Abstract
Extraction and depolymerisation of chitin and chitosan from shrimp waste material was carried out using fish proteases aided process. A high deproteinization level (80 %) was recorded with an Enzyme/Substrate ratio of 10 U/mg. The demineralization of shrimp waste was completely achieved within 6 h at room temperature in HCl 1.25 M, and the residual content of calcium in chitin was below 0.01 %. The degree of N-acetylation, calculated from the 13C CP/MAS-NMR spectrum, was 85 %. The chitin obtained was converted to chitosan by N-deacetylation. X-ray diffraction patterns also indicated two characteristics crystalline peaks approximately at 10° and 20° (2θ). Chitosan was then evaluated in the treatment of unhairing effluents from the tanning industry. A result showed that chitosan as a coagulant has good performance in alkaline pH and at concentration of 0.5 g/L. Within these conditions, chitosan could decrease turbidity value, total suspended solids (89 % at 1.5 g/L), biological oxygen demand (33.3 % at 1.5 g/L) and chemical oxygen demand (58.7 % at 1.5 g/L).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
About 45 % of processed seafood consists of shrimp, the waste of which is composed of exoskeleton and cephalothoraxes [1, 2], The latter has become a problem for the environment. This waste represents 50–70 % of the weight of the raw material; however it contains valuable components such as protein and chitin [3, 4].
Chitin, the second most abundant biopolymer next to cellulose and its derivatives like chitosan, carboxymethyl chitin, etc., are widely recognized to have immense applications in many fields [5]. They are widely used in the food industry, medicinal fields, chemical industries, textiles, water treatment plants, etc. [6, 7]. Glucosamine is another value-added product prepared from chitin by hydrolysis and it has versatile applications in pharmaceutics [8]. The advantages for the greater use of these biopolymers in various industries are cost of the manufacturing process and the technical [9]. The commercial method of preparation of chitin from shrimp shell involves strong acid and alkali treatment to remove the minerals and proteins, respectively [10]. However, the use of these chemicals causes depolymerisation of the product and therefore affects properties such as molecular weight, viscosity and degree of deacetylation [5]. These chemical treatment methods ring about hazardous environmental problems like disposal of wastewater. The cost of the chemicals is another drawback of this approach.
Potential and usual applications of chitin and its derivatives, mainly chitosan, are estimated to be more than 200 [11]. These biopolymers have antimicrobial activity, besides being biocompatible and biodegradable [12–14]. They display a wide range of applications in different fields, e.g. in cosmetics, agriculture, food, pharmacy, biomedical, paper industry and also as absorbent materials for wastewater treatment [15–17]. Chitosan has been used to modify the surface of nonwoven fabrics and polypropylene films to improve antimicrobial properties [18, 19].
Chitosan also possesses several intrinsic characteristics that make it an effective coagulant and/or flocculant for the removal of contaminants in the dissolved state [14, 20, 21]. It has characteristics of both coagulants and flocculants, i.e., high cationic charge density, long polymer chains, bridging of aggregates and precipitation (in neutral or alkaline pH conditions). Its uses are justified by two important advantages: firstly, its non-toxicity and biodegradability [22]; secondly its outstanding chelation behaviour [23, 24]. Its unique physico-chemical properties render it very efficient in interactions with various contaminants including both particulate and dissolved substances. These properties have been exploited for the design of coagulation/flocculation processes applied to the treatment of various effluents. For example, chitosan has been successfully used, for precipitative flocculation at pH above the pKa of the macromolecule, in the treatment of mineral and organic suspensions [25–27] and the coagulation of negatively charged contaminants in acidic solutions containing dyes [28] or humic acid [29, 30]. The main reasons for the success of biopolymers such as chitosan in wastewater treatment using coagulation/flocculation processes are: chitosan has the advantage of being non corrosive and safe to handle well (non hazardous product, not irritating for skin and eyes…) [22, 31].
The main objective of the present work is to isolate the useful polymer chitin from the waste by-products of the seafood industry in Tunisia using fish proteases aided process. The obtained chitin will be characterized and deacetylated to the more useful chitosan, which was used for the treatment of effluent from tannery industry.
Materials and Methods
Raw Material
The shrimp (Penaeus longirostris) shell from cephalothorax, abdomen and appendix were obtained in fresh condition from a local shrimp processing plant “Calembo” at Sfax, Tunisia. Prior to use, the shrimp shells were thoroughly washed with distilled water and grounded. The shells were then stored at −20 °C until further use.
