Abstract
In the meat production industry, large volumes of wastewater are generated containing great quantities of organic matter that requires y safe disposal or utilization. As a result, management of poultry wastewater is of great concern worldwide. However, problems associated with wastewater disposal are a well-known phenomenon. Nevertheless finding solutions to treat different waste types are not always an orderly method to solve and this have cause a lot of adverse effects on the receiving environment. In the current study, chitosan was synthesized from shrimp chitin to determine its usefulness in removing heavy metals from meat wastewater. Factors for example yield, moisture and ash content and deacetylation (DDA) were tested as well and results showed that chitosan was a source from shrimp chitin. Structural properties such FTIR, SEM and XRD were used to determine the structural morphology, and the final results implies successful isolation of chitosan. Modification of chitosan product was then accomplished via cross-linked chitosan with a series of cross-linking agent; glutaraldehyde, epichlorohydrine, p-benzoquinone s-methylbutylamine and 1,3-dichloroaceone adsorbents. Satisfactory percentages were obtained from shrimp chitosan cross-linked s-methylbutylamine, glutaraldehyde and epichlorohydrine (63–72%) whereas lower yield was observed from chitosan starch cross-linked p-benzoquinone (57%). The usefulness of the chitosan modified products were then investigated in purifying wastewater effluent using HG-AAS. Results of qualitative and quantitative analysis on the elemental content showed the presence of the following elements present in different concentrations: Pb, Cr, Cu, Fe and Zn in the meat wastewaters. Lower concentration ranges (0.01–0.9 mg/L) of these heavy metals were observed for Pb(II), Cr(VI), Cu(II), Fe(II) and Zn(II) after testing the different chitosan cross-linked products (A–E). Among all the metals tested, shrimp chitosan cross-linked with 1,3-dichloroacetone was found to be the most effective product for heavy metals removal. These results also revealed that there is a decrease in the amount of heavy metals present in meat wastewater effluent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution in general is a serious threat to the environment. These pollutions can come from different sources including but not limited to water courses (river lakes damp, stream), industrial and domestics waste. Nevertheless, environmentalist and government globally are more concerned about those pollution leading to human health problems, for instance heavy metals. In South Africa for example surface water are mainly polluted by heavy metals from industrial wastewater effluent, which in general subsidizes approximately 85% of the wastewater [1]. In particular, liquid waste which contain several types of dissolved chemicals including heavy metals ions that often results in colored effluents. When these effluents are disposed or washed down in river systems, the adverse effects are devastating and can ranges from immediate to long term effects. Pollution problems has been of great concern over the last decades, firstly humankind failed to understand that nature does not absorb any artificial object, or elements [2]. One of the biggest environmental disaster cause by pollution was perhaps that of Love Canal, a situation that caused mankind many losses financially and many human life’s. It is very much believed that up to date the contaminated area is not efficiently cleaned up. This event was a warning to mankind, letting them know nature is not able to handle chemical compounds manufactured by men [3]. In particular, one will mention toxic heavy metal are known to be present in wastewater. We shall recall for those who are not acquainted to these topic that, toxic heavy metals can be referred to any relatively dense metalloid able to be noted for its potential poisonousness, for instance to health and environmental contexts. We can list here specific heavy metals including mercury, lead, arsenic and cadmium that are included in the list of world health organization, as they appear to be a potential threat and a concern to public [4]. We have to mention that such metals are found unsurprisingly in the earth crust. Nevertheless, they become concentrated in the environment due to human activities. Exposure pathways to heavy metals can be through plants, animals inhalation and diet. Therefore, disposal of wastewater is important issue since most of the used techniques do not completely address the issue of heavy metals due to the complexity of the chemistry involved and the problems with the separation techniques [5, 6]. According to Anthony and Kozlowski some of the environmental effects caused by these elements which amongst includes the toxicity of these elements to aquatic life and the disruption to the ecological balance in the aquatic environment. Heavy metals are easily absorbed by aquatic species and the effects caused thereof have been shown to inhibit growth and reproduction which eventually leads to extinction of aquatic life. Thus, the determination of the amounts of heavy metals is especially important where there is a risk of having anthropogenic influence on aquatic environment [7].
