Abstract
Breast microcalcification is a potential diagnostic indicator for non-palpable breast cancers. Microcalcification type I (calcium oxalate) is restricted to benign tissue, whereas type II (calcium hydroxyapatite) occurs both in benign as well as in malignant lesions. Microcalcification is a pathological complication of the mammary gland. Over the past few decades, much attention has been paid to exploit this property, which forms the basis for advances in diagnostic procedures and imaging techniques. The mechanism of its formation is still poorly understood. Hence, in this paper, we have attempted to address the molecular mechanism of microcalcification in breast cancer. The central theme of this communication is “how a subpopulation of heterogeneous breast tumor cells attains an osteoblast-like phenotype, and what activities drive the process of pathophysiological microcalcification, especially at the invasive or infiltrating front of breast tumors”. The role of bone morphogenetic proteins (BMPs) and tumor associated macrophages (TAMs) along with epithelial to mesenchymal transition (EMT) in manipulating this pathological process has been highlighted. Therefore, this review offers a novel insight into the mechanism underlying the development of microcalcification in breast carcinomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer ranks second among different types of cancers worldwide, and is still one of the major leading causes of morbidity and mortality among women. The mortality rate could be reduced markedly if the breast cancers were diagnosed and treated at early stages. Mammography is widely used for early screening and detection of breast cancers [1] via the utilization of X-rays (at low dose) to visualize breast tissues clearly, and to screen various subtle abnormalities including pathological lesions. Use of mammography provides a benefit to women by reducing the mortality by as much as 30 % [2, 3]. Mammography detects both palpable and non-palpable breast lesions, based on abnormalities like the appearance of microcalcification [4–6].
Bright white flecks that appear on mammograms are a signature of calcification. They are broadly categorised as ‘macro’ and ‘micro’ calcification [7–13]. Macrocalcifications are coarse, large white dots or specks in nature (>0.5 mm in diameter) [11] that are often randomly dispersed throughout the breast tissue, and are most often found in non-cancerous tissues [13–15]. Microcalcification clusters are tiny specks (<0.5 mm in diameter) of calcium deposits [10, 11] appearing on the mammographic image [16, 17]. Accumulating evidence shows a positive association between microcalcification and malignancy, along with the grade of the breast cancer [18–20]. Moreover, literature reports suggest that the presence of microcalcification in breast tissue can be a diagnostic marker for breast cancer [4, 21, 22]. Microcalcification also correlates with increased cancer progression and metastasis of breast cancer [18–20, 23]. Thus, there is an urgent need to understand the underlying molecular mechanism of this pathophysiological mineralization.
In this review article, we investigate a subpopulation of heterogeneous breast tumor cells that acquires osteoblastic properties. These osteoblast-like cells drive the process of pathological calcification in breast cancer tissues [24–28]. Based on literature reports, we have outlined herein how potent osteoinducers of bone (morphogenetic proteins; BMPs), and tumor associated macrophages (TAMs) play a crucial role in enhancing the pathological mineralization.
Classification of Microcalcifications
Based on their morphological appearance, microcalcifications have been classified into the following five categories: i) “Ring shaped” in 100 % of benign lesions; ii) “Round microcalcification” in 22 % of malignant lesions; iii) “Pulverulent” (too fine) in 40 % of malignant lesions; iv) “Punctate” in 66 % of malignant lesions, and v) “Vermicular” in 100 % malignant lesions [29]. Breast Imaging Reporting and Data System (BI-RADS) classifies tumors based on morphology descriptors, mammographic density, presence of calcification, and their distribution on mammograms (calcification presents in BI-RADS I and BI-RADS II) [30]. Every morphology descriptor assigns a category to a lesion which helps in determining its malignant potential as: 1) benign, 2) intermediate, or 3) malignant [30]. Based on mammographic appearances, primary calcifications have also been classified as a: 1) powdery form, 2) crushed stone-like, or 3) casting-type [21].
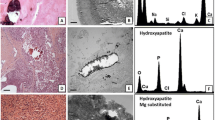
On the basis of chemical composition and physical properties, microcalcifications are also categorized into two types, as type I (calcium oxalate) and type II [calcium hydroxyapatite (HA)] [31]. Light microscopic views show that calcium oxalate crystals are amber in color and are partially transparent, while HA crystals are grey/white in color and are opaque [31]. Under polarized light, type I is birefringent, whereas type II is non-birefringent [31]. Deposition of calcium in the form of calcium oxalate occurs mostly in benign ducts, whereas the HA form often occurs both in benign and in proliferative lesions of breast carcinoma [31–34]. Further, some studies provide evidence that type II HA crystals are often found in the invasive infiltrating cells of breast cancer [18–20]. Moreover, compact clusters (20 microcalcification/cm2) represent a malignant condition [35, 36]. It is not yet understood whether the microcalcification functionally modulates the pathophysiology of this disease, or if this is just a consequence of disease development.
It is important to note here that microcalcification has been found in different body organs/tissues such as iliac artery, medial artery, thyroid nodules, testis, ovary, brain and kidney in association with various pathophysiologies depending on the tissue/organ it is found in. Beside mammography, these microcalcifications can be detected by other techniques such as ultrasonography, H&E staining, Von Kossa staining, etc. as summarized in supplementary Table S1.
HA Microcalcification and Malignancy of Breast Cancer
HA microcalcification contains a lower amount of carbonate in malignant lesions when compared to benign tissues [18, 19]. A possible explanation for the association of HA with malignant lesions was given by Morgan et al., where they found that HA has the potential to induce mitogenesis in MCF-7 and Hs578T breast cancer cells [20]. Moreover, treatment of breast cancer cells with HA enhanced matrix metalloproteinase (MMP) activity, and stimulated prostaglandin production to intensify its effect [20]. As a mechanism, it was demonstrated that the elevation of prostaglandin levels by HA treatment was due to upregulation of cyclooxygenase-2 (Cox-2), and that HA crystals can induce MMP activity by upregulating the inflammatory cytokine interleukin-1β (IL-1β) [37]. A recent study by Cox et al., documented that an invasive sub-clone of breast cancer Hs578T cells shows more competency to have (HA containing) mineralization when compared to parental Hs578T cells and normal breast epithelial MCF10A cells. Lung metastasizing breast cancer (4 T1 cells) also exhibited formation of mineralization sooner than that of the invasive sub-clone of Hs578T cells [102]. These data suggest that microcalcification can be a strong predictor for malignancy of breast cancers, and these studies also help to understand the role of HA in malignant tissues.
Role of Matrix Vesicles in Microcalcification
The mechanism for the deposition of calcium crystals in the form of microcalcification is poorly understood. To discover this mechanism, much attention has been paid to the role of matrix vesicles (MVs). MVs are small (20–200 nm) membrane enclosed structures, where various mechanisms for their biogenesis have been proposed. The most widely accepted mechanism is that MVs are derived by the process of budding off or being pinched out from selected sites of the plasma membrane of calcifying competent cells like osteoblasts [38, 39], odontoblasts [40–42], chondrocytes [43, 44], or embryonic stem cells [45] during the period of mineralization. We have listed all types of known calcifying cells which produce MVs in Table 1 [38–57]. Many studies show that breast tumor cells also produce MVs [50, 52, 53, 56]. The lipid membrane composition of these synthesized MVs differs significantly from the parent plasma membrane. The membranes of MVs are enriched in tissue non-specific alkaline phosphatase (TNAP) [58], phosphatidylserine (PS) [59], annexins [60], NaPi transporter [61], nucleotide pyrophosphatase phosphodiesterase 1(NPP1; PC1), and phospho1 (PE/PC phosphatase) [62], all of which facilitate the formation of HA crystals by supplying calcium and phosphate ions to MVs.
An important step in mineralization is the formation of the first crystal of HA (i.e., starting material for calcification) which is synthesized inside MVs by calcifying cells [63]. These MVs act as vehicles for the transfer of newly synthesized monocrystal from inside to the outside of the cell, and form a nucleational core of HA in the extracellular fluid [64]. The phenomenon of propagation of this monocrystal to appear as mature crystallized calcium is largely unexplored. However, it is thought that when HA crystals are exposed to the extracellular matrix, they serve as a template for the synthesis of the mature crystal [65].
MVs are involved both in normal as well as in ectopic calcification. Using transmission electron microscopy, it was found that MVs in mouse atheroma and human fibrous caps that were associated with solid microcalcification [66]. It was suggested that sortilin 1 (a type I transmembrane protein which belongs to the family of vacuolar protein sorting 10) induces MVs to progress in this process [67]. Moreover, recent findings by New et al., report that in atherosclerotic plaques, macrophages release MVs, and these MVs drive the formation of microcalcification [68]. All these findings suggest that MVs play a pivotal role in pathophysiological mineralization of different organs/tissues.
