Abstract
Background
To distinguish malignant from benign branch duct (BD)-intraductal papillary mucinous neoplasm (IPMN) still remains difficult. Recently, we revealed that MSX2 was frequently expressed in pancreatic cancer and its expression was correlated with aggressive behavior of the cancer. The aim of this study was to assess the involvement of MSX2 in IPMN development and whether its expression would differentiate malignant from benign IPMN.
Methods
Seventeen microdissected lesions and 45 IPMN tissues were used for quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR) and immunohistochemistry, respectively. The role of MSX2 in the pancreatic duct cell was assessed by the induced expression of MSX2 in a normal human pancreatic duct epithelial cell line (HPDE).
Results
Malignant IPMN expressed significantly higher levels of MSX2 mRNA than benign IPMN lesions. MSX2 protein expression was frequently found in borderline and malignant lesions (20/29, 68.9%), while its expression was seen in only one of 16 benign IPMN tissues. Univariate analysis showed that nodules of 6 mm or more and MSX2 expression were significantly correlated with the malignancy of BD-IPMN (P = 0.022 and 0.0026, respectively), and multivariate analysis revealed that only MSX2 expression was identified as an independent factor to predict malignant BD-IPMN. HPDE cells expressing MSX2 showed increased cellular proliferation compared to control cells.
Conclusions
Based on our results, MSX2 plays a pivotal role in the development of IPMN through growth stimulation of tumor cells, and its expression was identified as an independent predictive factor for malignancy of BD-IPMN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraductal papillary-mucinous neoplasia (IPMN) of the pancreas is a unique neoplasm that is considered to be a precancerous lesion analogous to adenomatous polyps of the colon [1]. IPMNs are classified as main-duct type (MD-IPMN) or branch-duct type (BD-IPMN) based on the location of the main tumor detected by imaging studies or histology. BD-IPMN is likely to have less aggressive biological behavior than MD-IPMN because about 70% (range, 57%–92%) of MD-IPMNs were shown to have contained carcinoma cells, while a low prevalence of cancer cells (6%–46%) has been detected in BD-IPMN [2–8]. We have demonstrated, however, the activation of oncogenes such as K-ras [9, 10], sonic hedgehog [11], and c-erb B-2 [12]; the accumulation of p53 [10], and the expression of a member of the inhibitor of apoptosis family, survivin [13], as well as loss of chromosome 18q [14], in BD-IPMN, suggesting the malignant potential of this neoplasm. In addition, stromal infiltration and distal metastasis have been reported even in this type of tumor [15, 16]. Consequently, some authors have recommended surgical treatment for all BD-IPMNs as preventive, stressing their potential malignancy [17, 18], while others have advocated nonsurgical management with close observation because of the infrequent carcinoma cells in BD-IPMN [19–21]. Therefore, the appropriate management of BD-IPMNs still remains unclear. Recent consensus guidelines suggest that the presence of one or more of the following is an indication for resection of BD-IPMN: cyst-related symptoms, main pancreatic duct diameter 10 mm or more, cyst size 30 mm or more, intramural nodules, or cyst fluid cytology suspicious/positive for malignancy [22]. This indication was able to identify all patients with malignancy; however, its specificity was low (23%) [23], suggesting that a more sensitive indication for resection of BD-IPMN would be required.
Msx2, a member of the homeobox gene (Hox gene) family, is present in a variety of sites, including premigratory cranial neural crest, tooth, retina and lens, apical ectodermal ridge, and mammary gland [24–27]. In the development of these organs, the expression patterns of this gene suggest its active involvement in epithelial–mesenchymal interactions. On the other hand, enhanced levels of transcripts for MSX2, the human homologue of Msx2 (HOX-8), have been shown in a variety of carcinoma cell lines of epithelial origin compared to their corresponding normal tissues [28]. Recently, we have revealed that MSX2 is expressed in pancreatic duct cell carcinoma and that its expression enhanced pancreatic cancer cell aggressiveness through the induction of epithelial-to-mesenchymal transition (EMT) [29, 30]. This raises the question whether MSX2 could be involved in the development of IPMN and whether the expression of this gene could differentiate malignant from benign IPMN. In the current study, therefore, we examined the expression of MSX2 in 45 BD-IPMN tissues and investigated the correlation between its expression and clinicopathological features to assess whether MSX2 expression could predict malignant BD-IPMNs.