In the last years, several methods and techniques have been developed to understand at which extend the polluted water can be purified. One of the most promising technique so far is perhaps the use of chitosan derivative as the method can be utilized to isolate heavy metals from the wastewaters. Nevertheless, such method weakness is the financial cost required to be implemented, additionally more time are required to achieve the purification [8, 9]. Nevertheless, this approach appears to be more promising leading to better results than cellulose and lignin, which are biopolymers. This is due to the fact that polymers are recognized as more efficient, less expensive, friendly user in respect to the environment. More importantly, the method also does not introduce chemical and physical exchanges in the purified water [10,11,12]. It is recognized that, in the recent decade, chitosan has attracted attention for its wider use and applicability in various sectors and fields of science, technology and engineering. We can point out its application to wastewater treatment due to its biocompatibility, biodegradability, bioactivity and its ability to act as a polyatomic electrolyte in acidic solution [13]. The use of chemically modified chitosan such as N-carboxymethyl, N-benzyl sulphonated as adsorbents are widely efficient in the removal of heavy metals from wastewaters. These modifications are reported to improve the efficiency of the polymer’s metal reduction characteristics also its stability in acidic environments. For instance Chen et al. reported on the adsorption of Cu(II), Zn(II), and Pb(II) ions using glutaraldehyde-cross-linked chitosan with maximum adsorption of the metal Cr ions occurring at pH 5 [14]. The focus of the manuscript is to investigate the effectiveness of chitosan derivatives to get rid of impurities in wastewaters from wastewater effluent. Some of the common elements that affect animals and aquatic life such as Fe, Cu, Zn, Pb, and Cd, which will be identified and also their presence in the purified wastewater [4, 15, 16]. To be certain, the investigation will focus on the performance o of each derivative cross-linked products, grounded on the number of each element adsorbed.
Instrumentation
General Lab Ware Laboratory Practice
Shimadzu Analytical balance (AW320) was used for all the weight measurement while a hotplate magnetic stirrer (H3760-HS) was used for heating and stirring the reaction mixtures. Safety measure was ensure for handling reagent, standards, chemicals and solutions. These precautions were taken to avoid contamination of analytical results. This was done to avoid cross contamination and to ensure quality assurance of all analytical results. All glassware were soaked in nitric acid solution (10%) for about 2 days and rinse with distilled water before usage [17].
Fourier Transform Infrared Spectroscopy (FTIR)
The sample was recorded on a Thermo Scientific Nicolet (6700 FTIR). Sample preparation was done using potassium bromide as pellet, whiles the sample spectrum was done at a scan rate of 64 repetition with baseline corrections. The spectrum with frequency ranges between 500 and 4000 cm−1 was then plotted [17].
Scanning Electron Microscope (SEM)
The morphological structure of the chitosan sample was done using Model JSM-7800F, scanning electron microscope, United Kingdom. The electron micrograph was taken at 25.0 kV (acceleration voltage) and specimen photos was also done at different magnification period respectively.
X-ray Diffractometer (XRD)
Characterization of the chitosan product was done by means of X-ray diffraction (XRD) technique using Bruker AXS D8 ADVANCE (Bruker Corporation of Germany) with a 40 kV Cu anode and a current of 40 mA producing. X-ray diffraction of wavelength (λ) of 1.5406 Å was used for the analysis of chitosan. Scanning ranges were between 5 and 30 °C and a frequency of 1 s were was used for all measurement that were taken.
HG-AAS
A computer controlled HG-AAS sequential plasma spectrometer was used for the determination of metal adsorbed present in the effluent wastewater obtained from the poultry wastewater.
Materials and Methods
Materials, Reagent and Glassware
The following products were bought for the experiment: Shrimp chitin (500 g) from Kapikapa International Tuticorin, India. Starch vitex (500 g), glutaraldehyde (25%), p-benzoquinone (98%), (−) (α)-s-methylbutylamine (95%), (±) epichlorohydrine (99%), 1,3-dichloroacetone (99%) from sigma Aldrich, South Africa, and were used as absorbance’s for heavy metals purification from meat wastewater. Drain effluent wastewater discharge was supplied to us in a fresh neat polyethylene bottles (5 L) and was store in the refrigerator until usage. Other chemicals used included HNO3 acids and distilled water solvents were all of analytical grade. Multi-elemental standard, hydride gas atomic adsorption spectroscopy (1000.00 mg/L) in stabilized nitric acid (7% v/v), Durban Schott grade A Erlenmeyer beakers was bought from Merck chemical supplier, and used for all the wet chemical analysis. Pastured pipettes with high accuracy volume and precession, glass burette (50 mL), were used for the measurement of dispersed volumes of the chemicals during analysis.
Description of the Wastewater Sample
Wastewater samples used in the current study were obtained from the local poultry company (poultry wastewater) situated in the outskirts of Bloemfontein, Free State Province in South Africa. Samples were provided in a large container (5 L) after having been collected from the effluent pipe. Strict regulations items of confidentiality were of high priority in securing samples as most of the poultry companies do not want bad publicity.