Basic Mechanism for Microcalcification in Breast Cancer Tissues
As we have discussed above, breast tumor cells produce MVs. What has not yet been reported is whether the composition of the lipid membrane of MVs from breast tumors matches those MVs which are derived from calcifying cells. Recent findings document that secreted MVs from osteosarcoma cancer cells contained similar kinds of components as those from osteoblast cells [69]. The composition of MVs of breast cancer cells has not yet been investigated. However, breast cancer tissues/cells showed increased levels of MV components (which are known to be involved in the calcification process) as compared to control tissues/normal breast epithelial cells (Table 2) [70–86] For example, many Ca2+ ion channels such as the transient receptor potential (TRP) cation channels and associated proteins annexin A2, A4, and A5, were found to be increased in breast cancer cells. These proteins can increase the concentration of Ca2+ ions inside MVs/cells [76, 87]. Other evidence shows that expression of the transient receptor potential cation channel 7 (TRPM7) is increased in breast cancer cells, and promotes cell proliferation, migration, and metastasis [112, 113, 116, 120, 121]. Similarly, other studies indicate that breast tumor cells accumulate more phosphate ions inside cells by increasing the expression of NaPi-IIb (SLC34A2) cotransporter as compared to noncancerous cells [79]. Moreover, calcification of breast cancer 4 T1 cells was aborted when cells were treated with phosphonophormic acid, an inhibitor of type-II Na-Pi cotransporter [71].
Collectively, these findings suggest that microcalcification in breast tumors, similar to other organs/tissues, may proceed through a similar process i.e., mediated through MVs. Moreover, metastatic cancer cells/tissues showed increased levels of several components of MVs (such as TRP channel, annexins, ALP etc.,) compared to non-metastatic cancer cells/tissues (Table 2). It is currently reported that expression of two Ca2+ channels (i.e. TRPM7 and TRPC1) are increased in infiltrating ductal carcinoma with microcalcification [83]. This evidence supports the idea that the metastatic/invasive breast cancer cells might have more competency for pathological microcalcification as compared to non-metastatic/non-invasive cancer cells.
Switch of Breast Cancer Cells into Osteoblast-Like Cells during Microcalcification
The literature states that at the time of pathological calcification (of different tissues), one cell type needs to transform into osteoblast-like cells, which mimics the process of physiological calcification [88, 89]. For example, vascular smooth muscle cells (VSMCs) transdifferentiate into osteoblast-like cells which process calcification in vascular or arterial walls [88, 89].
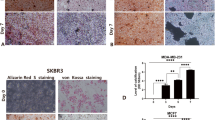
Thus, the existence of osteomimetic cells in breast tumors correlates with the occurrence of microcalcification. In fact, the presence of cells with osteoblastic and chondroblastic characteristics in breast tumor isolated from a cancer patient has been reported, and osteoblastic cells derived from this tumor show expression of ALP and OPN, both of which are markers of osteoblastic differentiation [70]. Various experimental studies have revealed that at the time of pathological mineralization, osteomimetic cells express many transcription factors and bone matrix proteins involved in physiological calcification, just the same as that of osteoblasts, (Table 3) [24–28, 90–134]. For instance, the MCF-7 breast cancer cell line showed expression of ALP when cells were treated with different agents such as 17β-estradiol [74]. A study by Cox et al., recently reported that metastatic breast cancer 4 T1 cells expressed a high level of ALP while MCF10A normal breast epithelial cells were unable to express ALP, when both cells were treated with an osteogenic cocktail [71]. They also found that treatment of 4 T1 cells with levamisole, an inhibitor of ALP, inhibited mineralization, a late marker for osteoblast differentiation [71]. Many findings have shown elevated levels of ALP in, i) the serum of breast cancer patients when compared to controls [75], ii) in patients with bone metastases when compared to patients without bone metastases [72, 73], and, iii) advanced stages of breast cancer as compared to early stages and/or healthy controls [135, 136]. These data indicate that in certain circumstances, a subpopulation of epithelial breast cancer cells may switch to osteoblast-like cells.
During bone formation, osteoblastic transcription factors such as Runx2 and Msx2 (which are expressed by osteoblast cells), drive ALP expression, mineralization, and also augment expression of osteoblastic matrix proteins such as osteocalcin (OCN), osteopontin (OPN), osteonectin (OSN) and bone sialoprotein (BSP). These matrix proteins mainly form the bone matrix, and also manipulate the calcification process [110, 137–140]. Accumulating evidence reveals that both breast cancer cells and tumor tissues expressed these osteoblastic transcription factors and matrix proteins [94, 123, 141–144]. For instance, Runx2 activity was found in LCC15-MB and MDA-MB-231 breast cancer cells, but not in normal human mammary epithelial cells (HMECs) [94]. Expression of Msx2 was found to be increased in MCF7, T47-D, SKBR3, and ZR75-1 breast cancer cells [26]. Moreover, increased expression of OPN and OSN have been shown to be associated with breast cancer microcalcification, and OPN expression was upregulated in infiltrating carcinomas with microcalcification [123, 124]. Similarly, infiltrating ductal carcinomas showed an increased expression of BSP [27]. Moreover, the levels of OCN and BSP, similar to ALP, were found to be increased in the serum of breast cancer patients as compared to benign cancer patients [28, 71, 120].
All these evidence suggests that in the pathophysiologic condition, breast cancer cells have a propensity to gain osteoblast characteristics (Fig. 1). Next, we discuss how breast cancer cells acquire the osteoblast-like phenotype.
Basic model of microcalcification formation. a A subpopulation of epithelial breast tumor cells potentially acquires the mesenchymal phenotype through epithelial to mesenchymal transition (EMT). b These mesenchymal cells acquire osteoblast like properties, and secret hydroxyapatite crystals which are deposited at the invasive front of breast tumors
Breast Cancer Cells Acquire Osteoblastic Characteristics during Epithelial to Mesenchymal Transition
It is important to mention here that osteoblasts are generated from the differentiation of mesenchymal cells [145]. A sub-population of heterogeneous epithelial cancer cells of a tumor usually undergoes epithelial to mesenchymal transition (EMT). This subpopulation of cells governs a more invasive potential, and are responsible for metastasis [124]. Invasive MDA-MB-231 breast cancer cells showed an increased expression of the osteoblastic transcription factor Runx2 as compared to non-invasive breast cancer MCF7 cells. MDA-MB-231 cells are more mesenchymal in nature, as compared to MCF7 [96]. Similarly, Runx2 DNA binding activity was also higher in MDA-MB-231 cells as compared to normal HMECs [146]. Hassan et al., recently demonstrated that the microRNA miR-218 increases the metastatic potential of breast cancer cells by enhancing the expression of Runx2 [90]. Moreover, expression of Runx2 in cancer cells positively associates with the EMT phenotype and the metastatic properties of these cells, with a concomitant increase of OCN [143]. Ectopic expression of Runx2 also converts mesenchymal stem cells to osteoblast cells [147]. Thus, expression of Runx2 in breast cancer cells might increase invasive potential, and also transdifferentiate cancer cells to osteoblast-like cells.
Similarly, another osteoblastic transcription factor, Msx2, was found to be frequently dysregulated in cancers [148]. Msx2 is also a potent inducer of the EMT phenotype of cancer cells [149, 150]. The expression of Msx2 was found to be increased in infiltrating breast cancer cells which are more invasive by nature, when compared to non-infiltrating breast cancer cells [150]. Moreover, the level of Msx2 could be an indicator for malignancy as it was elevated gradually from benign to malignant lesions [151]. Overexpression of Msx2 inhibits cell growth [26]. These data indicate that Msx2 might have a significant role in promoting EMT of cancer cells by halting cell growth. Similar to Runx2, expression of Msx2 converts mesenchymal progenitor cells to osteoblast cells [152].
We have discussed above that metastatic breast cancer cells show high osteoblastic gene expression of Runx2, Msx2, OPN, OSN, BSP, and ALP, with increased mineralization [26, 27, 96, 123, 124]. Moreover, it has been reported that the microcalcification surrounding breast tissue correlates with mineralized malignant cells [153]. These findings suggest that during tumorigenesis, a population of epithelial cancer cells of a breast tumor gains the mesenchymal phenotype through EMT, and at least a few of these invasive cancer cells which acquire mesenchymal characteristics may differentiate into “osteoblast-like” cells; presumably driven by osteoblastic factors.