Materials and methods
The study subjects were a total of 45 patients with BD-IPMN who underwent surgery at the Division of Hepato-Biliary-Pancreatic Surgery at Tohoku University Hospital from January 1995 to March 2008. Informed consent was obtained from all patients before surgery. The tissues collected at the time of surgery were fixed in 10% paraformaldehyde overnight and embedded in paraffin wax. Seventeen lesions from 12 IPMN samples and 45 IPMN tissues were used for quantitative real-time reverse transcription-polymerase chain reaction (QRT-PCR) and immunohistochemistry, respectively. The IPMNs were histopathologically classified according to the WHO classification, with slight modification [31] as: intraductal papillary-mucinous adenoma (IPMA, papillary hyperplasia, n = 16); borderline intraductal papillary-mucinous neoplasm (IPMB, atypical hyperplasia, n = 5), intraductal papillary-mucinous carcinoma (IPMC, carcinoma in situ, n = 19), and invasive carcinoma derived from intraductal papillary-mucinous carcinoma (IC-IPMC, n = 5). Because IPMN tissue showed various cellular dysplasias within the same tumor, the specimen including the highest grade of cell atypia in each patient was used for the immunohistochemistry.
Microdissection and one-step quantitative real-time RT-PCR
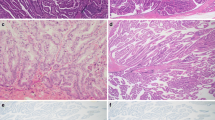
The paraffin-embedded tissues were cut into 10 μm sections under RNAase-free conditions and deparaffinized with xylene, followed by washings with 100% ethanol. Five to eight sequential sections from the same paraffin block were subjected to RNA extraction. Histologically, adenoma to borderline (IPMA and IPMB, n = 6) and carcinoma cells (IPMC, n = 5) and normal to hyperplastic duct cells (nontumor cells, n = 6) from IPMNs, respectively, were microdissected using a 22-gauge needle under a stereomicroscope (Olympus SZX16; Olympus, Tokyo, Japan) as shown in Fig. 1. Total RNA extraction was undertaken using the RecoverAll kit (Ambion., Austin, TX, USA) according to the manufacturer’s instructions. One-step quantitative real-time RT-PCR was performed on each sample by applying 20 ng total RNA with a QuantiTect SYBR Green RT-PCR Kit (Qiagen, Hilden, Germany), using a LightCycler (Roche diagnostics, Basel, Switzerland). RNA concentration was determined with an ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA). The primer pairs used were MSX2, forward 5′-CCGCCTCGGTCAAGTCGGAAAAT-3′ and reverse 5′-TGGAGAGGTACTGTTTCTGACGG-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward 5′-GGCGTCTTCACCACCATGGAG-3′ and reverse 5′-AAGTTGTCATGGATGACCTTGGC-3′. All reactions were performed according to the manufacturer’s protocol. The amplified DNA fragments of MSX2 and GAPDH were subcloned into pCR 2.1-TOPO (Invitrogen, Carlsbad, CA, USA) and used as standards. The copy number of MSX2 in each sample was normalized to the respective GAPDH copy number. The specificity of each PCR reaction was confirmed by melting curve analyses. Only specimens with adequate RNA (GAPDH copy numbers ≥1000) were included in this study.