The barbel (Barbus callensis) used in the present work were obtained from Barrage SIDI SAAD, Kairouan, Tunisia. The samples were packed in polyethylene bags, placed in ice [sample/ice ratio of about 1:3 (w/w)], and transported to the laboratory within 2 h after collection. The internal organs were separated and then stored in sealed plastic bags at −20 °C.
Preparation of Alkaline Crude Protease Extract
Crude protease extract was prepared according to the method of Sila et al. [32]. Viscera from B. callensis were washed with water then with buffer A (10 mM Tris–HCl, pH 8.0). The cleaned viscera (100 g) were defatted by homogenization with cold acetone at a ratio of 1:2 (w/v) for 30 s using a Moulinex R62 homogenizer (Organotechnie, Courneuve, France). The homogenate was filtered using Whatman No. 4 paper. The acetone insoluble material was washed three times with cold acetone and then dried at room temperature overnight. The acetone dried powder was homogenized for 2 h with buffer A at 4 °C (at a concentration of 1:10 (w/v)). The homogenate was centrifuged (MED-instrument MPW-350 R) at 8,500×g and 4 °C for 30 min. The resultant supernatant was collected and used as the crude protease extract.
Chemical Analysis
The moisture and ash content were determined according to the AOAC [33] standard methods 930.15 and 942.05, respectively. Total nitrogen content was determined by using the Kjeldahl method. Protein was estimated by multiplying total nitrogen content by the factor of 6.25. Lipids were determined gravimetrically after Soxhlet extraction of dried samples with hexane.
Deproteinization of Shrimp Wastes by Fish Proteases
Shrimp wastes were mixed with water at a ratio of 1:2 (w/v), minced then cooked for 20 min at 90 °C. The cooked sample was then homogenized in a Moulinex® blender for about 2 min. The pH of the mixture was adjusted to 8.0. Then, the shrimp wastes proteins were digested with barbel proteases using different E/S ratio (Units of enzyme/mg of protein). Enzyme activity expressed as units was calculated according to Khembavi and Kulkarni [34]. Protein content was determined by Kjedahl method.
After incubation for 3 h at 40 °C, the reaction was stopped by heating the solution at 90 °C during 20 min to inactivate the enzyme. The shrimp wastes protein hydrolysates were then centrifuged at 5,000g for 20 min to separate insoluble and soluble fractions. The solid phase was washed and then dried for 1 h at 60 °C. The supernatant was lyophilized and used for analysis of protein concentration.
Deproteinization (DP) was expressed as percentage and computed by the following equation as described by Rao et al. [35].
where P O and P R are protein concentrations (%) before and after hydrolysis; while, O and R represent the mass (g) of original sample and hydrolyzed residue in dry weight basis, respectively.
Demineralization
Demineralization was carried out in a dilute HCl (1.5 M) solution. Solid fractions obtained after hydrolysis by barbel proteases were treated with HCl in 1:10 (w/v) ratio for 6 h at room temperature (25 °C) under constant stirring. The chitin product was filtered through four layers of gauze with the aid of vacuum pump and washed to neutrality with deionized water and then freeze-dried.
13C CP/MAS-NMR Spectroscopic Analysis
Chitin structural analysis was carried out by 13C NMR with CP/MAS technique (cross-polarization, magic-angle-spinning) using a BRUKER-ASX300 instrument. NMR spectra were recorded at a 13C frequency of 75.5 MHz (field of 7.04 T). CP/MAS sequence was used with the following parameters: the 13C spin lattice relaxation time was 5 s, powdered samples were placed in an alumina rotor used for the double air-bearing-type MAS system and spun as fast as 8 kHz. Contact time was 8 ms.
The degree of acetylation (DA) of the samples was determined by dividing the intensity of the resonance of the methyl group carbon by the average intensity of the resonances of the glycosyl ring carbon atoms. The DA was calculated using the following relationship Ottøy et al. [36]
(I is the intensity of the particular resonance peak).
Deacetylation of chitin
The purified chitin was treated with 50 % (w/v) NaOH at 80 °C for 4 h until it was deacetylated to chitosan. After filtration, the residue was washed with distilled water and the crude chitosan was obtained by drying in a dry heat incubator at 50 °C overnight.