A recent study supports this concept, since the co-existence of mesenchymal markers (vimentin and β-Catenin) and osteoblastic proteins (OPN and BMP-2) was greater in infiltrating carcinomas with microcalcification when compared to infiltrating ductal carcinomas without microcalcifications [124]. These studies propose that neoplastic osteoblast-like cells are responsible for the pathophysiological mineralization.
Significance of BMP Signalling in Osteoblastic Differentiation of Breast Cancer Cells
It was earlier reported that parathyroid hormone related protein (PTHrP) has a role in regulating pathological microcalcification in breast cancer [154]. However, detailed studies have not yet been conducted to show the mechanism for PTHrP-mediated mineralization. Emerging evidence suggests that BMP-2 might play a significant role in regulating breast cancer microcalcification [23, 71, 124, 155, 156].
BMPs are multifunctional growth factors that belong to the transforming growth factor-β (TGF-β) superfamily. Earlier studies established that BMPs are potent osteoinducers, and play a vital role in physiological and pathophysiological calcification of different tissues such as cartilage, bone, and arteries [157, 158]. BMPs can transduce signalling through canonical and non-canonical pathways to perform various physiological and pathological functions [159–171], which have been briefly described in Fig. 2.
Proposed molecular mechanism for microcalcification. BMPs transmit messages by canonical and non-canonical pathways to perform several physiological and pathological functions. Using non-canonical pathway (NCP), BMPs activate proliferative, cell survival, mitogenic signalling and induces EMT of epithelial breast cancer cells. Using canonical pathway (CP), BMPs activates Smad signalling to transcribe different transcription factors which eventually upregulate ALP and bone matrix proteins, involved in mineralization of transformed cells (EMT cells). BMP also increases MVs component TRPC channel and ALP. Subsequently, all proteins necessary for microcalcification formation accumulate within a microdomain of the membrane. This microdomain (enriched with these proteins) can be pinched out from the parental membrane, which results in the formation of MVs. The pH of MVs differs from the pH of the cytoplasm. Thus, at the appropriate pH, a monocrystal of HA forms, and then this MV is released into the extracellular matrix. This monocrystal acts as nucleus for the further HA deposition. Tumor cell derived cytokines such as MCP-1 and CSF-1 recruit macrophages at the tumor site; these macrophages further propagate this process by secreting MVs to the nucleation site of crystal. BMP also increases apoptosis, and the apoptotic bodies also accelerate the process of microcalcification
Recent findings by Cox et al. show that BMP-2 treatment potentiates osteogenic cocktail-induced mineralization in 4 T1 metastatic breast cancer cells [23, 71]. Similarly, another research group observed that inoculation of the R3230 rat mammary carcinoma cells overexpressing BMP-2 into the mammary fat pads resulted in breast tumors with microcalcification, as compared to the control group [155]. The same group also demonstrated that treatment with recombinant BMP-2 induced microcalcification in breast cancer tissue of all rats bearing tumors [156]. All these findings suggest that BMP-2 can induce microcalcification in breast cancer. Recently Scimeca et al., reported a key finding that BMP-2 expression was upregulated in infiltrating carcinoma with microcalcification as compared to infiltrating carcinoma without microcalcification, and these calcified infiltrating carcinoma tissues showed expression of both mesenchymal markers and osteoblastic proteins [124]. Based on these findings, it was proposed that a subpopulation of cancer cells which underwent EMT showed the osteoblast-like phenotype, a transition that is presumably driven by BMP-2 [124].
There is also evidence that BMP heightens the expression of transient receptor potential cation channel (TRPC) which may facilitate microcalcification by supplying Ca2+ ions to the cells/MVs [172]. Recent literature shows a positive association with serum BMP and cancer metastasis and/or advanced stage of cancer [173]. Many studies have demonstrated that BMPs inhibit cancer cell proliferation [174–177], but augment migration and invasion of breast cancer cells [178–181], presumably by inducing EMT [167, 182–186].
These findings suggest that BMP-2 not only induces EMT of epithelial breast cancer cells, but also can transdifferentiate EMT-cells to osteoblast-like cells. This subpopulation, which acquires osteoblastic properties, seems to be more competent for pathological microcalcification in breast cancer (schematically described in supplementary Figure S1).
Macrophage Recruitment Accelerates Microcalcification
Heterogeneous tumors consist of tumorigenic, non-tumorigenic, cancer stem cells, and non-cancer cells. Breast tumor cells secrete many inflammatory cytokines such as CCL2, CCL5 and CSF-1 [187, 188], which recruit macrophages to the tumor site, increasing the malignancy of cancers [189–191]. Moreover, metastatic breast cancer cells secrete more CSF-1 when compared to non-metastatic breast cancer cells [188]. Elevation of CSF-1 levels in human serum has been linked with the malignancy of different cancers including endometrial, breast, and ovarian carcinoma [192]. The elevation of CSF-1 results in dense macrophage infiltration to the tumor site [193]. These recruited macrophages, known as tumor associated macrophages (TAMs), play a pivotal role in microcalcification as they supply MVs to the nucleation centre of microcalcification [194]. Other studies also support this idea since breast biopsy samples were shown to have an accumulation of macrophages surrounding microcalcification [195]. Thus, these TAMs could provide additional support for the development of microcalcification in malignant breast tumors.
Proposed Mechanism of Microcalcification
Based on all the literature discussed above, we herein propose a mechanism that the accretion of microcalcification of breast cancer is preceded by a few specialized cells, which have undergone EMT, and also have acquired osteoblastic characteristics. These rare cells with mesenchymal characteristics, become osteoinductive in response to BMP, and function like osteoblasts which may also express NaPi-IIb transporter, NPP, TNAP, TRPC, and annexin channels [76, 79, 86, 87, 135, 196–198], to facilitate calcification. Finally, lipid rafts containing these protein molecules may be pinched out from the membrane to form MVs inside the cells. These MVs move to the extracellular environment, and unload the crystal molecules on the top of extracellular matrix proteins. Other MVs, along with Ca2+ and PO4 3− ions found in the extracellular fluid may support the propagation of crystal formation, which subsequently leads to microcalcification (Fig. 2).
BMPs sometime increase apoptosis of cancer cells, but these apoptotic bodies may, in turn, promote microcalcification [199]. BMPs obstruct tumor growth by inhibiting cancer cell proliferation. This infers that BMPs may have anticancer activity, but when the growth of cancer cells is halted, it may allow differentiation of epithelial cells to a mesenchymal phenotype (Supplementary Figure S1). Therefore, BMPs may augment an invasive phenotype, as well as the calcifying property of cancer cells by increasing by apoptotic bodies that promote microcalcification, and by inhibiting cell growth which may allow epithelial cells to undergo EMT. These cells may be transduced into osteoblast-like cells.
In addition, some cytokines such as CSF-1, CCL2 and CCL5 recruit macrophages to the site of microcalcification [189, 193]. These recruited macrophages might accelerate this pathophysiological mineralization by supplying MVs to the site of crystallization (Fig. 2).
Future Prospects
More basic and clinical research work is required to confirm the presence of microcalcification as a diagnostic and/or a prognostic marker for breast cancer progression and metastasis. In fact, only a causal link between the occurrence of microcalcification and malignancy of cancer has been shown. Thus, it has to be investigated whether the presence/occurrence of mineralization or microcalcification in aggressive cancer tissues is a consequence of the metastatic nature of cancerous tissues. A few studies support the positive role of HA in cancer progression [19, 20, 37]. However, more research is needed to define the function of HA in the regulation of invasiveness/malignancy of cancers. Since cancer cells having osteoblastic properties drive the calcification process, and BMPs are known potent osteoinductive agents, BMPs might promote the microcalcification process. What needs to be resolved is how epithelial cells gain osteoblastic properties. Furthermore, validation is required to know whether EMT is a prerequisite for gaining the osteoblast-like properties of cancer cells. The molecular mechanisms need to be elucidated to determine whether BMPs drive osteoblastic transdifferentiation of epithelial cancer cells followed by EMT or if it induces osteoblastic properties in EMT cells. Future studies will confirm if targeting microcalcification in breast cancer will be a promising therapeutic intervention. Breast cancer often occurs in postmenopausal women. At this age, the risk of osteoporosis is also quite high. Thus, a special strategy should be taken to design a therapeutic drug which prevents microcalcification of breast tissues without debilitating bone quality.
Abbreviations
- TRPC:
-
Transient receptor potential cation channel
- NPP1:
-
Nucleotide pyrophosphatase phosphodiesterase 1
- TNAP:
-
Tissue non-specific alkaline phosphatase
- HA:
-
Hydroxyapatite
- NTP:
-
Nucleoside triphosphate
- iPP:
-
Inorganic pyrophosphate
- iP:
-
Inorganic phosphate
- EMT:
-
Epithelial to mesenchymal transition
References
Tabar L, Yen M-F, Vitak B, Chen H-HT, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. The Lancet. 2003;361(9367):1405–10.