Expression of MSX2 mRNA in microdissected lesions was detected by quantitative real-time reverse transcription polymerase chain reaction (RT-PCR). RNA extraction was performed from the microdissected lesions. Hematoxylin-stained borderline lesions (×66) (a) were selectively dissected (b) using a 22-gauge needle under a stereomicroscope and recovered in lysis buffer. Seventeen lesions from 12 paraffin-embedded samples were microdissected and RNAs were extracted. RNAs were subjected to one-step quantitative real-time RT-PCR and the target gene was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. MSX2 mRNA expression was significantly higher in intraductal papillary-mucinous carcinoma (IPMC) than in nontumorous regions and nonmalignant intraductal papillary-mucinous tumor of the pancreas (IPMN; intraductal papillary-mucinous adenoma [IPMA] and borderline intraductal papillary-mucinous neoplasm [IPMB]), (P = 0.000003 and 0.00002, respectively, Bonferroni analysis). MSX2/GAPDH indicates MSX2 copy number/GAPDH copy number/μl
Immunohistochemistry
Localization of MSX2 in BD-IPMNs was investigated by immunohistochemistry. The tissue sections were deparaffinized and antigens were retrieved by boiling the sections in Target Retrieval Solution (Dako, Carpinteria, CA, USA) in a microwave oven. Then the sections were incubated in methanol with 0.3% hydrogen peroxide for 30 min in order to block the endogenous peroxidase activity. Subsequently, a histofine kit (Nichirei, Tokyo, Japan) was used. After treatment with 10% serum block solution for 30 min at room temperature, the slides were incubated with polyclonal goat anti-human MSX2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. After treatment with biotinylated anti-goat IgG for 30 min at room temperature, the sections were incubated with peroxidase-conjugated streptavidin for 30 min at room temperature. Between the incubations, the specimens were washed in phosphate-buffered saline (PBS) three times. Visualization of the immunoreaction was carried out in 0.06 mM 3,3′-diaminobenzidine tetrahydrochloride (Dojin, Kumamoto, Japan) containing 2 mM hydrogen peroxide in PBS for several minutes at room temperature. For the negative control, the immunostaining processes were performed by replacing the primary antibody with PBS. The negative control sections showed no specific immunoreactivity. In addition, the specificity of MSX2 antibody was determined in an absorption test using an excess amount of blocking peptide for MSX2 antibody (sc-17729 P; Santa Cruz Biotechnology) as shown previously [30].
The immunostaining of MSX2 was evaluated as positive when more than 10% of cells showed immunoreactivity in the nuclei. The evaluation of immunostaining was done independently by 2 observers (K. S. and A. K.) who had not been informed of the histological diagnosis.
Cell culture and generation of MSX2-expressing cells
A normal human pancreatic duct epithelial cell line (HPDE cells) was provided by Dr. M. S. Tsao and maintained as described previously [32]. The 293T cell line was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA), and routinely grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (Miles, Kankakee, IL, USA). Cells were incubated in a humidified incubator at 37°C, 5% CO2. An MSX2-expressing retrovirus vector was constructed by introducing the MSX2 cDNA [30] into the pMEI-5 Neo vector (Takara Bio, Ohtu, Japan). A replication defective retroviral particle was obtained by cotransfection to the 293T cells with the pMEI-5 Neo MSX2 vector, amphotropic env expression vector, and gag-pol expression vector from the Retrovirus packaging kit (Takara Bio). The HPDE cells were transfected with the MSX2-introducing retrovirus by using a retronectin (Takara Bio)-coated dish. The expression of MSX2 was confirmed by QRT-PCR.
Cell growth
Ten thousand MSX2-expressing or control HPDE cells were seeded per well in 12-well plates (Corning, Corning, NY, USA) in normal cell growth media. The numbers of cells in each well were counted every 48 h.
Statistical analysis
Differences between the mRNA expression of MSX2 and histological grade of BD-IPMN were analyzed by Bonferroni analysis, and a P value of less than 0.017 was considered to be statistically significant. Correlation between MSX2 protein expression and the histological grade of BD-IPMN was analyzed by the χ 2 test, and a P value of less than 0.05 was considered to be statistically significant. The association of clinical parameters and MSX2 expression with malignancy of IPMN was analyzed by Log rank regression analysis, and a P value of less than 0.05 was considered to be statistically significant.
