000 h with other samples having a perceptive loss in coating gloss. After 4000 h exposure, all samples had some degree of checking and were marred by some whiteness.
For the styrene-acrylic copolymer series, samples visually degraded between 1000 and 1500 h of accelerated weathering. As observed with the acrylic coating series, the base styrene-acrylic coating and Commercial samples were also first to visually degrade losing gloss and whitening with cracks evident after 1000 h. These coatings were deemed to have failed prior to 1250 h. All tannin modified coatings proved satisfactory to at least 1250 h, but extended exposure led to some degree of coating failure for most samples. At 1375 h the 0.1% tannin sample had significantly deteriorated, while only the 0.2% TanMal and 0.2% TanCarbMeth samples had no cracking evident at this time. After completing 1500 h exposure, all samples had also begun to crack, lose gloss and whiten, with only the 0.2% TanMal sample retaining reasonable condition.
Discussion and Summary
Incorporating modified tannins as functional additives in coating formulations has revealed that condensed tannins in either native or modified form could be readily added to acrylic and styrene-acrylic coating emulsions. While some tannin additives introduce colour to the coatings (Table 2; Fig. 3), at typical additive loadings this colour was considered relatively minimal, particularly when modified formulations were applied as clear coatings over wood (L*, a*, b* values, Figs. 3, 4). Water immersion of cured coatings suggest tannins do not leach from the coating nor did the presence of tannins inhibit coating cross-linking and cure (Fig. 5). Being inherent UV absorbers, the presence of tannins enhanced the UV absorption profile of coatings across UVA and UVB wavelengths in both liquid and cured states.
Under accelerated weathering conditions the clear coated wood generally darkens, but the presence of tannin in the coating had a positive influence, with samples retaining a relatively lighter appearance than control reference samples. This colour stability also had a dependency on tannin content with greater tannin contributing to lower rate of wood darkening on UV exposure. This indicates the presence of the tannin may confer a degree of photo-stabilisation of wood components such as lignin and offset the darkening of these components. Practically, this finding may have a commercial application for the photo-stabilisation of clear coated wood surfaces.
As found for the photo-stabilisation of clear coated wood, the tannin content also appeared pivotal to the physical appearance and coating performance. While each modified tannin proved beneficial to improved coating performance, native tannin, TanMal and TanCarb provided greater efficacy than the fully esterified tannins TanAc and TanMalAc. In both coating series the presence of tannin extended coating longevity >25% than offered by the commercial additive at similar additive loading (0.2%). Similar trends in coating efficacy for the different tannin materials were evident for both acrylic and styrene-acrylic systems. Additionally, native tannin and TanMalAc (0.1–0.4% content) reveal a dependence of tannin concentration on this performance. Efficacy was evident at 0.2% content, but at 0.1%, the content may be considered too low, particularly given the calculated tannin content of TanMalAc (Table 1).
Overall, results suggest the UV absorption provided by tannins in both native and modified form was important to coating longevity with antioxidant capacity likely playing a minor role. This beneficial effect was also observed for esterified tannins introduced into plastics [8]. However, unlike with plastics where ester chain length was important for UV stability, it was likely native tannins have become homogeneously dispersed within coating formulations aided by an alkaline pH coating and aqueous solubility without the need for chemical modification. The commonality of extended coating longevity provided by the modified tannins suggest their broader UV absorption profile contributed to this performance. While predominantly UVB, the alkaline coating pH and partial substitution of some modified tannins shift the UV absorption toward the UVA region. Retention of some antioxidant activity compared with commercial additives (Table 1) was likely also pivotal in performance as TanAc was shown to have relatively poor efficacy compared to other modified tannins added at similar tannin content which possess a lower degree of substitution and a minimal antioxidant activity (Table 1). The broader UV absorption and antioxidant capacity of native tannin and presence of carboxy groups of TanMal and TanCarb may explain the greater efficacy of these tannin materials as functional additives to the acrylic and styrene-acrylic coatings.