L T, Gad A, Holmberg L, Ljungquist U, Fagerberg C, Baldetorp L, et al. Reduction in mortality from breast cancer after mass screening with mammography: randomised trial from the breast cancer screening working group of the Swedish national board of health and welfare. Lancet. 1985;325(8433):829–32.
Tabar L, Vitak B, Chen TH, Yen AM, Cohen A, Tot T, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011;260(3):658–63. doi:10.1148/radiol.11110469.
Morgan MP, Cooke MM, McCarthy GM. Microcalcifications associated with breast cancer: an epiphenomenon or biologically significant feature of selected tumors? J Mammary Gland Biol Neoplasia. 2005;10(2):181–7.
Cheng H-D, Cai X, Chen X, Hu L, Lou X. Computer-aided detection and classification of microcalcifications in mammograms: a survey. Pattern Recogn. 2003;36(12):2967–91.
Gulsun M, Demirkazik FB, Ariyurek M. Evaluation of breast microcalcifications according to breast imaging reporting and data system criteria and Le Gal’s classification. Eur J Radiol. 2003;47(3):227–31.
Suhail Z, Sarwar M, Murtaza K. Automatic detection of abnormalities in mammograms. BMC Med Imaging. 2015;15. doi:10.1186/s12880-015-0094-8.
Homer MJ. Mammographic Interpretation: A Practical Approach. vol v. 766. McGraw-Hill, Health Professions Division; 1997.
Benson JR, Gui GP, Tuttle T. Early breast cancer: from screening to multidisciplinary management. CRC Press: London; 2013.
Davies SG, Chapman S. Aids to radiological differential diagnosis. UK: Elsevier Health Sciences; 2013.
Bhargava S, Bhargava SK. Differential diagnosis in radiology. Jaypee brothers, medical publishers Pvt. New Delhi: Limited; 2014.
Institute NC. Understanding breast changes: health guide for all women. National Institutes of Health, National Cancer Institute: U.S. Department of Health and Human Services; 2004.
Dikshith TSS. Hazardous chemicals: safety management and global regulations. CRC Press:Boca Raton; 2013.
Judd SJ. Breast cancer sourcebook: basic consumer health information about breast cancer. Incorporated: Omnigraphics; 2004.
MC M, CM d C. A woman’s concise guide to common medical tests. New Brunswick: Rutgers University Press; 2005.
Panda RN, Panigrahi BK, Patro MR. Feature extraction for classification of microcalcifications and mass lesions in mammograms. IJCSNS Int J Comput Sci Netw Secur. 2009;9(5):255–65.
Leborgne R Diagnosis of tumors of the breast by simple roentgenography; calcifications in carcinomas. Am J Roentgenol Radium Ther. 1951;65(1):1.
Haka AS, Shafer-Peltier KE, Fitzmaurice M, Crowe J, Dasari RR, Feld MS. Identifying microcalcifications in benign and malignant breast lesions by probing differences in their chemical composition using Raman spectroscopy. Cancer Res. 2002;62(18):5375–80.
Baker R, Rogers K, Shepherd N, Stone N. New relationships between breast microcalcifications and cancer. Br J Cancer. 2010;103(7):1034–9.
Morgan MP, Cooke MM, Christopherson PA, Westfall PR, McCarthy GM. Calcium hydroxyapatite promotes mitogenesis and matrix metalloproteinase expression in human breast cancer cell lines. Mol Carcinog. 2001;32(3):111–7.
Tabar L, Tony Chen HH, Amy Yen M, Tot T, Tung TH, Chen LS, et al. Mammographic tumor features can predict long-term outcomes reliably in women with 1–14-mm invasive breast carcinoma. Cancer. 2004;101(8):1745–59.
Thurfjell E, Thurfjell MG, Lindgren A. Mammographic finding as predictor of survival in 1–9 mm invasive breast cancers. Worse prognosis for cases presenting as calcifications alone. Breast Cancer Res Treat. 2001;67(2):177–80.
Cox R. Cellular and molecular basis of mammary microcalcifications. 2011.
McDonald L, Ferrari N, Terry A, Bell M, Mohammed ZM, Orange C, et al. RUNX2 in subtype specific breast cancer and mammary gland differentiation. Dis Model Mech. 2014;7:525–34.
Clement JH, Marr N, Meissner A, Schwalbe M, Sebald W, Kliche K-O, et al. Bone morphogenetic protein 2 (BMP-2) induces sequential changes of id gene expression in the breast cancer cell line MCF-7. J Cancer Res Clin Oncol. 2000;126(5):271–9.
Lanigan F, Gremel G, Hughes R, Brennan DJ, Martin F, Jirström K, et al. Homeobox transcription factor muscle segment homeobox 2 (Msx2) correlates with good prognosis in breast cancer patients and induces apoptosis in vitro. Breast Cancer Res. 2010;12(4):R59.
Gillespie MT, Thomas RJ, Pu ZY, Zhou H, Martin TJ, Findlay DM. Calcitonin receptors, bone sialoprotein and osteopontin are expressed in primary breast cancers. Int J Cancer. 1997;73(6):812–5.
Suzuki S, Nomizu T, Nihei M, Rokkaku Y, Kimijima I, Tsuchiya A, et al. Clinical evaluation of serum osteocalcin in patients with bone metastasis of breast cancer. Nihon Gan Chiryo Gakkai shi. 1989;24(10):2386–93.
Le Gal M, Chavanne G, Pellier D. Diagnostic value of clustered microcalcifications discovered by mammography (apropos of 227 cases with histological verification and without a palpable breast tumor). Bull Cancer. 1984;71(1):57–64.
D’orsi C, Bassett L, Berg W, Feig S, Jackson V, Kopans D. Breast imaging reporting and data system: ACR BI-RADS-mammography. Am Coll Radiol Reston. 2003;4.
Frappart L, Boudeulle M, Boumendil J, Lin HC, Martinon I, Palayer C, et al. Structure and composition of microcalcifications in benign and malignant lesions of the breast: study by light microscopy, transmission and scanning electron microscopy, microprobe analysis, and X-ray diffraction. Hum Pathol. 1984;15(9):880–9.
Going J, Anderson T, Crocker P, Levison D. Weddellite calcification in the breast: eighteen cases with implications for breast cancer screening. Histopathology. 1990;16(2):119–24.
Radi MJ. Calcium oxalate crystals in breast biopsies. An overlooked form of microcalcification associated with benign breast disease. Arch Pathol Lab Med. 1989;113(12):1367–9.
Frouge C, Meunier M, Guinebretiere J, Gilles R, Vanel D, Contesso G, et al. Polyhedral microcalcifications at mammography: histologic correlation with calcium oxalate. Radiology. 1993;186(3):681–4.
Park J, Choi H, Bae S-J, Lee M-S, Ahn S-H, Gong G. Clustering of breast microcalcifications: revisited. Clin Radiol. 2000;55(2):114–8.
de Lafontan B, Daures JP, Salicru B, Eynius F, Mihura J, Rouanet P, et al. Isolated clustered microcalcifications: diagnostic value of mammography–series of 400 cases with surgical verification. Radiology. 1994;190(2):479–83.
Cooke MM, McCarthy GM, Sallis JD, Morgan MP. Phosphocitrate inhibits calcium hydroxyapatite induced mitogenesis and upregulation of matrix metalloproteinase-1, interleukin-1Î2 and cyclooxygenase-2 mRNA in human breast cancer cell lines. Breast Cancer Res Treat. 2003;79(2):253–63.
Boonrungsiman S, Gentleman E, Carzaniga R, Evans ND, McComb DW, Porter AE, et al. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci. 2012;109(35):14170–5.
Thouverey C, Strzelecka-Kiliszek A, Balcerzak M, Buchet R, Pikula S. Matrix vesicles originate from apical membrane microvilli of mineralizing osteoblast-like saos-2 cells. J Cell Biochem. 2009;106(1):127–38.
Kogaya Y, Furuhashi K. Ultrastructural localization of calcium in matrix vesicles and preodontoblasts of developing rat molar tooth germs during initial dentinogenesis. Cells Tissues Organs. 1988;132(2):100–8.
Sasagawa I The appearance of matrix vesicles and mineralization during tooth development in three teleost fishes with well-developed enameloid and orthodentine. Arch Oral Biol. 1988;33(2):75–86.