Results
MSX2 mRNA expression in microdissected IPMN lesions
The target lesions were carefully microdissected without contamination by different histological lesions and then RNAs were extracted (Fig. 1a, b). Thus, this enabled us to compare the accurate levels of RNA among various lesions. Using these samples, we investigated the expression levels of MSX2 mRNA in 17 lesions from 12 IPMNs by one-step QRT-PCR. The reliability of this method for evaluating clinical samples has been reported previously [33–35]. MSX2 mRNA was detected in all tumor samples examined at various levels. The expression levels of MSX2 mRNA were increased in a stepwise manner from benign to malignant IPMN. IPMC lesions expressed significantly higher levels of MSX2 mRNA than nontumorous regions and IPMA and IPMB cells did (P = 0.000003 and 0.00002, respectively, Fig. 1c), while no significant difference was found between nontumorous regions and IPMA-B cells (P = 0.265, Fig. 1c).
Immunoreactivity of MSX2 in IPMN tissues
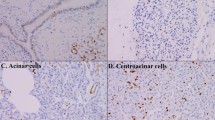
We then employed immunohistochemistry using the specific anti MSX2 antibody to clarify the relation of this gene expression and the tumorigenesis of BD-IPMNs in a large series of samples. Immunolocalization of MSX2 was basically found in the nuclei of tumor cells, while no immunoreactivity was found in normal ducts, acinar cells, or inflammatory cells neighboring the tumor cells (Fig. 2a, b). Consistent with the results of QRT-PCR, MSX2 immunoreactivity was frequently found in IPMB lesions (3/5, 60%), IPMC lesions (12/19, 63.2%), and IC-IPMC lesions (5/5, 100%), while its expression was seen in only one of 16 IPMA tissues. The number of cells expressing MSX2 was significantly increased as the histological grade of malignancy progressed (P = 0.00032, Table 1).
Expression of MSX2 in IPMN tissues. MSX2 expression was not found in IPMA tissues (a). In IPMC tissues, MSX2 expression was found in tumor cell nuclei, while this expression was not detected in stromal cells (b). Nuclear expression was clearly observed in both IPMC cells (arrows) and invasive carcinoma cells (arrowheads) in invasive carcinoma derived from intraductal papillary-mucinous carcinoma (IC-IPMN) tissues (c, d). Bars indicate 100 μm
Predictive factors for malignancy in IPMNs
To assess whether MSX2 expression could discriminate malignant from benign BD-IPMN, multivariate analysis was done among 7 clinical parameters; age, sex, branch duct size, nodule size, diameter of main pancreatic duct, serum carcinoembryonic antigen (CEA) level, and serum carbohydrate antigen (CA) 19-9 level, in addition to MSX2 expression. Univariate analysis showed that nodule size of 6 mm or more and MSX2 expression were significantly correlated with malignancy of BD-IPMN (P = 0.022 and 0.0026, respectively) and that branch 30 mm or more and main pancreatic duct (MPD) 6 mm or more tended to be associated with malignant BD-IPMN (P = 0.058 and 0.062, respectively), but the associations did not reach statistical significance. Other clinical parameters did not show any relation by this method. Consequently, we performed multivariate analysis among all these factors and this analysis revealed that MSX2 expression was the only factor identified as an independent factor to predict IPMC, as shown in Table 2.
MSX2 promotes pancreatic duct cell growth
We analyzed MSX2 function in pancreatic duct cells using HPDE cells retrovirally transfected with MSX2. As shown in Fig. 3a, b, MSX2 expression in transfected HPDE cells (HX) was confirmed by QRT-PCR. The effect of MSX2 on cell growth was analyzed by cell count every 48 h after seeding cells. HX cells demonstrated an approximately 2.5-fold greater number of cells compared to HPDE control cells at 4 days after seeding these cells (Fig. 3c).