Experimental Procedure
Synthesis of the Shrimp Chitosan
In separate round bottom flasks (250 mL), chitin (2.5 g; 0.0241 mmol) were transferred, dissolved in acetic acid (1%, 100 mL) using a magnetic stirrer. The above mixtures was allowed to stir at ambient (30 °C) temperature for 24 h awaiting for a viscous solution. To this mixture, a solution of sodium hydroxide solution (2%) was poured slowly and carefully in order to neutralize the acidic solution. The subsequent product was poured in a beaker, filtered and then rinsed thoroughly with plentiful amounts of distilled water. Resultant white precipitate product was formed, dried at room temperature and 90% yield for shrimp chitosan was obtained [18]. The white product formed was characterized by it physiochemical factors for instance yield, moisture and ash content, degree of deacetylation (Table 2), SEM, FT-IR and XRD spectroscopy.
Determination of the Physiochemical Properties of Chitosan
Yield
Yields of chitosan and cross-linked chitosan derivatives were calculated by weighing the dry weight product before and after treatment sample. Results were recorded as shown in Tables 1 and 2.
Ash Content
The ash content method used was modified according to the Association of Official Analytical Chemist. Prepared chitosan samples (0.9709 g) was burned, cooled and tarred crucible. Sample was then heated in a stifle furnace, warmed at 700 °C for about 8 h. The crucible was cooled down for about 100 °C, then positioned on top of an emitted desiccator. The empty crucible was then weighed followed by weighing of the ash. The percentage of ash content was then calculated as seen below and the results was also record as shown in Table 1 [19].
Moisture Content
The moisture content method used in the current study was the gravimetric method, modified from Black [20]. This was done as follows, the water mass was determined by drying the sample to constant weight and measuring the sample before and after drying. The water mass (or weight) was than calculated from the difference between the weights of the wet versus the dry samples and the result were recorded as seen in Table 1.
Degree of Deacetylation (DDA)
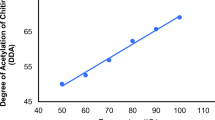
The method used for the degree of deacetylation was modified from Jiao et al. [21]. DDA of chitosan was analyzed using FT-IR instruments, frequency ranges between 4000 to 500 cm−1, with baseline equation corrected. The degree of acetylation for all the tested shrimp chitin products were found to increase with the increase in temperature (50–100 °C). A liner correlation between the temperature and the degree of acetylation was observed and maximum degree of acetylation for all the chitin products was obtained at 100 °C and the least was recorded at 50 °C (Table 1).
where T—Transmittance.
The result was recorded as shown in Table 1.
Solubility of Chitosan
The solubility test was done by dissolving chitosan (0.5 g) in acetic acid (1%, 100 mL), distilled water (100 mL) and NaOH (0.1 M). Each of these solutions were stirred for 60 min under vigorous at room temperature (30 °C), and their respective results were recorded in Table 1.
Synthesis of Shrimp Chitosan Derivatives
In separate glass beakers, two set of solutions of powered shrimp chitosan (2.0 g, 0.131 mmol) and starch (1%) was added to acetic acid (100 mL, 1%). In another set of solution of epichlorohydrine, p-benzoquinone, 1,3-dichloroacetone, glutaraldehyde and s-methyl-butyl amine are added to the preceding solution. The subsequent mixture form was then stirred at room temperature ranges between 24 and 48 h. The product formed was a viscous gel. This viscous solution was poured slowly into acetone (500 mL) in order to promote precipitate The final product formed was a white precipitate, was washed and dried at ambient temperature and the yield was recorded as shown in Table 1 [17].
Preparation of AAS Calibration Standards
Atomic adsorption spectroscopy, multi-elemental standard (1000.0 μg/L), stabilized in nitric acid (7% v/v), obtained from Merck supplier was used to prepared the calibration standard. Transferpette micro-pipette was used to prepared calibration standards; 5, 10, 20, 30 and 40 μg/L. Addition of HNO3 solution (5.0 mL; 65%) was done previously the addition of distilled water up to the mark. Then after homogenization took place and was kept for until usage.
Preliminary Treatment of the Wastewaters
A stock solution of the poultry meat effluent wastewaters (5 L) was first shaken to ensure homogeneity of the solution before filtered. The filtrate which contain most of the heavy metals were collected (1 L) in a separate beaker for HG-ASS analysis.