Garcés-Ortíz M, Ledesma-Montes C, Reyes-Gasga J. Presence of matrix vesicles in the body of odontoblasts and in the inner third of dentinal tissue: A scanning electron microscopyc study. Med Oral Patol oral Cir Bucal. 2013;18(3):e537.
Ali SY, Sajdera S, Anderson H. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci. 1970;67(3):1513–20.
Iannotti J, Naidu S, Noguchi Y, Hunt R, Brighton C. Growth plate matrix vesicle biogenesis: the role of intracellular calcium. Clin Orthop Relat Res. 1994;306:222–9.
Katsman D, Stackpole EJ, Domin DR, Farber DB. Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS One. 2012;7(11):e50417.
Chen NX, O’Neill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 2008;23(11):1798–805.
Chen NX, Chen X, O’Neill KD, Atkinson SJ, Moe SM. RhoA/Rho kinase (ROCK) alters fetuin-A uptake and regulates calcification in bovine vascular smooth muscle cells (BVSMC). Am J Physiol Ren Physiol. 2010;299(3):F674–F80.
Kapustin AN, Shanahan CM. Calcium regulation of vascular smooth muscle cell–derived matrix vesicles. Trends Cardiovasc Med. 2012;22(5):133–7.
Hutcheson JD, Maldonado N, Aikawa E. Small entities with large impact: microcalcifications and atherosclerotic plaque vulnerability. Curr Opin Lipidol. 2014;25(5):327.
Millimaggi D, Festuccia C, Angelucci A, D’Ascenzo S, Rucci N, Flati S, et al. Osteoblast-conditioned media stimulate membrane vesicle shedding in prostate cancer cells. Int J Oncol. 2006;28(4):909–14.
Dolo V, Adobati E, Canevari S, Picone MA, Vittorelli ML. Membrane vesicles shed into the extracellular medium by human breast carcinoma cells carry tumor-associated surface antigens. Clin Exp Metastasis. 1995;13(4):277–86.
Galindo-Hernandez O, Gonzales-Vazquez C, Cortes-Reynosa P, Reyes-Uribe E, Chavez-Ocaña S, Reyes-Hernandez O, et al. Extracellular vesicles from women with breast cancer promote an epithelial-mesenchymal transition-like process in mammary epithelial cells MCF10A. Tumor Biol. 2015;1-11.
Menck K, Scharf C, Bleckmann A, Dyck L, Rost U, Wenzel D, et al. Tumor-derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J Mol Cell Biol. 2015;7(2):143–53.
Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci. 2014;111(31):E3234–E42.
Wysoczynski M, Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer. 2009;125(7):1595–603.
Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71(15):5346–56.
Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6.
Register T, McLean F, Low M, Wuthier R. Roles of alkaline phosphatase and labile internal mineral in matrix vesicle-mediated calcification. Effect of selective release of membrane-bound alkaline phosphatase and treatment with isosmotic pH 6 buffer. J Biol Chem. 1986;261(20):9354–60.
Peress NS, Anderson HC, Sajdera SW. The lipids of matrix vesicles from bovine fetal epiphyseal cartilage. Calcif Tissue Res. 1974;14(1):275–81.
Kirsch T, Harrison G, Golub EE, Nah H-D. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem. 2000;275(45):35577–83.
Nielsen L, Pedersen F, Pedersen L. Expression of type III sodium-dependent phosphate transporters/retroviral receptors mRNAs during osteoblast differentiation. Bone. 2001;28(2):160–6.
Stewart AJ, Roberts SJ, Seawright E, Davey MG, Fleming RH, Farquharson C. The presence of PHOSPHO1 in matrix vesicles and its developmental expression prior to skeletal mineralization. Bone. 2006;39(5):1000–7.
Bonucci E Fine structure of early cartilage calcification. J Ultrastruct Res. 1967;20(1):33–50.
Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5(3):222–6.
Anderson HC. Mechanisms of pathologic calcification. Rheum Dis Clin N Am. 1988;14(2):303–19.
Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc Natl Acad Sci. 2013;110(26):10741–6.
Goettsch C, Hutcheson JD, Aikawa M, Singh S, Libby P, Aikawa E. Sortilin 1 Promotes Matrix Vesicle-Mediated Vascular Microcalcification via Rab11 Trafficking and C-Terminal Phosphorylation. Arterioscler Thromb Vasc Biol. 2014;34(Suppl 1):A51-A.
New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, et al. Macrophage-derived matrix vesicles an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113(1):72–7.
Agnieszka Strzelecka-Kiliszek LB. Rene buchet & slawomir pikula. European Calcified Tissue Society Conference: Chemical composition of apatites formed by matrix vesicles during bone mineralization; 2014. doi:10.1530/boneabs.3.PP133.
Sando N, Oka K, Moriya T, Saito H, Nagakura S, Mori N, et al. Osteosarcoma arising in the breast. APMIS. 2006;114(7–8):580–6.
Cox R, Hernandez-Santana A, Ramdass S, McMahon G, Harmey J, Morgan M. Microcalcifications in breast cancer: novel insights into the molecular mechanism and functional consequence of mammary mineralisation. Br J Cancer. 2012;106(3):525–37.
Korpela J, Tiitinen S, Hiekkanen H, Halleen J, Selander K, Väänänen H, et al. Serum TRACP 5b and ICTP as markers of bone metastases in breast cancer. Anticancer Res. 2006;26(4B):3127–32.
Sarvari B, Mahadev DS, Rupa S, Mastan S. Detection of bone metastases in breast cancer (BC) patients by serum tartrate-resistant acid phosphatase 5b (TRACP 5b), a bone resorption marker and serum alkaline phosphatase (ALP), a bone formation marker, in lieu of whole body skeletal scintigraphy with Technetium99m MDP. Indian J Clin Biochem. 2015;30(1):66–71.
Guerreiro S, Monteiro R, Martins MJ, Calhau C, Azevedo I, Soares R. Distinct modulation of alkaline phosphatase isoenzymes by 17β-estradiol and xanthohumol in breast cancer MCF-7 cells. Clin Biochem. 2007;40(3):268–73.
Usoro NI, Omabbe MC, Usoro CA, Nsonwu A. Calcium, inorganic phosphates, alkaline and acid phosphatase activities in breast cancer patients in Calabar, Nigeria. Afr Health Sci. 2010;10(1):9.
Deng S, Wang J, Hou L, Li J, Chen G, Jing B, et al. Annexin A1, A2, A4 and A5 play important roles in breast cancer, pancreatic cancer and laryngeal carcinoma, alone and/or synergistically. Oncol Lett. 2013;5(1):107–12.
Sharma MR, Koltowski L, Ownbey RT, Tuszynski GP, Sharma MC. Angiogenesis-associated protein annexin II in breast cancer: selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp Mol Pathol. 2006;81(2):146–56.
Deng S, Jing B, Xing T, Hou L, Yang Z. Overexpression of annexin A2 is associated with abnormal ubiquitination in breast cancer. Genom Proteomics Bioinform. 2012;10(3):153–7.
Chen D-R, Chien S-Y, Kuo S-J, Teng Y-H, Tsai H-T, Kuo J-H, et al. SLC34A2 as a novel marker for diagnosis and targeted therapy of breast cancer. Anticancer Res. 2010;30(10):4135–40.
Denoyer D, Perek N, Le Jeune N, Frere D, Dubois F. Evidence that 99mTc-(V)-DMSA uptake is mediated by NaPi cotransporter type III in tumour cell lines. Eur J Nucl Med Mol Imaging. 2004;31(1):77–84.
Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, et al. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem. 2011;28(5):813–22.
Guilbert A, Gautier M, Dhennin-Duthille I, Haren N, Sevestre H, Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol. 2009;297(3):C493–502.
Mandavilli S, Singh BB, Sahmoun AE. Serum calcium levels, TRPM7, TRPC1, microcalcifications, and breast cancer using breast imaging reporting and data system scores. Breast Cancer Targets Ther. 2013;5:1.
Middelbeek J, Kuipers AJ, Henneman L, Visser D, Eidhof I, van Horssen R, et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012;72(16):4250–61.
Helenius M, Jalkanen S, Yegutkin GG. Enzyme-coupled assays for simultaneous detection of nanomolar ATP, ADP, AMP, adenosine, inosine and pyrophosphate concentrations in extracellular fluids. Biochim et Biophys Acta (BBA)-Mol Cell Res. 2012;1823(10):1967–75.
Yang SY, Lee J, Park CG, Kim S, Hong S, Chung HC, et al. Expression of autotaxin (NPP-2) is closely linked to invasiveness of breast cancer cells. Clin Exp Metastasis. 2002;19(7):603–8.