X-Ray Diffraction (XRD) of Chitosan
The X-ray diffraction pattern of chitosan was recorded at room temperature on a X-ray diffractometer (D8 advance, Bruker, Germany). The data were collected in the 2θ range 2–70° with a step size of 0.02° and a counting time of 5 s/step.
Efficiency of Chitosan in the Treatment of Unhairing Effluents from the Tanning Industry
Raw Unhairing Wastewater
The wastewater used in this study was collected from a Tunisian operating tanning factory. The wastewater samples were collected from the liming processes of cow and sheep hides. Effluents were filtered through 140 μm mesh sieves to remove hair, pieces of skin, and fats. After collection, samples were stored in the dark at 4 ± 1 °C until use.
Jar tests
The coagulation–flocculation experiments were carried out in a jar test apparatus (OSK-Japan) with a six jars. The wastewater after a well mixing was allowed to settle for 30 min [37] and the supernatant was transferred to a clean container. Precise doses of flocculants aluminium polychloride and a precise doses of coagulants including aluminium sulphate (Al2(SO4)3) and chitosan, were added to 500 mL jars containing 250 mL of wastewater. A series of jar tests were carried out as follows: first rapid mixing stage carried out on jars at 100 rpm for 2 min and then slow mixing stage carried out at 30 rpm for 20 min and finally the solutions were settled for 30 min [37, 38]. The produced supernatants were used for the measurement of remained COD, TOC, BOD5 and TSS.
Experimental Design
Factors such as type and dose of coagulant influence the coagulation–flocculation process. In addition to these factors, stirring speed, stirring time, settling time, and temperature are effective, too. However, in this work the last ones considered constant and the effect of first ones (type and dose) were investigated. The tests were conducted at average room temperature of 25 °C. The samples of wastewater were transferred in succession to the six jars. Chitosan and aluminium sulphates of 0.5, 1.5, and 2 g/L were added to the jars, respectively. For each jar a concentration of flocculent was added to the wastewater sample.
Analytical Procedures
The influent and effluent quality parameters including COD (Chemical Oxygen Demand), BOD5 (Biological Oxygen Demand) and TSS (Total suspended Solids) were determined according to standard methods [39]. Total organic carbon (TOC) was measured by a Shimadzu-TOC-5000A analyzer (catalytic oxidation on Pt at 680 °C) via calibration using standards of potassium phthalate according to standard methods described in the Japanese International Standard (JIS) handbook (JIS, 1998).
Phytotoxicity Study
Unhairing effluent phytotoxicity was assessed for untreated unhairing wastewater and treated effluent with chitosan and aluminium sulphate against seed germination of tomato (Lycopersicon esculentum). Phytotoxicity was determined using a modified Zucconi test [39, 40] by measuring seed germination. Thirty seeds were placed on filter papers in 9 cm Petri dishes and 5 mL of each sample were then added to each dish. Dishes were incubated in the dark at 26 ± 2 °C for 5 days. Distilled water was used as control. All samples, including controls, were triplicated. A germination index (GI) was calculated according to the following formula:
Where SS, SC are the number of germinated seeds of the sample and the control, respectively, and LS, LC, are the average root length of seeds for the sample and the control, respectively.
Statistical Analysis
All experiments were carried out in triplicate, and average values with standard deviation errors are reported. Mean separation and significance were analyzed using the SPSS software package (SPSS, Chicago, IL). Correlation and regression analysis was carried out using EXCEL program.
Results and Discussion
Extraction of Chitin from Shrimp Waste
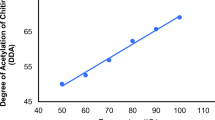
Chitin in the exoskeleton of shrimp shells is closely associated with proteins. Therefore, deproteinization in chitin extraction process is crucial. Chemical treatment requires the use of HCl and NaOH, which can cause deacetylation and depolymerization of chitin. Many reports have demonstrated the application of proteolytic microorganisms for the deproteinization of marine crustacean wastes to produce chitin [41, 42]. To the best of our knowledge, there are no available reports on the enzymatic deproteinization of shrimp wastes by fish proteases. The crude protease extract from barbel viscera was applied for the deproteinization of shrimp waste to produce chitin. Different E/S ratios (0.5, 1, 3, 5, 10 and 15 U/mg protein) were used to compare the deproteinization efficiency. As shown in Fig. 1, the deproteinization rate with a ratio of 10 was 80 %. Beyond a ratio of 10, no significant increase in the deproteinization rate was observed. The deproteinization activity of barbel crude proteases was better than many bacterial proteases reported in many previous studies [9, 43, 44]. The fact that deproteinization cannot reach 100 % may be explained by the non-accessibility of enzymes to some proteins protected by chitin.