MSX2 promotes cellular growth of pancreatic duct. An MSX2-expressing human normal pancreatic duct cell line (HPDE) was generated by transfection of the MSX2-introducing retrovirus. The expression of MSX2 was confirmed by quantitative real-time RT-PCR. After 40 cycles of PCR, the products were electrophoresed on 1.5% agarose gel (a). More than 6-fold higher expression was determined in MSX2-expressing HPDE cells (HX) compared to control cells (HC), N negative control (b). The cell growth rate was evaluated by cell count assay. MSX2-expressing HPDE cells showed enhanced cell growth compared to control cells (c)
Discussion
IPMN is distinct from pancreatic cancer because of its intraductal growth in the main pancreatic duct (MPD) or secondary branches. This type of tumor usually shows a better prognosis compared with pancreatic cancer because of its slow growth and rare invasion to parenchyma [1]. However, recent evidence has indicated that once IPMN shows stromal invasion, it progresses like pancreatic cancer [7, 8]. Therefore, it is important to know how IPMN obtains an aggressive phenotype. MSX2, a member of the homeobox gene family, is suggested to be a downstream target for the ras signaling pathway [36] and is expressed in carcinoma of epithelial origin [28], suggesting the association of this gene with the development of pancreatic carcinoma, because pancreatic carcinoma harbors frequent mutations of the K-ras gene [9]. In accordance with these data, we demonstrated that MSX2 was expressed in about 70% of pancreatic carcinoma tissues, and its expression was correlated with less differentiation of carcinoma cells and venous infiltration; we also found that the induced expression of MSX2 in pancreatic carcinoma cells enhanced an aggressive phenotype both in vitro and in vivo, suggesting that MSX2 is involved in pancreatic carcinoma development rather than carcinogenesis [30]. Thus, we speculated that MSX2 was also involved in the development of IPMN, because this neoplasm exhibited frequent K-ras mutations [9, 10].
In the current study, real-time RT-PCR analysis for microdissected lesions from IPMNs clearly revealed that the expression of MSX2 mRNA was significantly higher in IPMC than in nontumorous ducts or IPMA, while no difference was found between nontumorous ducts and IPMA cells. Consistently, MSX2 immunoreactivity was detected frequently in borderline IPMN (3/5, 60%), IPMC (12/19, 63.1%), and in all IC-IPMC (5/5, 100%), whereas it was rarely observed in IPMA (1/16, 6.3%), indicating that MSX2 plays a crucial role in the transition from the benign to the malignant phenotype of IPMN. To address this hypothesis, we analyzed the role of MSX2 in a nontransformed pancreatic duct cell line, HPDE, because no IPMN cell lines have been generated so far. The cellular proliferation was enhanced when MSX2 was overexpressed in HPDE cells compared to control HPDE cells. MSX2 has been suggested to stimulate the proliferation of osteoprogenitors [37], as well as osteoblast cells [38], and this molecule has been shown to enhance branching morphogenesis of mouse mammary ducts [39, 40], indicating that this gene’s function is associated with regulation of the proliferation of epithelial cells as well as osteogenic cells. In addition, MSX2 stimulated the proliferation of pancreatic cancer cells [30], suggesting that MSX2 has a function to promote the cellular growth of benign as well as malignant pancreatic duct cells. In the current study, together with the results of QRT-PCR, the results of the immunohistochemical analyses suggest that MSX2 contributes to the transition from benign to malignant duct cells and facilitates the tumorigenesis of IPMN cells through stimulating pancreatic duct cell growth. In addition, the current result that MSX2 was expressed in all invasive lesions of BD-IPMN suggests that MSX2 is involved in the invasion of IPMC cells, because MSX2 promotes EMT of pancreatic carcinoma cells [29, 30].