Ouadid-Ahidouch H, Dhennin-Duthille I, Gautier M, Sevestre H, Ahidouch A. TRP calcium channel and breast cancer: expression, role and correlation with clinical parameters. Bull Cancer. 2012;99(6):655–64.
Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, et al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56(3):453–62.
Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114(1 suppl):I-547-I-52.
Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang W, Jafferji M, Gordon JA, et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem. 2012;287(50):42084–92.
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–64.
Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107(17):2181–4.
Lee H-L, Woo KM, Ryoo H-M, Baek J-H. Tumor necrosis factor-α increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem Biophys Res Commun. 2010;391(1):1087–92.
Barnes GL, Javed A, Waller SM, Kamal MH, Hebert KE, Hassan MQ, et al. Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 2003;63(10):2631–7.
Chang C-H, Fan T-C, Yu J-C, Liao G-S, Lin Y-C, Shih A, et al. The prognostic significance of RUNX2 and miR-10a/10b and their inter-relationship in breast cancer. J Transl Med. 2014;12(1):257.
Pratap J, Wixted JJ, Gaur T, Zaidi SK, Dobson J, Gokul KD, et al. Runx2 transcriptional activation of Indian hedgehog and a downstream bone metastatic pathway in breast cancer cells. Cancer Res. 2008;68(19):7795–802.
Inman CK, Shore P. The osteoblast transcription factor Runx2 is expressed in mammary epithelial cells and mediates osteopontin expression. J Biol Chem. 2003;278(49):48684–9.
Selvamurugan N, Kwok S, Partridge NC. Smad3 interacts with JunB and Cbfa1/Runx2 for transforming growth factor-β1-stimulated collagenase-3 expression in human breast cancer cells. J Biol Chem. 2004;279(26):27764–73.
Pratap J, Javed A, Languino LR, Van Wijnen AJ, Stein JL, Stein GS, et al. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol. 2005;25(19):8581–91.
Pilloni A, Pompa G, Saccucci M, Di Carlo G, Rimondini L, Brama M, et al. Analysis of human alveolar osteoblast behavior on a nano-hydroxyapatite substrate: an in vitro study. BMC Oral Health. 2014;14(1):22.
Suzuki A, Ghayor C, Guicheux J, Magne D, Quillard S, Kakita A, et al. Enhanced expression of the inorganic phosphate transporter pit-1 is involved in BMP-2–induced matrix mineralization in osteoblast-like cells. J Bone Miner Res. 2006;21(5):674–83.
Wang EA, Rosen V, D’Alessandro JS, Bauduy M, Cordes P, Harada T, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci. 1990;87(6):2220–4.
Hadjicharalambous C, Kozlova D, Sokolova V, Epple M, Chatzinikolaidou M. Calcium phosphate nanoparticles carrying BMP-7 plasmid DNA induce an osteogenic response in MC3T3-E1 pre-osteoblasts. J Biomed Mater Res A. 2015;103:3834–42.
Kaden JJ, Bickelhaupt S, Grobholz R, Vahl C, Hagl S, Brueckmann M, et al. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis. 2004;13(4):560–6.
Rong S, Zhao X, Jin X, Zhang Z, Chen L, Zhu Y, et al. Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the Wnt/β-catenin pathway. Cell Physiol Biochem. 2014;34(6):2049–60.
Arnold S, Tims E, McGrath B. Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: importance of BMP2. Cytokine. 1999;11(12):1031–7.
Clement JH, Sänger J, Höffken K. Expression of bone morphogenetic protein 6 in normal mammary tissue and breast cancer cell lines and its regulation by epidermal growth factor. Int J Cancer. 1999;80(2):250–6.
Alarmo E-L, Kuukasjärvi T, Karhu R, Kallioniemi A. A comprehensive expression survey of bone morphogenetic proteins in breast cancer highlights the importance of BMP4 and BMP7. Breast Cancer Res Treat. 2007;103(2):239–46.
Alarmo EL, Rauta J, Kauraniemi P, Karhu R, Kuukasjärvi T, Kallioniemi A. Bone morphogenetic protein 7 is widely overexpressed in primary breast cancer. Genes Chromosom Cancer. 2006;45(4):411–9.
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29.
Oh J-H, Park S-Y, de Crombrugghe B, Kim J-E. Chondrocyte-specific ablation of osterix leads to impaired endochondral ossification. Biochem Biophys Res Commun. 2012;418(4):634–40.
Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24(4):391–5.
Wilkie AO, Tang Z, Elanko N, Walsh S, Twigg SR, Hurst JA, et al. Functional haploinsufficiency of the human homeobox gene MSX2 causes defects in skull ossification. Nat Genet. 2000;24(4):387–90.
Goupille O, Saint Cloment C, Lopes M, Montarras D, Robert B. Msx1 and Msx2 are expressed in sub-populations of vascular smooth muscle cells. Dev Dyn. 2008;237(8):2187–94.
Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression of bone sialoprotein (BSP) in developing human tissues. Calcif Tissue Int. 1991;49(6):421–6.
Chen J, Shapiro HS, Sodek J. Developmental expression of bone sialoprotein mRNA in rat mineralized connective tissues. J Bone Miner Res. 1992;7(8):987–97.
Severson AR, Ingram RT, Fitzpatrick LA. Matrix proteins associated with bone calcification are present in human vascular smooth muscle cells grownin vitro. In Vitro Cell Dev Biol Anim. 1995;31(11):853–7.
Kovacheva M, Zepp M, Berger SM, Berger MR. Sustained conditional knockdown reveals intracellular bone sialoprotein as essential for breast cancer skeletal metastasis. Oncotarget. 2014;5(14):5510.
J-H ZHANG, Wang J, Tang J, Barnett B, Dickson J, Hahsimoto N, et al. Bone sialoprotein promotes bone metastasis of a non-bone-seeking clone of human breast cancer cells. Anticancer Res. 2004;24(3 A):1361–8.
Diel IJ, Solomayer E-F, Seibel MJ, Pfeilschifter J, Maisenbacher H, Gollan C, et al. Serum bone sialoprotein in patients with primary breast cancer is a prognostic marker for subsequent bone metastasis. Clin Cancer Res. 1999;5(12):3914–9.
Erik H, Jared SG, Yinyin L, Esben SS, Frank B, Graeme KH, et al. Osteopontin mediates mineralization and not osteogenic cell development in vitro. Biochem J. 2014;464(3):355–64.
Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures inhibition by osteopontin. Circ Res. 1999;84(2):166–78.
Bellahcene A, Castronovo V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol. 1995;146(1):95.
Scimeca M, Giannini E, Antonacci C, Pistolese CA, Spagnoli LG, Bonanno E. Microcalcifications in breast cancer: an active phenomenon mediated by epithelial cells with mesenchymal characteristics. BMC Cancer. 2014;14(1):286.
Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, et al. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143(3):420–30.
Liu T, Lin J, Ju T, Chu L, Zhang L. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves matrix metalloproteinase-2 modulation by homocysteine. Mol Cell Biochem. 2015;1-11.
Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H, et al. Induction of bone-type alkaline Phosphatase in human vascular smooth muscle cells roles of tumor necrosis factor-α and oncostatin M derived from macrophages. Circ Res. 2002;91(1):9–16.
Accorsi-Mendonça T, da Silva Paiva KB, Zambuzzi WF, Cestari TM, Lara VS, Sogayar MC, et al. Expression of matrix metalloproteinases-2 and-9 and RECK during alveolar bone regeneration in rat. J Mol Histol. 2008;39(2):201–8.
Chen NX, O’Neill KD, Chen X, Kiattisunthorn K, Gattone VH, Moe SM. Activation of arterial matrix metalloproteinases leads to vascular calcification in chronic kidney disease. Am J Nephrol. 2011;34(3):211–9.
Tsai C-L, Chen W-C, Hsieh H-L, Chi P-L, Hsiao L-D, Yang C-M. TNF-α induces matrix metalloproteinase-9-dependent soluble intercellular adhesion molecule-1 release via TRAF2-mediated MAPKs and NF-κB activation in osteoblast-like MC3T3-E1 cells. J Biomed Sci. 2014;21:12.
Ishigaki R, Takagi M, Igarashi M, Ito K. Gene expression and immunohistochemical localization of osteonectin in association with early bone formation in the developing mandible. Histochem J. 2002;34(1–2):57–66.
Farrokhi E, Ghatreh Samani K, Hashemzadeh Chaleshtori M, Tabatabaiefar MA. Effect of oxidized low density lipoprotein on the expression of Runx2 and SPARC genes in vascular smooth muscle cells. Iran Biomed J. 2015;19(3):160–4.