Centrifuge Technique for the Isolation of Heavy Metals Ions Present in Poultry Meat Wastewaters
Cross-linked chitosan derivatives products (0.2 g) were each mixed with the industrial effluent (50 mL) (obtained from poultry wastewater in Bloemfontein, South Africa). The pH of the collected samples were taken and the samples were incubated for about 4 h for 25 °C. The contents were then centrifuged at 10,000 rpm for 10 min, and the resultant supernatant were filtered with a Whatman filter paper. The heavy metals Pb(II), Cr(VI), Cu(II) Zn(II), Fe(II), concentrations in all samples were determined by atomic absorption spectrophotometer (Model HG-ASS) Shimadzu Corporation, Japan using air-acetylene flame with digital read out system [22].
Abbreviation list of shrimp chitosan-starch derivatives cross-linked products used for the removal of heavy metals in poultry wastewaters:
A—Glutaraldehyde
B—Epichlorohydrine
C—S-methylbutylamine
D—p-Benzoquinone
E—1,3-Dichloroacetone
Quantitative Analysis of the Effluent Solutions from the Poultry Meat Wastewaters Using HG-AAS
The collected effluent solutions absorbed from poultry meat wastewater was transferred into a volumetric flask (100.0 mL). HNO3 solution (5.0, 65%) was added and the preceding solution, homogenized and was later analyses using the HG-ASS technique.
Result and Discussion
Physiochemical Properties of Chitosan Products
Yield
The overall yield of chitosan and chitosan–starch cross-linked polymer ranges from 57 to 78%. Satisfactory percentage of high yield was obtained from shrimp chitosan (78%), followed by shrimp chitosan cross-linked s-methylbutylamine, 1,3-dichloroacetone, glutaraldehyde and epichlorohydrine ranges from 63 to 72% whereas shrimp chitosan cross-linked p-Benzoquinone (57%) has the lowest yield. The lower yield occur as a result of the potential loss of small particle sizes product which may be due to products lost during the filtration, washing and grinding of the products.
Ash Content
Prepared chitin from shrimp shell waste had an ash content of 2.3% (see Table 1), which is reasonable associated to commercial chitosan, having ash content of close to 2%. Ti is then suggested that prepared chitosan had for used for industrial wastewater treatment.
Moisture Content
Synthesized chitosan had a moisture content of 7.21% (Table 1). This value lies within the range of commercial chitosan from literature study [23].
Solubility
Preliminary solubility test reveal that prepared shrimp chitosan was soluble in 1% acetic acid, shows less solubility in water and non-soluble in 0.1 M NaOH (Table 1).
Degree of Deacetylation (DDA)
Chitosan degree of deacetylation obtained during this research work show satisfactory results, with a percentage of 72% (Table 1). DDA parameter is important to this study since it present influences other parameters such as solubility, biodegradability and so on. Commercial available chitosan are known to have DDA values ranges from 72 to 85%. This parameter varies on the source, method of preparation with results range from 30 to 95% [24]. The parameter also depend on the sample preparation, type of analytical method as well as the instrument used [25].
FT-IR Analysis
The chitosan shrimp was described utilizing FTIR (Fig. 1). It was observed that the OH and NH2 accompanying by the essential amine group extending tremors brought about solid bands occurring between 3200 and 3700 cm−1. The corresponding vibrations having stretching rates of 2870 and 2340 cm−1 illustrate that methylene group in CH2OH, NHCOCH3 found in methyl, and the pyranose ring containing the methyl group respectively. CO vibrations obligations of the RNHCO present in the amide group, the bond at 1657 cm−1 is responsible for the secondary amide, that depicted by groups inside the range 1651–1558 cm−1 [17]. The groups occurred at 1421, 1379 and 1389 cm−1 and were ascribed by twisting vibrational stretch of CH2 groups. The absorption groups inside the range 1160–1000 cm−1 are associated with the extending vibrations of CO groups. OH and NH twisting vibration yielded tiny peaks appearing at 610 cm−1 and 647 cm−1. This is associated with the flapping of the polysaccharide structure of chitosan. The chitosan plane in Fig. 1 plainly introduces the perceptions clarified above [26]. Regarding the previously mentioned author [26] the confined chitosan product were absent at the 3425 cm−1 peak, which describe the extending OH group, and 2921 cm−1 which relate to CH2OH present in methylene group. However, the bond located at 1630 cm−1 peak was related with the amide group, which was caused by the spreading of the CO bond, and the peaks happening around 1656–1628 cm−1 compare to the amide group which represent the NH stretching vibration. According to this product, small peaks occurring between 1257 to 1380 cm−1 in the NHCOCH3 group, equate the pyranose ring complex containing the CH stretching frequencies of the NHCO group. The 1158 cm−1 and 1154 cm−1 peaks resulted from COC glycosidic linkages characterization. Peak appearing at 1099 cm−1 is associated with the auxiliary hydroxyl group (OH) while those found at 1027, is due to the OH essential group. CO vibration bending, occurring in the CH ring was locate at 894 cm−1 (pyranose ring skeletal vibrations) whereas those occurring out of the plane where located at 603 and 665 cm−1 [17].