Interestingly, the pattern of MSX2 expression in the current study was similar to that of of the reduction of nuclear phosphoprotein 32 (pp32) expression in pancreatic tumors of ductal origin [41]. The reduced expression of pp32 was observed in IPMN with moderate dysplasia, as well as in moderately and poorly differentiated pancreatic carcinoma, while it was not found in IPMN with mild dysplasia or in well-differentiated pancreatic cancer. The authors postulated that pp32’s ability to inhibit K-ras-induced transformation [42] may have been the reason for this expression pattern because the majority of pancreatic ductal adenocarcinomas have an active K-ras mutation and IPMNs with moderate dysplasia have a greater likelihood of harboring K-ras mutations than IPMNs with mild dysplasia. This speculation could also partly account for why MSX2 was expressed in well-differentiated neoplastic (dysplastic and carcinoma) lesions of IPMN, while its expression was frequently observed in moderately and poorly differentiated but rarely in well-differentiated pancreatic carcinoma, because MSX2 is also a K-ras-associated gene [36].
In our study, nodule size 6 mm or more was significantly associated with malignancy of BD-IPMN by univariate analysis, although the significance disappeared when multivariate analysis was performed. In addition, branch size 30 mm or more and MPD dilatation of 6 mm or more tended to correlate with malignant BD-IPMN, although they did not show statistical significance. A branch duct size cutoff of 30 mm has been widely accepted as a factor to predict the malignancy of BD-IPMN [22]; however, its low sensitivity for malignancy has also been demonstrated [23]. Therefore, more specific predictive factors for BD-IPMC have been explored by various approaches. Clinical findings such as branch size, as described above, presence of a nodule, or dilatation of the MPD have been demonstrated as signs of malignant BD-IPMN [7, 8, 21, 23]. On the other hand, molecular analyses revealed that various events including mutation of K-ras [9, 10], inactivation of p53 [10] or smad 4 [43], and activation of the sonic hedgehog pathway [11] were correlated with malignant BD-IPMN. However, little is known about whether or not molecular markers would be better predictive factors for the malignancy of BD-IPMN than clinical parameters such as nodule size or branch dilatation. In the current study, we clearly revealed that MSX2 expression was identified as an independent predictive factor for IPMC compared to the clinical parameters that were previously shown to be associated with malignant IPMN. It is difficult to obtain histological confirmation of BD-IPMN cells, because it has been reported that endosonography-guided fine-needle aspiration biopsy caused dissemination [44]. On the other hand, MSX2 expression can be detected in small numbers of cells in pancreatic juice by QRT-PCR (manuscript in preparation). Therefore, the detection of MSX2-expressing cells in clinical samples such as pancreatic juice collected under endoscopic retrograde pancreatography for BD-IPMN could be a useful application to predict the malignancy of BD-IPMN.
Abbreviations
- IPMN:
-
Intraductal papillary-mucinous tumor of the pancreas
- QRT-PCR:
-
Quantitative real-time reverse transcription-polymerase chain reaction
- IPMA:
-
Intraductal papillary-mucinous adenoma
- IPMB:
-
Borderline intraductal papillary-mucinous neoplasm
- IPMC:
-
Intraductal papillary-mucinous carcinoma
- IC-IPMN:
-
Invasive carcinoma derived from intraductal papillary-mucinous carcinoma
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- PBS:
-
Phosphate-buffered saline
References
Loftus JEV, Olivares-Pakzad BA, Batts KP, Adkins MC, Stephens DH, Sarr MG, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Gastroenterology. 1996;110:1909–18.
Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–7.
Hara T, Yamaguchi T, Ishihara T, Tsuyuguchi T, Kondo F, Kato K, et al. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology. 2002;122:34–43.
Kobari M, Egawa S, Shibuya K, Shimamura H, Sunamura M, Takeda K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–6.
Doi R, Fujimoto K, Wada M, Imamura M. Surgical management of intraductal papillary mucinous tumor of the pancreas. Surgery. 2002;132:80–5.
Matsumoto T, Aramaki M, Yada K, Hirano S, Himeno Y, Shibata K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261–5.
Kitagawa Y, Unger TA, Taylor S, Kozarek RA, Traverso LW. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7:12–8. discussion 18–19.
Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244–9.