Li X, Weaver O, Desouki MM, Dabbs D, Shyum S, Carter G, et al. Microcalcification is an important factor in the management of breast intraductal papillomas diagnosed on core biopsy. Am J Clin Pathol. 2012;138(6):789–95.
Chavkin NW, Chia JJ, Crouthamel MH, Giachelli CM. Phosphate uptake-independent signaling functions of the type III sodium-dependent phosphate transporter, PiT-1, in vascular smooth muscle cells. Exp Cell Res. 2015;333(1):39–48.
Singh A, Pandey A, Tewari M, Kumar R, Sharma A, Singh K, et al. Advanced stage of breast cancer hoist alkaline phosphatase activity: risk factor for females in India. 3 Biotech. 2013;3(6):517–20.
Choudhari A, Desai P, Indumati V, Kadi S. Activities of serum Ada, GGT and alp in carcinoma breast-a case control study for diagnostic and prognostic significance. Indian J Med Sci. 2013;67(5):123.
Komori T Regulation of skeletal development by the Runx family of transcription factors. J Cell Biochem. 2005;95(3):445–53.
S-i H, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349–55.
Koga T, Matsui Y, Asagiri M, Kodama T, de Crombrugghe B, Nakashima K, et al. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005;11(8):880–5.
Mandal CC, Drissi H, Choudhury GG, Ghosh-Choudhury N. Integration of phosphatidylinositol 3-kinase, Akt kinase, and smad signaling pathway in BMP-2-induced osterix expression. Calcif Tissue Int. 2010;87(6):533–40.
Barnes GL, Hebert KE, Kamal M, Javed A, Einhorn TA, Lian JB, et al. Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases-associated osteolytic disease. Cancer Res. 2004;64(13):4506–13.
Jauliac S, Lopez-Rodriguez C, Shaw LM, Brown LF, Rao A, Toker A. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4(7):540–4.
Yeung F, Law WK, Yeh C-H, Westendorf JJ, Zhang Y, Wang R, et al. Regulation of human osteocalcin promoter in hormone-independent human prostate cancer cells. J Biol Chem. 2002;277(4):2468–76.
Bellahcene A, Kroll M, Liebens F, Castronovo V. Bone sialoprotein expression in primary human breast cancer is associated with bone metastases development. J Bone Miner Res. 1996;11(5):665–70.
Neman J, Hambrecht A, Cadry C, Jandial R. Stem cell-mediated osteogenesis: therapeutic potential for bone tissue engineering. Biologics Targets Therapy. 2012;6:47.
Zanette DL, Rivadavia F, Molfetta GA, Barbuzano FG, Proto-Siqueira R, Silva-Jr WA, et al. miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Braz J Med Biol Res. 2007;40(11):1435–40.
Lee J-S, Lee J-M, Im G-I. Electroporation-mediated transfer of Runx2 and osterix genes to enhance osteogenesis of adipose stem cells. Biomaterials. 2011;32(3):760–8.
Abate-Shen C Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2(10):777–85.
Satoh K, Hamada S, Kimura K, Kanno A, Hirota M, Umino J, et al. Up-regulation of MSX2 enhances the malignant phenotype and is associated with twist 1 expression in human pancreatic cancer cells. Am J Pathol. 2008;172(4):926–39.
Di Bari M, Ginsburg E, Plant J, Strizzi L, Salomon D, Vonderhaar B. Msx2 induces epithelial-mesenchymal transition in mouse mammary epithelial cells through upregulation of Cripto-1. J Cell Physiol. 2009;219(3):659–66.
Satoh K, Hamada S, Kanno A, Hirota M, Umino J, Ito H, et al. Expression of MSX2 predicts malignancy of branch duct intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol. 2010;45(7):763–70.
Cheng S-L, Shao J-S, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278(46):45969–77.
Castronovo V, Bellahcene A. Evidence that breast cancer associated microcalcifications are mineralized malignant cells. Int J Oncol. 1998;12(2):305–13.
Bundred NJ, Walls J, Ratcliffe WA. Parathyroid hormone-related protein, bone metastases and hypercalcaemia of malignancy. Ann R Coll Surg Engl. 1996;78(4):354–8.
Liu F, Bloch N, Bhushan KR, De Grand AM, Tanaka E, Solazzo S, et al. Humoral BMP-2 is sufficient for inducing breast cancer microcalcification. Mol Imaging. 2008;7(4):175.
Inoue K, Liu F, Hoppin J, Lunsford EP, Lackas C, Hesterman J, et al. High-resolution CT imaging of single breast cancer microcalcifications in vivo. Mol Imaging. 2011;10(4):295.
Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105.
Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97(2):105–14.
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell Signal. 2011;23(4):609–20.
Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–41. doi:10.1080/08977190412331279890.
Choi ME, Ding Y, Kim SI, editors. TGF-β signaling via TAK1 pathway: role in kidney fibrosis. Seminars in nephrology; 2012: Elsevier.
Tian XY, Yung LH, Wong WT, Liu J, Leung FP, Liu L, et al. Bone morphogenic protein-4 induces endothelial cell apoptosis through oxidative stress-dependent p38MAPK and JNK pathway. J Mol Cell Cardiol. 2012;52(1):237–44. doi:10.1016/j.yjmcc.2011.10.013.
Hong J, Zhou J, Fu J, He T, Qin J, Wang L, et al. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res. 2011;71(11):3980–90.
Hipp S, Berg D, Ergin B, Schuster T, Hapfelmeier A, Walch A, et al. Interaction of snail and p38 mitogen-activated protein kinase results in shorter overall survival of ovarian cancer patients. Virchows Arch. 2010;457(6):705–13.
Hsu YL, Huang MS, Yang CJ, Hung JY, Wu LY, Kuo PL. Lung tumor-associated osteoblast-derived bone morphogenetic protein-2 increased epithelial-to-mesenchymal transition of cancer by Runx2/Snail signaling pathway. J Biol Chem. 2011;286(43):37335–46. doi:10.1074/jbc.M111.256156.
Liu J, Ben Q-W, Yao W-Y, Zhang J-J, Chen D-F, He X-Y et al. BMP2 induces PANC-1 cell invasion by MMP-2 overexpression through ROS and ERK. Front Biosci (Landmark Ed). 2012;17:2541–9.
Liao A, Wang W, Sun D, Jiang Y, Tian S, Li J, et al. Bone morphogenetic protein 2 mediates epithelial-mesenchymal transition via AKT and ERK signaling pathways in gastric cancer. Tumour Biol. 2014. doi:10.1007/s13277-014-2901-1.
Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL, Kim JS, et al. Metastatic function of BMP-2 in gastric cancer cells: the role of PI3K/AKT, MAPK, the NF-κB pathway, and MMP-9 expression. Exp Cell Res. 2011;317(12):1746–62.
Kang MH, Kim JS, Seo JE, Oh SC, Yoo YA. BMP2 accelerates the motility and invasiveness of gastric cancer cells via activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Exp Cell Res. 2010;316(1):24–37. doi:10.1016/j.yexcr.2009.10.010.
Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–40.
Schlange T, Matsuda Y, Lienhard S, Huber A, Hynes NE. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res. 2007;9(5):R63. doi:10.1186/bcr1769.
Li X, Lu W, Fu X, Zhang Y, Yang K, Zhong N, et al. BMP4 increases canonical transient receptor potential protein expression by activating p38 MAPK and ERK1/2 signaling pathways in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol. 2013;49(2):212–20. doi:10.1165/rcmb.2012-0051OC.
Choi YJ, Kim ST, Park KH, Oh SC, Seo JH, Shin SW, et al. The serum bone morphogenetic protein-2 level in non-small-cell lung cancer patients. Med Oncol. 2012;29(2):582–8.
Ghosh-Choudhury N, Woodruff K, Qi W, Celeste A, Abboud SL, Choudhury GG. Bone morphogenetic protein-2 blocks MDA MB 231 human breast cancer cell proliferation by inhibiting cyclin-dependent kinase-mediated retinoblastoma protein phosphorylation. Biochem Biophys Res Commun. 2000;272(3):705–11.
Chen A, Wang D, Liu X, He S, Yu Z, Wang J. Inhibitory effect of BMP-2 on the proliferation of breast cancer cells. Mol Med Rep. 2012;6(3):615–20.
Takahashi M, Otsuka F, Miyoshi T, Otani H, Goto J, Yamashita M, et al. Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 mitogen-activated protein kinase activation. J Endocrinol. 2008;199(3):445–55.
Ampuja M, Jokimäki R, Juuti-Uusitalo K, Rodriguez-Martinez A, Alarmo E-L, Kallioniemi A. BMP4 inhibits the proliferation of breast cancer cells and induces an MMP-dependent migratory phenotype in MDA-MB-231 cells in 3D environment. BMC Cancer. 2013;13(1):429.