In the recovery of chitin from shrimp waste, associated minerals should be removed as a second stage. As a consequence, shrimp wastes deproteinized by enzymatic treatment was subjected to mild acid treatment in order to remove minerals. The demineralization was completely achieved within 6 h at room temperature after treatment with 1.25 M HCl solution at a ratio of 1:10 (w/v). One of the factors determining the good quality of chitin is the low mineral content [45].
Chitin Characterization
The characteristics of shrimp waste and chitin that was prepared by enzymatic treatment are shown in Table 1. The ground shrimp wastes before pre-treatment contained a relatively high content of protein (31.3 ± 0.61 %) and ash (28.61 ± 0.79 %). These results are comparable with those reported by previous studies [46, 47]. The demineralization conditions used in this study reduce the mineral content to permissible limits in the chitin. Indeed, the ash content was reduced to about 0.22 %. This value was lower than that found by Sini et al. [5]. This low ash content for chitin indicated the suitability of removal of calcium carbonate and other minerals from the raw material. The residual protein content must be corrected, and the percentage of the non-protein nitrogen fraction (nitrogen from chitin) must be withdrawn from the total nitrogen value as reported by Rødde et al. [48].
13C CP/MAS-NMR Spectroscopic Analysis of Extracted chitin
The structure of the chitin and its purity was evaluated by using 13C CP/MAS solid-state NMR spectroscopy. 13C CP/MAS-NMR spectrum of the chitin sample prepared by enzymatic treatment is shown in Fig. 2. Each spectrum consisted of eight well-defined resonances of C1–C6 carbons of the N-acetylglucosamine monomeric unit, which were observed between 50 and 110 ppm, indicating high structural homogeneity. The C=O signal appears as a sharp and symmetric profile indicating a unique conformational state, typical of α-chitin structure. In addition, the 13C signals for C3 (72.88 ppm) and C5 (73.69 ppm) are clearly separated into two signals. These are similar to the commercial α-chitin and to chitins reported by Cardenas et al. [49] and Focher et al. [50]. However, for the β-chitin from Illex argentinus squid pens, the C3 and C5 merge into single resonance centered at 75.0 ppm [51]. The degree of acetylation is the most important characteristic of chitin, and its value depends on the raw material and the processes used for the deproteinization and demineralization. Solid-state 13C CP/MAS-NMR spectroscopy appears to be suitable for the evaluation of the degree of acetylation [52] and is known to be very sensitive to changes in the local structure. The degree of acetylation from the NMR spectra of chitin isolated by enzymatic deproteinization was 85 % (Table 1). These values are similar to those obtained for the chitin produced using B. subtilis fermentation [5] and chitin from shrimp Crangon crangon shells deproteinized by Alcalase® [53]. Compared with the commercial α-chitin [54], the obtained chitin was more deacetylated.
Preparation of Chitosan
The deacetylation degree, molecular weight and also the order of its repetitive units are important parameters for chitosan, as they affect its properties [55]. The deacetylation degree of chitosan is important for its use in the industry. From this regard, certain researchers [56, 57] suggested that the term chitosan should be used when the degree of deacetylation is above >70 %. In the present study, the deacetylation degree of the extracted chitosan was determined as 85 %.
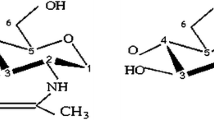
Figure 3a shows the chitosan spectrum, in which the deacetylation of chitin is evident, since there are no peaks at 23 and 173 ppm that correspond to the CH3 and C = O groups, respectively. The other peaks correspond to C1 (105.3 ppm), C2 (57.9 ppm), C3 (75.8 ppm), C4 (82.3 ppm), C5 (75.8 ppm) and C6 (61.1 ppm). C3 and C5 peaks appear as an only signal at 75.8 ppm. The presence of a peak at 33.5 ppm (not expected), could be due to the presence of a possible by–product or impurity in the sample (Fig. 3a).
The X-ray diffraction pattern for chitosan was given between 10 and 60 of 2θ in Fig. 3b. The prepared chitosan has two peaks at 2θ = 10 and at 2θ = 20. In the literature, many XRD patterns of chitosan have two characteristic peaks which are usually around 2θ = 10 and 2θ = 20 [58].