Satoh K, Sawai T, Shimosegawa T, Koizumi M, Yamazaki T, Mochizuki F, et al. The point mutation of c-Ki-ras at codon 12 in carcinoma of the pancreatic head region and in intraductal mucin-hypersecreting neoplasm of the pancreas. Int J Pancreatol. 1993;14:135–43.
Satoh K, Shimosegawa T, Moriizumi S, Koizumi M, Toyota T. K-ras mutation and p53 protein accumulation in intraductal mucin-hypersecreting neoplasms of the pancreas. Pancreas. 1996;12:362–8.
Satoh K, Kanno A, Hamada S, Hirota M, Umino J, Masamune A, et al. Expression of Sonic hedgehog signaling pathway correlates with the tumorigenesis of intraductal papillary mucinous neoplasm of the pancreas. Oncol Rep. 2008;19:1185–90.
Satoh K, Sasano H, Shimosegawa T, Koizumi M, Yamazaki T, Mochizuki F, et al. An immunohistochemical study of the c-erbB-2 oncogene product in intraductal mucin-hypersecreting neoplasms and in ductal cell carcinomas of the pancreas. Cancer. 1993;72:51–6.
Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271–8.
Fukushige S, Furukawa T, Satoh K, Sunamura M, Kobari M, Koizumi M, et al. Loss of chromosome 18q is an early event in pancreatic ductal tumorigenesis. Cancer Res. 1998;58:4222–6.
Sugiyama M, Atomi Y, Saito M. Intraductal papillary tumors of the pancreas: evaluation with endoscopic ultrasonography. Gastrointest Endosc. 1998;48:164–71.
Yasuda H, Takada T, Amano H, Yoshida M. Surgery for mucin-producing pancreatic tumor. Hepatogastroenterology. 1998;45:2009–15.
Lee SY, Lee KT, Lee JK, Jeon YH, Choi D, Lim JH, et al. Long-term follow up results of intraductal papillary mucinous tumors of pancreas. J Gastroenterol Hepatol. 2005;20:1379–84.
Traverso LW. Surgical treatment of intraductal papillary mucinous neoplasms of the pancreas: the aggressive approach. J Gastrointest Surg. 2002;6:662–3.
Bernard P, Scoazec JY, Joubert M, Kahn X, Le Borgne J, Berger F, et al. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274–8.
Irie H, Yoshimitsu K, Aibe H, Tajima T, Nishie A, Nakayama T, et al. Natural history of pancreatic intraductal papillary mucinous tumor of branch duct type: follow-up study by magnetic resonance cholangiopancreatography. J Comput Assist Tomogr. 2004;28:117–22.
Kobayashi G, Fujita N, Noda Y, Ito K, Horaguchi J, Takasawa O, et al. Mode of progression of intraductal papillary-mucinous tumor of the pancreas: analysis of patients with follow-up by EUS. J Gastroenterol. 2005;40:744–51.
Tanaka M. International consensus guidelines for the management of IPMN and MCN of the pancreas. Nippon Shokakibyo Gakkai Zasshi. 2007;104:1338–43.
Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–64.
Takahashi Y, Le Douarin N. cDNA cloning of a quail homeobox gene and its expression in neural crest-derived mesenchyme and lateral plate mesoderm. Proc Natl Acad Sci USA. 1990;87:7482–6.
Davidson DR, Crawley A, Hill RE, Tickle C. Position-dependent expression of two related homeobox genes in developing vertebrate limbs. Nature. 1991;352:429–31.
Jowett AK, Vainio S, Ferguson MW, Sharpe PT, Thesleff I. Epithelial-mesenchymal interactions are required for msx 1 and msx 2 gene expression in the developing murine molar tooth. Development. 1993;117:461–70.
Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, et al. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122:2729–37.
Suzuki M, Tanaka M, Iwase T, Naito Y, Sugimura H, Kino I. Over-expression of HOX-8, the human homologue of the mouse Hox-8 homeobox gene, in human tumors. Biochem Biophys Res Commun. 1993;194:187–93.