Scanning Electron Microscope (SEM)
The SEM results for shrimp chitosan under × 100 low magnification, display crystalline assembly filament with scathing surfaces, which are tightly close together. When this image was zoom in at a high magnification, of × 15,000, the preceding product shows smooth surfaces with non-pores or semi-pores phase containing micro fibrils dome shape orifices and crystallite structures (Fig. 2).
Diffractomenter (XRD)
The XRD pattern of chitosan in this study displayed two sharp diffraction peaks at 2θ = 8° and 20°, revealing the high crystallinity of chitosan. The strong reflection intensity at 8° and 20° is due to the incorporation of bound water molecule into crystal lattice. The peak later decreases latter after 20° to 60°. This sharp decrease was a result of the decrease in inter and intra molecule hydrogen bond. These peaks values were indexed as 022,115, from the lower angle for chitosan (Fig. 3). From this results, maximum peak intensity was observed at the 115 reflection point. This peak decreases with increase in degree of deacetylation but later appeared back round the same point of reflection. This result is consistent with the result of a number of previous studies, which reported the typical X-ray diffraction pattern of chitosan. This results is consistent with the result of a number of previous studies, which reported the typical X-ray diffraction pattern of chitosan [27, 28].
Qualitative and Quantitative Analysis of the Poultry Wastewaters
Qualitative analysis of the poultry wastewater samples detect the presence of five elements namely; Cr(II), Cu (II), Pb(II), Fe(II) and Zn(II), with an estimated low concentration 0–1 mg/L. (Table 3). From Table 3, it is seen that these elements plays an important role in the proper growth of living species, variable extends stated by the world health organization [29]. Heavy metals originated from the washing of intestines and from a variety of chemicals used in the meat processing industry causes toxic effect to human and the environment. Before heavy metal analysis, the poultry wastewater sample were filtered and poured into volumetric flasks (100 mL) and hydrochloric acid was used to adjust the pH range to 3.5. The sample was later stored at ambient temperature until analysis took place [30]. The following specific wavelength hallow cathode lamp were used during the analysis of the samples. Pb (220.35 nm), Cu (324.75 nm), Cr (205.55 nm), Cd (214.44 nm). Fe (239.56 nm), Zn (228.61 nm), Mn (257.61 nm). The detection limit ranged from 0.01 to 0.1 mg/L, and their individual concentrations were discussed as seen below.
Lead
Lead is well known to be the oldest element known to mankind. It is toxicant, non-essential metal having no nutritional value to living organisms. It is mostly source from the atmospheric upshot [4]. From the analysis result it was found that lead concentration lies between 0.01 and 0.03 mg/L). This results is in agreement with the standard value given by the WHO organization, which was < 0.05 mg/L. The concentration of lead need to be lower than expected since high concentration of lead affect aquatic animals who feeds on the disposed wastewater which are usually dumped by these abattoirs farmers into nearby ponds, river and stream. Since the above metal Pb is known to be very toxic even at low concentration, abattoir farmers will need to recycle their wastewater so that it can be used again in the meat abattoir for domestic purposes in order to avoid dumping. The study also indicated that ingestion of large quantity of lead (up to 12 mg/L) is fatal to human health and also result in diminution in haemoglobin production and also affects the kidneys together with the cardiovascular systems [31,32,33,34].
Chromium (Cr)
In the present study, Chromium concentration was observed within the range 0.01–0.02 mg/L (Table 1), thus this concentration did comply with the WHO guidelines of < 0.05 mg/L for domestic purposes [29]. Therefore wastewater effluent from meat abattoir can be recycle and used back again for washing and cleaning of instrument used in the abattoir. Heavy metals such as chromium can be easily absorbed by aquatic species and the effects caused thereof have been shown to inhibit growth and reproduction which eventually leads to extinction of aquatic life. From the literature research, it was observed the oxidation of Cr(VI) compounds affects the health and survival of aquatic species [35]. It was also observed in fishes, where high concentrations of heavy metals ions present constitute health problems such as damage to the nervous system, kidneys and cardiovascular [36].