Effects of Chitosan and Aluminium Sulphate on the Treatment of Unhairing Effluents
Leather tanning generates many complex and high-loaded effluents that require treatment before being discharged into receiving waters [38]. The main characteristics of tannery effluents are high organic loading, high ammonia and organic nitrogen content and the presence of specific inorganic compounds (sulphide, chromium, sodium chloride, etc.). In particular, the unhairing stage associated with beam house processes generates a highly toxic, alkaline wastewater containing high concentrations of proteins, sulphide, suspended solids and salts (e.g. sodium chlorine) [59]. In this study the wastewater used was collected from a Tunisian operating tanning factory.
Coagulation is mainly induced by inorganic metal salts, e.g., aluminium and ferric sulphates and chlorides. The most common additives are aluminium sulphate (generally known as alum), ferric chloride and ferric sulphate [22, 60]. The addition of these cations results in colloidal destabilization, as they specifically interact with and neutralise the negatively charged colloids. Chitosan has unique properties among biopolymers especially due to the presence of primary amino groups and it is a commercially interesting compound because of its high nitrogen content in comparison to cellulose [3]. The main parameters influencing the characteristics and properties of chitosan is the degree of deacetylation (DD), representing the molar fraction of deacetylated units, and crystallinity [61, 62]. Chitosan is widely applied in water and wastewater treatment because it can be conditioned and used for pollutant complexation in different forms.
COD and BOD5 Removals
Figure 4 shows the percent removal of COD and BOD as a result of unhairing wastewater treatment by coagulation–flocculation process. Figure 4a depicts the variation of COD removal with different doses of chitosan and aluminium sulphates coagulants. Result showed that, at a dose of 0.5 g/L, chitosan results in a maximum COD removal of 53 %, while aluminium sulphate leads to a minimum COD removal efficiency of 24 %. COD removal efficiencies increased as the sulphate aluminium coagulant dose increased. Indeed, maximum COD removal of 58.7 % was reached at a 1.5 g/L, compared with the lowest COD removal of 24 %, attained at a concentration of 0.5 g/L. However, the minimum dose of 0.5 g/L chitosan was sufficient to obtain higher COD removal. In the same context, Haydar and Aaziz [63] showed that COD removal varied between 53.3 and 60.9 % during coagulation of tannery wastewater by alumine in the range dose of 200 and 240 mg/L. Whereas, Song et al. [37] showed that COD removal varied between 15 and 35 % for the range dose of aluminium sulphate 400–1,200 mg/L at pH 8, during tannery wastewater. These results can be explained by the fact that the quality of raw tannery wastewater affects on the efficiency of coagulation process [36]. The optimum dose of coagulant is dependent on the amount of parameters such as TSS, TDS and concentration of pollutants (Cr, S2 −, N, etc.), so it is better determined by jar test [37, 64].
The removal of BOD5 (Fig. 4b) does not compare well with that of COD during coagulation assay. Thus, the BOD5 removals efficiencies raises as both aluminium sulphate and chitosan coagulants concentration increased. Indeed, maximum BOD5 removal of 33.3 and 27.7 % was reached with 1.5 g/L of sulphate alumine and chitosan, respectively, compared with the lowest COD removals of 14 and 11 % attained at 0.5 g/L of sulphate aluminium and chitosan, respectively.
TOC Removal
Figure 5 depicts the TOC removal efficiency after treatment with coagulation–flocculation process of unhairing wastewater. Result showed that chitosan dose of 1 g/L is enough to achieve important TOC removals (31 %). However, the dose of sulphate aluminium affects the TOC removal. Thus, it reaches its maximum at the highest tested dose of 1.5 g/L. The comparison of sulphate aluminium and chitosan effects showed that the TOC removal efficiencies are 20.6 and 34.4 % for a 1.5 g/L, respectively. Therefore, chitosan, which have biological origin, is more efficient as coagulant than the aluminium sulphate.