Study findings reveal the inclusion of condensed tannins in coatings was found to offer a photo-oxidative stabilisation to acrylic and styrene-acrylic polymer coatings promoting longevity. The tannin materials provided enhanced efficacy over a commercial antioxidant and UV stabiliser. While native and modified tannins contribute colour to coatings this can be minimised, particularly on clear coating wood. The presence of tannins also has the beneficial effect of providing a photostabilisation role, reducing the effects of wood darkening on exposure to UV light.
References
Mash A (2015) Sustainability in the Coatings Industry. PCI Magazine
Lligadas G et al (2013) Renewable polymeric materials from vegetable oils: a perspective. Mater Today 16(9):337–343
Tullo AH (2010) Paints from Plants: with biobased-coatings raw materials, companies get more than just independence from petroleum. Chem Eng News 88(15):16–19
Challener C (2010) The natural approach: Renewable resources in coatings. JCT CoatingsTech 7(9):42–47
Hayes DG, Dumont MJ (2016) Polymeric products derived from industrial oils for paints, coatings, and other applications, in Industrial Oil Crops. Elsevier Inc., Amsterdam, p. 43–73
Hare CH, Protective Coatings (1994) Fundamentals of chemistry and composition, edn. S.f.p. coatings. Technology Publishing Company, Pittsburgh
Grigsby WJ et al (2013) Esterification of condensed tannins and their impact on the properties of poly(lactic acid). Polymers 5(2):344–360
Grigsby WJ et al (2014) Evaluating modified tannin esters as functional additives in polypropylene and biodegradable aliphatic polyester. Macromol Mater Eng 299(10):1251–1258
Grigsby WJ, Bridson JH, Schrade C (2015) Modifying biodegradable plastics with additives based on condensed tannin esters. J Appl Polym Sci 132(11):41626
Hagerman AE (2002) Tannin Handbook ftp://217.148.94.129/: University of Miami, Ohio
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13(10):572–584
Hemingway RW, Karchesy JJ, Branham SJ (1989) The chemistry and significance of condensed tannins. Plenum Press, New York
Grigsby WJ (2016) Simulating the protective role of bark proanthocyanidins in surface coatings: Unexpected beneficial photo-stabilisation of exposed timber. J Org Coat. doi:10.1016/j.porgcoat.2017.03.007
Luo C et al (2010) Synthesis, characterization, and thermal behaviors of tannin stearates prepared from quebracho and pine bark extracts. J Appl Polym Sci 117(1):352–360
Arbenz A, Avérous L (2015) Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem 17(5):2626–2646
Bridson JH (2007) Derivatisation of polyphenols, University of Waikato
Bridson JH, Grigsby WJ, Main L (2013) Synthesis and characterization of flavonoid laurate esters by transesterification. J Appl Polym Sci 129(1):181–186
Grigsby WJ, Kadla JF (2013) Evaluating poly(lactic acid) fiber reinforcement with modified tannins. Macromol Mater Eng 299:368–378
Gülçin I (2012) Antioxidant activity of food constituents: an overview. Arch Toxicol 86(3):345–391
Shukla S, Rai JSP (2014) Environmentally-friendly acrylates-based polymer latices. In Tiwari A, Syväjärvi M (eds) Advanced materials for agriculture, food and environmental safety. Wiley Blackwell, Hoboken pp 145–176
Monaghan G (2008) Environmentally advanced technology for semitransparent deck stains. JCT CoatingsTech 5(3):30–37
Tolvaj L, Mitsui K (2005) Light source dependence of the photodegradation of wood. J Wood Sci 51(5):468–473
Acknowledgements
This work was supported by Biopolymer Network Ltd, through funding by the New Zealand Ministry of Business, Innovation and Employment. The authors are thankful to the contributions of Jaime-Anne Elliot (UV spectra) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grigsby, W., Steward, D. Applying the Protective Role of Condensed Tannins to Acrylic-based Surface Coatings Exposed to Accelerated Weathering. J Polym Environ 26, 895–905 (2018). https://doi.org/10.1007/s10924-017-0999-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-0999-0