Zinc (Zn)
The average concentration of zinc obtained during the study ranges between 0.010 and 0.06 mg/L. It is known that concentration of Zn > 5 mg/L present in water provides a biting taste, and the water has a cloudy form. It is then recommendation that the concentration of Zn should be < 5.5 mg/L. The outcome denotes zinc centration present in industrial effluent were higher than the United State Public Health (USPH) standard of 5.5 mg/L. Using this technique, hydride gas atomic absorption spectroscopy was successful in the isolation of metal zinc from the environment. This results also in agreement with the permissible concentration required by USPH. Concentration of Zinc higher than the permissible standard may results in serious health illness such as vomiting, abnormal cramps, inhibit plant growth, diarrhoea, necrosis and chlorosis [37].
Copper (Cu)
The concentration of cadmium in the study ranges between 0.011 and 0.06 mg/L. Copper is also toxic to marine and freshwater aquatic life environment. The concentration of Cu found in present abattoir wastewater effluent discharge comply with the WHO standard guidelines which was assume to be below 0.05 mg/L for domestic usage [29]. The obtained concentration suggest unscrupulous performance from the meat industries industries, strategy of disposing wastewater which was inadequate for the environment health. The chronic exposure levels of Cu ranges from 0.02 to 0.3 mg/L an can be toxic for plants, fish and invertebrates [38].
Iron (Fe)
The maximum concentration of iron ranges from 0.50 to 0.80 mg/L. Iron is an essential element required for the uptake of hemoglobin that supply blood to the tissue. The concentration of Fe found in the present abattoir wastewater effluent discharge was higher than the WHO standard guidelines which was assumed to be below 0.5 mg/L [29]. High concentration of iron can be a threat in pathogenic species due to the fact that most of these species depend on iron for their growth survival. Fe(II) is also responsible for the unpleasant organoleptic properties in drinking water [37].
Analysis of the Adsorbed Element from Poultry Wastewater by Means of Chitosan Cross-Linked Derivatives Products
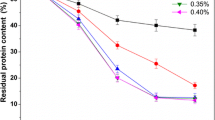
It was earlier mentioned that heavy metals detected in the poultry wastewater was from the washing of the intestine and also different chemicals used in the meat processing industries. Results after qualitative and quantitatively analyses indicates the presence of Pb, Cd, Cu, Cr and Zn, that were previously discussed to be the key elements adversely affecting aquatic life and animals [39]. From these atomic absorption spectroscopy analysis, it was clearly observed that that chitosan-cross-linked products A–E were good adsorbers of Cr, Cu and Pb, containing negligible proportion of the poultry wastewater tested (0.01–0.9 mg/L). There was significant reduction in the amount of Cr, Pb which were 0.03 mg/L and Cu, 0.05 mg/L respectively. Element Cr, Cu and Pb concentration were found in faultless amount from the wastewater tested while the concentration of Fe was slightly higher, up to 0.9 mg/L. The obtained results shows that chitosan produces has the ability to adsorb these heavy metals, even though the concentration of these elements obtained were not very low. These element were also detected in trace and ultra-trace amount (< 0.05 mg/L) from the wastewater during experimental analysis using the chitosan cross-linked product. Existence of these elements even at very small amount in wastewater causes a long term effect on polluted water. The discharge of the above elements into the water courses causes increase in the concentration via the process of evaporation. However, the presence of these metals confirmed the reports of water pollution due to the presence of heavy metals. The presence of Pb and Cu was of great concern as these elements have high toxicity levels in the environment. The effect of Pb and Cu causes some devastating diseases to animals and human. Approximately the element Cu and Pb earlier mentioned above, absorbed by the cross-linked chitosan products (A–E) suggest possible application in the adsorption process. Chitosan cross-linked 1,3-dichloroacetone showed to be the best product compared to all the other cross-linked product tested in the adsorption of Cr, Cu, Zn and Pb. Ranking of the top chitosan cross-linked products for the adsorption of heavy metals were as follows product E (Pb), product E(Zn) and A (Cu, Fe and Cr), Figs. 4, 5, 6, 7 and 8.
Evaluation of Results
Evaluation of the experimental results shows the various ways on which the modified chitosan products can be executed. These ways depend on the chemical composition of each of the chitosan products. Evaluation of the statistical results revealed standard deviation range of 0–1%, showing a reasonable degree of precision in all the experimental analysis. There was an increase in the heavy metal concentrations of; Fe, Cr, Zn and Pb which were ascribed by the easily ionisable element effect present in the atomic absorption spectroscopy measurement. These effects were more prominent at higher concentration whereas at lower concentration it works in the opposite way. Most of the heavy metals were adsorbed by the shrimp chitosan cross-linked 1,3-dichlroacetone products, with adsorption capacity ranges from 80 to 90%. The existence of the amine and hydroxyl group (NH2 and OH), present in the preceding cross-linked product was thought to have play a vital role thereby increasing the binding sites of the chitosan cross-linked product. From the data generated it was evident that the shrimp chitosan cross-linked 1, 3-dichlroacetone product showed the show high adsorption capacity compared to all the other products tested.