TSS Removal
Figure 6 shows that chitosan and sulphate aluminium coagulants contributed to TSS removal of 89 and 68 % at a dose of 1.5 g/L, respectively. Thus, chitosan is more efficient coagulant than aluminium sulphate to remove suspended solid. Furthermore, the important SS removal was obtained from a dose of 0.5 g/L when using chitosan as coagulant. However, the SS removal increased as the concentration of sulphate aluminium increased. Thus, SS removals are 32, 50 and 68 % after adding 0.5, 1 and 1.5 g/L of aluminium sulphate, respectively. It is noted that the effects of both tested coagulants on the SS removal is more significant than that on COD, BOD and TOC removal during coagulation–flocculation process. This result proved that coagulation serves to agglomerate the very small particles into sizes that are settable or can be removed by filters or absorption [65].
Phytotoxicity Study (Germination Test)
Toxicity of raw and treated unhairing wastewater was tested using a seed germination assay. Seed germination of tomato species was strongly inhibited by raw waste water and treated wastewater by sulphate alumina coagulant (Fig. 7). In treated effluent by chitosan coagulant, however, a high germination index was noticed (98 %). Thus, the coagulation of unhairing wastewater by chitosan had a beneficial effect on seeds germination. This is confirmed by the significant role of chitosan to remove al tested parameters such as COD, BOD, TOC and TSS during coagulation–flocculation assay.
Conclusion
In this paper, enzymatic deproteinization process was applied for chitin recuperation. To our knowledge, the use of fish proteases for this purpose has never been demonstrated before. Barbel proteases were found to deproteinize 80 % of the shell proteins with an Enzyme/Substrate of 10 U/mg. Chitin obtained by enzymatic deproteinizations was then converted to chitosan by deacytilation.
The work demonstrated that chitosan can be used as coagulants in the treatment of unhairing effluents. It is important to correspond that it has natural biological origin. Also, the minimum used dose (0.5 g/L) of chitosan is enough to obtain maximum performance.
References
Ibrahim HM, Salama MF, El-Banna HA (1999) Shrimp’s waste: chemical composition, nutritional value and utilization. Nahrung 43:418–423
Venugopal V, Shahidi F (1995) Value-added products from underutilized fish species. Crit Rev Food Sci 35:431–453
Roberts GAF (1992) Chitin chemistry, 1st edn. Macmillan, London
Shahidi F, Synowiecki J (1991) Isolation and characterization of nutrients and value-added products from snow crab (Chinoecetes opilio) and shrimp (Pandalus borealis) processing discards. J Agric Food Chem 39:1527–1532
Sini TK, Santhosh S, Mathew PT (2005) Study of the influence of processing parameters on the production of carboxymethyl chitin. Polymer 46:3128–3131
Santhosh S, Sini TK, Anandan R, Mathew PT (2006) Effect of chitosan supplementation on antitubercular drugs-induced hepatotoxicity in rats. Toxicology 219:53–59
Muzzarelli RAA (1996) Chitin chemistry. In: Salamone JC (ed) The polymeric materials encyclopedia. CRC Press, Inc., Boca Raton, FL, USA, pp 312–314
Santhosh S, Anandan R, Sini TK, Mathew PTJ (2007) Protective effect of glucosamine against ibuprofen-induced peptic ulcer in rats. Gastroenterol Hepato 22:949–953
Bustos RO, Healy MG (1994) Chitin World. Wirtschafts verlag NW-Verlag fur neue issenschaft Gmbh, Bremerhaven, pp 15–25
Bautista J, Jover M, Gutierrez JF, Corpas R, Cremades O, Fontiveros E, Iglesias F, Vega J (2001) Preparation of crayfish chitin by in situ lactic acid production. Process Biochem 37:229–234
Brzeski M (1987) Chitin and chitosan-putting waste to good use. Infofish Int 5:31–33
Mathur NK, Narang CK (1990) Chitin and chitosan, versatile polysaccharides from marine animals. J Chem Educ 67:938–942
Muzzarelli RAA (1977) Chitin. Pergamon Press, New York
Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym 46:1–273
Bautista-Baños S, Hernández-Lauzardo AN, Velázquez-del Valle MG, Hernández-López M, Ait Barka E, Bosquez-Molina E, Wilson CL (2006) Chitosan as a potential natural compound to control pre and postharvest disease of horticultural commodities. Crop Prot 25:108–118