Hamada S, Satoh K, Hirota M, Kimura K, Kanno A, Masamune A, et al. Bone morphogenetic protein 4 induces epithelial–mesenchymal transition through MSX2 induction on pancreatic cancer cell line. J Cell Physiol. 2007;213:768–74.
Satoh K, Hamada S, Kimura K, Kanno A, Hirota M, Umino J, et al. Up-regulation of MSX2 enhances the malignant phenotype and is associated with twist 1 expression in human pancreatic cancer cells. Am J Pathol. 2008;172:926–39.
Longnecker DS, Adler G, Hruban RH, Kloppel G. Intraductal papillary mucinous neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, editors. Pathology and genetics. Tumours of the digestive system. WHO classification of tumours. Lyon: IARC Press; 2000. p. 237–40.
Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol. 1996;148:1763–70.
Specht K, Richter T, Muller U, Walch A, Werner M, Hofler H. Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol. 2001;158:419–29.
Kim J, Reber HA, Hines OJ, Kazanjian KK, Tran A, Ye X, et al. The clinical significance of MAGEA3 expression in pancreatic cancer. Int J Cancer. 2006;118:2269–75.
Ohuchida K, Mizumoto K, Fujita H, Yamaguchi H, Konomi H, Nagai E, et al. Sonic hedgehog is an early developmental marker of intraductal papillary mucinous neoplasms: clinical implications of mRNA levels in pancreatic juice. J Pathol. 2006;210:42–8.
Takahashi C, Akiyama N, Matsuzaki T, Takai S, Kitayama H, Noda M. Characterization of a human MSX-2 cDNA and its fragment isolated as a transformation suppressor gene against v-Ki-ras oncogene. Oncogene. 1996;12:2137–46.
Dodig M, Tadic T, Kronenberg MS, Dacic S, Liu YH, Maxson R, et al. Ectopic Msx2 overexpression inhibits and Msx2 antisense stimulates calvarial osteoblast differentiation. Dev Biol. 1999;209:298–307.
Liu YH, Tang Z, Kundu RK, Wu L, Luo W, Zhu D, et al. Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: a possible mechanism for MSX2-mediated craniosynostosis in humans. Dev Biol. 1999;205:260–74.
Satoh K, Ginsburg E, Vonderhaar BK. Msx-1 and Msx-2 in mammary gland development. J Mammary Gland Biol Neoplasia. 2004;9:195–205.
Satoh K, Hovey RC, Malewski T, Warri A, Goldhar AS, Ginsburg E, et al. Progesterone enhances branching morphogenesis in the mouse mammary gland by increased expression of Msx2. Oncogene. 2007;26:7526–34.
Brody JR, Witkiewicz A, Williams TK, Kadkol SS, Cozzitorto J, Durkan B, et al. Reduction of pp32 expression in poorly differentiated pancreatic ductal adenocarcinomas and intraductal papillary mucinous neoplasms with moderate dysplasia. Mod Pathol. 2007;20:1238–44.
Bai J, Brody JR, Kadkol SS, Pasternack GR. Tumor suppression and potentiation by manipulation of pp32 expression. Oncogene. 2001;20:2153–60.
Biankin AV, Biankin SA, Kench JG, Morey AL, Lee CS, Head DR, et al. Aberrant p16 (INK4A) and DPC4/Smad4 expression in intraductal papillary mucinous tumours of the pancreas is associated with invasive ductal adenocarcinoma. Gut. 2002;50:861–8.
Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323–4.
Acknowledgments
We thank Dr. M.S. Tsao for providing HPDE cells. This work was supported in part by Grants-in-Aid #21590870 and #20390202 from the Ministry of Education, Science, Sports and Culture in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satoh, K., Hamada, S., Kanno, A. et al. Expression of MSX2 predicts malignancy of branch duct intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol 45, 763–770 (2010). https://doi.org/10.1007/s00535-010-0200-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-010-0200-1