Fourier Transform Infrared Spectroscopy (FT-IR) Spectrum of Prepared Chitosan
See Fig. 1.
Scanning Electron Microscope (SEM)
See Fig. 2.
X-ray Diffractometer (XRD)
See Fig. 3.
Chitosan Cross-Linked Products for Heavy Metals Adsorption
Proposed Structure of Shrimp Chitosan Cross-Linked 1,3-Dichloroacetone
See Fig. 9.
Shrimp Chitosan Cross-Linked 1,3-Dichloroacetone Purified Poultry Wastewater Samples Before and After Treatment
See Fig. 10.
Conclusion
Shrimp chitosan synthesizing has been successfully achieved from shrimp domestic waste. Characterization by percentage yield, moisture and ash content denoted that the respectable source was chitosan. Structural and Functional properties; Fourier Transform infrared spectroscopy (FT-IR), scanning electron microscope and X-ray diffractometer pattern were analyzed. Results shown characteristic peaks and morphologic structures which correspond to those cited in the literature study. A successful modification of chitosan was done by crosslinking with; glutaraldehyde, 1,3-dichloroacetone, epichlorohydrine, s-methybutylamine, p-benzoquinone products. Determination of the effectiveness of these modified chitosan cross-linked derivatives products in the purification of poultry abattoir wastewater was also studied. Qualitative analysis of above poultry wastewaters was achieved using HG-AAS, which revealed the existence of five elements; Cr, Zn Cu, Pb and Fe and in different concentrations.
Analysis by quantitative technique using the above cross-linked products, shows lower concentrations for metal Cr, Pb, Cu Fe and Zn ranging from 0.01 to 0.09 mg/L. Even though the adsorption results was completely achieved, there was considerable reduction in the quantity of heavy metals present. Shrimp chitosan cross-linked 1,3-dichloroacetone show good correlation results compared to all the other cross-linked product tested. HG-AAS analysis of cross-linked product, as an example in Fig. 10, shows the purification of poultry wastewater before and after treatment with shrimp chitosan cross-linked 1,3-dichloroacetone. One can conclude that, the tested cross-linked product is a better adsorbent of heavy metals and can be used in the purification of industrial effluent wastewater discharge. Furthermore, we recommended that there should be safe disposal of abattoir wastewater to minimize environmental impact and to limit the methods of disposal to those that are internationally permitted and suggested.
References
Chukwu O, Adeoye PA, Chidiebere I (2011) Abattoir wastes generation, management and the environment: a case of Minna, North Central Nigeria. Int. J. Biosci. 1:100–109
Mouzdahir Y, Elmchaouri A, Mahboub R, Gil A, Korili SA (2010) Equilibrium modelling for the adsorption of methylene blue from aqueous solution on activated clay minerals. Desalination 250:335–338
Princeton University Water Resources Program (1984) Groundwater Contamination from Hazardous Wastes. Prentice-Hall Inc., Englewood Cliffs
Us EPA 2005 (2016) Guidelines for carcinogen risk assessment. Risk Assessment Forum, United States Environmental Protection Agency, Washington, DC.EPA/630/P-03/001F.