Rashidova SS, Milusheva RY, Voropaeva NL, Pulatova SR, Nikonovich GV, Ruban IN (2004) Isolation of chitin from a variety of raw materials, modification of the material, and interaction its derivatives with metal ions. Chromatographia 59:783–786
Sashiwa H, Aiba S (2004) Chemistry modified chitin and chitosan as biomaterials. Prog Polym Sci 29:887–908
Abdou ES, Elkholy SS, Elsabee MZ, Mohamed E (2008) Improved antimicrobial activity of polypropylene and cotton nonwoven fabrics by surface treatment and modification with chitosan. J Appl Polym Sci 108:2290–2296
Elsabee MZ, Abdou ES, Nagy KSA, Eweis M (2008) Surface modification of polypropylene films by chitosan and chitosan/pectin multilayer. Carbohydr Polym 71:187–195
No HK, Meyers SP (2000) Application of chitosan for treatment of wastewaters. Rev Environ Contam Toxicol 163:1–28
Wase DAJ, Forster CF (1997) Biosorbents for metal ions. CRC Press, Boca Raton
Bratby J (2007) Coagulation and flocculation in water and wastewater treatment, 2nd edn. IWA Publishing, London
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purifi Technol 38:43–74
Roussy J, Van Vooren M, Dempsey BA, Guibal E (2005) Influence of chitosan characteristics on the coagulation and the flocculation of bentonite suspensions. Water Res 39:3247–3258
Roussy J, Van Vooren M, Guibal E (2005) Influence of chitosan characteristics on coagulation and flocculation of organic suspensions. J Appl Polym Sci 98:2070–2079
Roussy J, Van Vooren M, Guibal E (2004) Chitosan for the coagulation and flocculation of mineral colloids. J Dispers Sci Technol 25:663–677
Guibal E, Roussy J (2007) Coagulation and flocculation of dye-containing solutions using a biopolymer (chitosan). React Funct Polym 67:33–42
Bratskaya SY, Schwarz S, Chervonetsky D (2004) Comparative study of humic acids flocculation with chitosan hydrochloride and chitosan glutamate. Water Res 38:2955–2961
Bratskaya SY, Avramenko VA, Sukhoverkhov SV, Schwarz S (2002) Flocculation of humic substances and their derivatives with chitosan. Colloid J 64:756–761
Bolto B, Gregory J (2007) Organic polyelectrolytes in water treatment. Water Res 41:2301–2324
Sila A, Nasri R, Jridi M, Balti R, Nasri M, Bougatef A (2012) Characterisation of trypsin purified from the viscera of Tunisian barbel (Barbus callensis) and its application for recovery of carotenoproteins from shrimp wastes. Food Chem 132:1287–1295
AOAC (1995) Official methods of analysis, 16th edn. Association of Official Analytic Chemist, Washington
Kembhavi AA, Kulkarni A (1993) Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM No.64. Appl Biochem Biotechnol 327:83–92
Rao MS, Munoz J, Stevens WF (2000) Critical factors in chitin production from biomaterials of black tiger shrimp (Penaeus monodon) by fermentation. App Microbiol Biotechnol 54:808–813
Ottøy MH, Vårum KM, Smidsrød O (1996) Compositional heterogeneity of heterogeneously deacetylated chitosans. Carbohydr Polym 29:7–24
Song Z, Williams CJ, Edyvean RGJ (2004) Treatment of tannery wastewater by chemical coagulation. Desalination 164:249–259
Song Z, Williams CJ, Edyvean RGJ (2000) Sedimentation of tannery wastewater. Water Res 34:2171–2176
AFNOR (1997) Recueil des normes afnor, Qualité de l’eau, Méthodes d’analyse. Ed Afnor
Zucconi FA, Pera MF, Bertoldi M (1981) Evaluating toxicity of immature compost. Biocycle 22:54–57
Oh KT, Kima YJ, Nguyen VN, Jung WJ, Park RD (2007) Demineralization of crab shell waste by Pseudomonas aeruginosa F722. Process Biochem 42:1069–1074
Jo GH, Jung WJ, Kuk JH, Oh KT, Kim YJ, Park RD (2008) Screening of protease- producing Serratia marcescens FS-3 and its application to deproteinization of crab shell waste for chitin extraction. Carbohydr Polym 74:504–508
Jung WJ, Jo GH, Kuk JH, Kim YJ, Oh KT, Park RD (2007) Production of chitin from red crab shell waste by successive fermentation with Lactobacillus paracasei KCTC-3074 and Serratia marcescens FS-3. Carbohydr Polym 68:746–750