Clement JH, Raida M, Sänger J, Bicknell R, Liu J, Naumann A, et al. Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. Int J Oncol. 2005;27(2):401–7.
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27(49):6322–33.
Montesano R Bone morphogenetic protein-4 abrogates lumen formation by mammary epithelial cells and promotes invasive growth. Biochem Biophys Res Commun. 2007;353(3):817–22.
Ketolainen JM, Alarmo E-L, Tuominen VJ, Kallioniemi A. Parallel inhibition of cell growth and induction of cell migration and invasion in breast cancer cells by bone morphogenetic protein 4. Breast Cancer Res Treat. 2010;124(2):377–86.
Kang MH, Kang HN, Kim JL, Kim JS, Oh SC, Yoo YA. Inhibition of PI3 kinase/Akt pathway is required for BMP2-induced EMT and invasion. Oncol Rep. 2009;22(3):525–34.
Richter A, Valdimarsdottir L, Hrafnkelsdottir HE, Runarsson JF, Omarsdottir AR, Ward-van Oostwaard D, et al. BMP4 promotes EMT and mesodermal commitment in human embryonic stem cells via SLUG and MSX2. Stem Cells. 2014;32(3):636–48. doi:10.1002/stem.1592.
Xu T, Yu CY, Sun JJ, Liu Y, Wang XW, Pi LM, et al. Bone morphogenetic protein-4-induced epithelial-mesenchymal transition and invasiveness through Smad1-mediated signal pathway in squamous cell carcinoma of the head and neck. Arch Med Res. 2011;42(2):128–37. doi:10.1016/j.arcmed.2011.03.003.
Theriault BL, Shepherd TG, Mujoomdar ML, Nachtigal MW. BMP4 induces EMT and rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007;28(6):1153–62. doi:10.1093/carcin/bgm015.
Hamada S, Satoh K, Hirota M, Kimura K, Kanno A, Masamune A, et al. Bone morphogenetic protein 4 induces epithelial-mesenchymal transition through MSX2 induction on pancreatic cancer cell line. J Cell Physiol. 2007;213(3):768–74. doi:10.1002/jcp.21148.
Ghirelli C, Reyal F, Jeanmougin M, Zollinger R, Sirven P, Michea P et al. Breast cancer cell-derived GM-CSF licenses regulatory Th2 induction by plasmacytoid pre-dendritic cells in aggressive disease subtypes. Cancer Research. 2015:canres. 2386.014.
Mandal CC, Ghosh-Choudhury T, Dey N, Choudhury GG, Ghosh-Choudhury N. miR-21 is targeted by omega-3 polyunsaturated fatty acid to regulate breast tumor CSF-1 expression. Carcinogenesis. 2012:bgs198.
Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, et al. The CC chemokine RANTES in breast carcinoma progression regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62(4):1093–102.
Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, et al. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59(18):4681–7.
Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21(6):836–47.
Kacinski BM. CSF-1 and its receptor in breast carcinomas and neoplasms of the female reproductive tract. Mol Reprod Dev. 1997;46(1):71–4.
Tang R, Kacinski B, Validire P, Beuvon F, Sastre X, Benoit P, et al. Oncogene amplification correlates with dense lymphocyte infiltration in human breast cancers: a role for hematopoietic growth factor release by tumor cells? J Cell Biochem. 1990;44(3):189–98.
Shih J-Y, Yuan A, Chen JJ-W, Yang P-C. Tumor-associated macrophage: its role in cancer invasion and metastasis. J Cancer Mol. 2006;2(3):101–6.
Yam M, Tchou J, English R, Highnam R, Highnam R, Roskell D, et al. A mammographic dilemma: calcification or haemosiderin as a cause of opacities? Validation of a new digital diagnostic tool. Br J Radiol. 2001;74(887):1048–51.
Chen M, O’Connor KL. Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene. 2005;24(32):5125–30. doi:10.1038/sj.onc.1208729.
Kiyamova R, Shyian M, Lyzogubov VV, Usenko VS, Gout T, Filonenko V. Immunohistochemical analysis of NaPi2b protein (MX35 antigen) expression and subcellular localization in human normal and cancer tissues. Exp Oncol. 2011;33(3):157–61.
Wang C-Y, Lin C-F. Annexin A2: its molecular regulation and cellular expression in cancer development. Dis Markers. 2014;2014.
Schlieper G, Aretz A, Verberckmoes SC, Krüger T, Behets GJ, Ghadimi R, et al. Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol. 2010;21(4):689–96.
Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007;18(5):444–8.
Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30(10):542–50.
Balcerzak M, Hamade E, Zhang L, Pikula S, Azzar G, Radisson J, et al. The roles of annexins and alkaline phosphatase in mineralization process. Acta Biochim Pol. 2003;50(4):1019–38.
Lieben L, Carmeliet G. The involvement of TRP channels in bone homeostasis. Front Endocrinol. 2012;3:99.
Yoshiko Y, Candeliere GA, Maeda N, Aubin JE. Osteoblast autonomous P(i) regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol. 2007;27(12):4465–74. doi:10.1128/mcb.00104-07.
Chen D, Harris M, Rossini G, Dunstan C, Dallas S, Feng J, et al. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in culturesof fetal rat calvarial osteoblasts. Calcif Tissue Int. 1997;60(3):283–90.
Ghosh-Choudhury N, Mandal CC, Choudhury GG. Statin-induced Ras activation integrates the phosphatidylinositol 3-kinase signal to Akt and MAPK for bone morphogenetic protein-2 expression in osteoblast differentiation. J Biol Chem. 2007;282(7):4983–93.
Mandal C, Ganapathy S, Gorin Y, Mahadev K, Block K, Abboud H, et al. Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochem J. 2011;433:393–402.
Ghosh-Choudhury N, Mandal CC, Das F, Ganapathy S, Ahuja S, Choudhury GG. c-Abl-dependent molecular circuitry involving Smad5 and phosphatidylinositol 3-kinase regulates bone morphogenetic protein-2-induced osteogenesis. J Biol Chem. 2013;288(34):24503–17.
Ciceri P, Elli F, Brenna I, Volpi E, Romagnoli S, Tosi D, et al. Lanthanum prevents high phosphate-induced vascular calcification by preserving vascular smooth muscle lineage markers. Calcif Tissue Int. 2013;92(6):521–30.
Acknowledgment
Authors thank the Editors-in-Chief Prof. Russell C. Hovey (Department of Animal Science, University of California, Davis, USA) and the reviewers for their critical comments and suggestions. CCM is supported by UGC Grant [30-49/2014 (BSR)], DBT [6242 P9/RGCB/PMD/DBT/CCML/2015] and Central University of Rajasthan, India, and TS is supported by DST-INSPIRE fellowship [IF140765] provided by the Department of Science and Technology, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Supplementary Fig. S1
BMPs as inducers of hydroxyapatite formation. BMPs are sourced in tumor microenvironment from different types of cells including breast tumor cells, cancer associated cells such as macrophages, and blood. BMPs molecules may act on the breast tumor cells to inhibit cell proliferation and to induce apoptosis of cancer cells. BMPs may also induce EMT in a few cancer cells. Cells which have undergone EMT attain osteoblast properties in response to BMP. These osteoblast cells secrete HA to the extracellular fluid spaces which results in microcalcification. Moreover, this process of HA formation is further accelerated by apoptotic bodies which may be produced due to the action of BMPs. Abbreviation: OB-Osteoblast, HA-Hydroxyapatite, EMT-Epithelial to mesenchymal transition. (TIFF 326 kb)
Supplementary Table S1
Detection of microcalcification in different tissues by different techniques. Abbreviations: HE-Hematoxylin & eosin, VKS-Von Kossa stain, PET-Positron emission tomography, US-Ultrasonography, LM-Light microscopy, SEM-Scanning electron microscopy, TEM-Transmission electron microscopy, CNB-Core needle biopsy, MRI-Magnetic resonance imaging, FTIR-Fourier transform infrared spectroscopy, DCIS-Ductal carcinoma in situ, IC-Invasive carcinoma, LCIS-Lobular carcinoma in situ, IDC-Invasive ductal carcinoma, ILC-Invasive lobular carcinoma, CT-Computed tomography. (DOCX 58 kb)
Rights and permissions
About this article
Cite this article
Sharma, T., Radosevich, J.A., Pachori, G. et al. A Molecular View of Pathological Microcalcification in Breast Cancer. J Mammary Gland Biol Neoplasia 21, 25–40 (2016). https://doi.org/10.1007/s10911-015-9349-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-015-9349-9