Adeyemo OK (2002) Unhygiene operation of a city abattoir in south western Nigeria: environment implication. Afr J Environ Assess Manage 4:23–28
Nkansah MA, Ansah JK (2014) Determination of Cd, Hg, As, Cr and Pb levels in meat from the Kumasi Central Abattoir. Int J Sci Res Publ 4:1–4
Anthony RG, Kozlowski R (1982) Heavy metals in tissues of small mammals inhabiting wastewater-irrigated habitats. J Environ Qual 11:20–22
Denkbas EB, Odabasi M, Kiliçay E, Özdemir N (2002) Human serum albumin (HSA) adsorption with chitosan microspheres. J Appl Polym Sci 86:3035–3039
Urbaniak M, Sakson G (1999) Preserving sludge from meat industry waste waters through lactic fermentation. Process Biochem 34:127–132
Zhang Y, Xue Q, Li F (2019) Removal of heavy metal ions from wastewater by capacitive deionization using polypyrrole/chitosan composite electrode. Adsorp Sci Technol 37(3–4):205–216
Aktaş N, Gürses A (2005) Moisture adsorption properties and adsorption isosteric heat of dehydrated slices of Pastirma (Turkish dry meat product). Meat Sci 71:571–576
Vieira RS, Beppu MM (2005) Mercury ion recovery using natural and cross-linked chitosan membranes. Adsorption 11:731–736
Chen RH (1998) Manipulation and application of chain flexibility of chitosan. In: Chen RH, Chen HC (eds) Advances in chitin science, vol III. Department of Food Science, National Taiwan Ocean University, Keelung, pp 39–46
Chen A, Yang C, Chen C, Chen CW (2009) The chemically cross-linked metal-complexed chitosan for comparative adsorptions of Cu(II), Zn(II), Ni(II) and Pb(II) ions in aqueous medium. J Hazard Mater 163:1068–1075
Igberase E, Osifo OP (2019) Mathematical modelling and stimulation of packed bed column for the efficient adsorption of Cu(II) ions using modified bio-polymeric material. J. Environ Chem Eng 7:103–129
Igberase E, Ofomaja A, Osifo PO (2019) Enhanced heavy metal ions adsorption by 4-animobenzoic acid grafted on chitosan/epichlorohydrine composite: kinetic, isotherms thermodynamics and desorption studies. Int J Biol Macromol 123:664–676
Atangana E, Chiweshe TT, Roberts H (2019) Modification of novel chitosan-starch cross-linked derivatives polymers: synthesis and characterization. J Polym Environ 27:979–995
Alabaraoye E, Achilonu M, Roberts H (2017) Biopolymer (chitin) from various marine seashell wastes. J Polym Environ 26:2207–2218
AOAC (1990) Official methods of analysis. Association of Official Analytical Chemists, Washington DC
Black CA (1965) Methods of soil analysis: part I physical and mineralogical properties. American Society of Agronomy, Madison, pp 671–698
Jiao TF, Zhou J, Zhou J, Gao L, Xing Y, Li X (2011) Synthesis and characterization of chitosan-based schiff base compounds with aromatic substituent groups. Iran Polym J 20:123–136
Gamage A, Shahidi F (2007) Use of chitosan for the removal of metal ion contaminants and proteins from water. Food Chem 104:989–996
Li Q, Dunn ET, Grandmaison EW, Goosen MFA (1992) Applications and properties of chitosan. J Bioact Compat Polym 7:370–397
Martino AD, Sittinger M, Risbud MV (2005) Chitosan: a versatile biopolymer for orthopedic tissue engineering. Biomaterials 26:5983–5990
Khan T, Peh K, Che’ng HS (2002) Reporting degree of deacetylation values of chitosan: the influence of analytical methods. J Pharm Pharm Sci 5:205–212
Zvezdova D (2010) Synthesis and characterization of chitosan from marine sources in Black Sea. Sci Work Russ Univ 49:65–69
Wada M, Saito Y (2001) Lateral thermal expansion of chitin crystals. J Polym Sci Part B 39:168–174
Feng F, Liu Y, Hu K (2004) Influence of alkali-freezing treatment on the solid state structure of chitin. Carbohyd Res 339:2321–2324
World Health Organization (2018) A global overview of national regulations and standards for drinking-water quality. World Health Organization, Geneva
Atangana E, Chiweshe TT (2019) Metal adosrbance in abattoir wastewater using crosslinked chitosan derivatives. J Polym Environ. https://doi.org/10.1007/s10924-019-01548-2
Paar A (1998) Microwave sample preparation system’—instruction handbook. Anton Paar GmbH, Graz, p 128
Nolan KR (1983) Copper toxicity syndrome. J Orthomol Psychiat 12:270–282
Galadima A, Garba ZN (2012) Heavy metals pollution in Nigeria: causes and consequences. Pollution 45:7919–7922
Okegye JI, Gajere JN (2015) Assessment of heavy metal contamination in surface and ground water resources around Udege Mbeki Mining District, North-Central Nigeria. J Geol Geop 4:1–7
Khansari FE, Ghazi-Khansari M, Abdollahi M (2005) Heavy metals content of canned tuna fish. Food Chem 93:293–296
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Tiwana NS, Jerath N, Singh G, Ravleen M (2005) Heavy metal pollution in Punjab rivers. Newslett Environ Inf Syst 3(1):3–7
Moore JW, Ramamoorthy S (1984) Heavy metals in natural waters. Appl Monit Impact Assess. 28:246
Atangana E (2019) Adsorption of Zn(II) and Pb(II) ions from aqueous solution using chitosan cross-linked formaldehyde adsorbent to protect the environment. J Polym Environ 27:2281–2291
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors proclaims that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Atangana, E., Oberholster, P.J. Modified Biopolymer (Chitin–Chitosan Derivatives) for the Removal of Heavy Metals in Poultry Wastewater. J Polym Environ 28, 388–398 (2020). https://doi.org/10.1007/s10924-019-01616-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01616-7