Oh YS, Shih IL, Tzeng YM, Wang SL (2000) Protease produced by Pseudomonas aeruginosa K-187 and its application in the deproteinization of shrimp and crab shell wastes. Enzyme Microb Technol 27:3–10
Tolaimate A, Debrieres J, Rhazi M, Alagui A (2003) Contribution to the preparation of chitin and chitosan with controlled physico–chemical properties. Polymer 44:7939–7952
Percot A, Viton C, Domard A (2003) Optimization of chitin extraction from shrimp shells. Biomacromolecules 4:12–18
Fanimo AO, Oduguwa OO, Onifade AO, Olutunde TO (2000) Protein quality of shrimp waste meal. Bioresour Technol 72:185–188
Rødde RH, Einbu A, Vårum KM (2008) A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohydr Polym 71:388–393
Cardenas G, Cabrera G, Taboada E, Miranda SP (2004) Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J Appl Polym Sci 93:1876–1885
Focher B, Beltrame PL, Naggi A, Torri G (1990) Alkaline N-deacetylation of chitin enhanced by flash treatments. Reaction kinetics and structure modifications. Carbohydr Polym 12:405–418
Cortizo MS, Berghoff CF, Alessandrini JL (2008) Characterization of chitin from Illex argentinus squid pen. Carbohydr Polym 74:10–15
Heux L, Brugnerotto J, Desbrieres J, Versali MF, Rinaudo M (2000) Solid state NMR for determination of degree of acetylation of chitin and chitosan in A. Niger. Biomacromolecules 1:746–751
Synowiecki J, Al-Khateeb NAAQ (2008) The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chem 68:147–152
Lavall RL, Assis OBG, Campana-Filho SP (2007) β-Chitin from the pens of Loligo sp.: extraction and characterization. Bioresour Technol 98:2465–2472
Lamarque G, Lucas JM, Viton C, Dormard A (2005) Physicochemical behavior of homogeneous series of acetylated chitosans in aqueous solution: role of various structural parameters. Biomacromolecules 6:131–142
Li Q, Dunn ET, Grandmaison SW, Goosen MFA (1997) Application and properties of chitosan. In: Goosen MFA (ed) Applications of chitin and chitosan. Technomic Publishing Co. Inc., Lancaster, PA, pp 1–21
Muzzarelli RAA (1985) Chitin. In: Aspinall GO (ed) The polysaccharides. Academic Press, New York, NY, pp 417–450
Yen MT, Yang JH, Mau JL (2009) Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr Polym 75:15–21
Cooman K, Gajardo M, Nieto J, Bornhardt C, Vidal G (2003) Tannery wastewater characterization and toxicity effects on Daphnia spp. Environ Toxicol 17:45–51
Stechemesser H, Dobiáš B (2005) Coagulation and flocculation Surfactant science series, vol. 126, 2nd edn. CRC Press, Boca Raton
Kurita K (2006) Chitin and chitosan: functional biopolymers from marine crustaceans. J Marine Biotechnol 8:203–206
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Haydar S, Aziz JA (2009) Characterization and treatability studies of tannery wastewater using chemically enhanced primary treatment (CEPT)—a case study of Saddiq Leather Works. J Hazard Mater 163:1076–1083
Abera S, Salari D, Parsa MR (2010) Employing the Taguchi method to obtain the optimum conditions of coagulation–flocculation process in tannery wastewater treatment. Chem Eng J 162:127–134
Corbitt RA (1989) In: Crawford HB, Gleason D (eds) Stand handbook of environment engineering. McGraw Hill, New York. pp 671–698
Acknowledgments
This work is a part of a master by Assaad Sila whose research was supported financially by Ministry of Higher Education and Scientific Research, Tunisia through a grant to Laboratoire de Génie Enzymatique et de Microbiologie-ENIS. We warmly thank Mr. Rachid Hajji (Unité de Recherche de Service Commun RMN des Solides) for his invaluable help with RMN analysis. We thank Ms. Amira Zineddine (Laboratoire Génie des Matériaux et Environnement) for his help in the X-Ray analysis. The authors would like to thank Mr. Baha Eddine Abdelmalek (Shrimp Processing Plant, Sfax-Tunisia) for providing of raw materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sila, A., Mlaik, N., Sayari, N. et al. Chitin and Chitosan Extracted from Shrimp Waste Using Fish Proteases Aided Process: Efficiency of Chitosan in the Treatment of Unhairing Effluents. J Polym Environ 22, 78–87 (2014). https://doi.org/10.1007/s10924-013-0598-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-013-0598-7