Abstract
In the present work, we examined the impact of Fe2O3 on the mechanical and photon shielding characteristics of lead-phosphoaluminate glasses. Due to water corrosion, the use of lead-phosphosilicate glasses may be limited.The addition of Fe2O3 significantly improves its water resistance. The radiation and mechanical of 20PbO – 45P2O5 − 15 Al2O3– (\(20-x\)) Na2O- \(x\) Fe2O3glass system doped with \(x\)Fe2O3reflect the originality of this article. The molar volume of these samples is declining whereas the density is an increment. Ultrasonic velocity and elastic modulus were found to be enhanced. The shielding parameters were established using the Phy-X / PSD. The mace attenuation coefficient is the highest in the sample with the maximum Fe2O3 content. The (Zeff) and (Neff) values decreased and then increased slowly at lower energy. Because of the Compton scattering interaction, the (Zeq)-value decreases as the energy and Fe2O3content increase. Small deviations from the glass samples are observed at higher energy levels to decrease the value (ΣR). Finally, the increment of Fe2O3 in prepared glasses increases the γ-radiation & neutronattenuation rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Glass is an amorphous material that melts without crystallizing. High-manufacture phosphate glasses are used intended for numerous configurations [1,2,3,4,5,6,7]. The features of iron-phosphosilicate mixed glass networks are different from those of single glass networks [8,9,10,11,12,13,14,15,16]. There are several physical-chemical features of the transition metal ions (TMIs) doped phosphosilicate glasses that lead to a wide range of discussions of those samples in material knowledge. In detail, transition metal oxides (TMO) are widely perceived in phosphate glasses that are used to improve their chemical resistance. Fe2O3 and PbO are important elements because of their physical and mechanical characteristics. Fe2O3 and PbO glasses have specific characteristics as a result of their mechanical and radiation applications [17,18,19,20,21,22,23,24].
Iron alkaline phosphosilicate glasses have significant scientific and technological advancements. The structural benefits involved with the existence of PO4 are responsible for this significance. Besides phosphate glass, the presence of TMIs, for example, Fe2O3 and PbO can provide new possibilities to extend these glasses’ features. Heavy metal oxide doped glasses as PbO exhibition these glasses are highly optical, and mechanical because they act as a former and modifier. The presence of TMIs, such as Fe2O3 and PbO, in phosphosilicate glasses, can provide novel potentials for extending the features of these glasses [17,18,19,20,21,22,23].
Radiation has an incredible influence and has extremely large requests in our daily lives for everything we do. Radiation depletion can be used to determine the risk impacts on living organisms. As a result of its special properties in our lives, the application of radiation has enhanced, and the application of radiation shielding is increasing day by day. Many researchers used the glasses for various radiation protection [25,26,27,28,29].
The addition of Fe2O3 enhances the strength of the glass materials are used in gamma-ray shielding. Fe2O3 is commonly used in phosphate glasses to increase their chemical resistance to moisture attacks. Phosphate glasses containing alkali, alkaline earth metals and TMIs are having great importance as they are used as cathode materials in batteries. Among the various TMIs, the (Fe2O3) ions have a vigorous bearing on the innumerable properties particularly mechanical, optical and structural properties of glassy materials and which can exist in two different oxidations Fe2+ and Fe3+ states with different co-ordinations or environments. By keeping in view all the above methodologies, the radiation, and mechanical features of \(20\)PbO – 45P2O5 − 15 Al2O3 – (\(20-x\)) Na2O- \(x\)Fe2O3 glass system doped with \(x\)Fe2O3 reflect the originality of this article.
2 Materials and Methods
The melting and quenching process was used to synthesize iron-lead-phosphoaluminate glasses with a chemical formula of 45P2O5 – 15Al2O3 –\(20\)PbO – (\({20}-{x}\)) Na2O- \(x\)Fe2O3 ( \(0\le x\le 2)\) mol%. The mixture in the alumina crucible was preheated for 1 h in an electric furnace at 450 °C to remove moisture, CO2, & NH3. As a result, the melting process began right away in the same furnace, which was kept at 1150 °C for 1 h. The melted glass is quenched in a mould (inner size: Height 2 cm x thickness 1 cm x 1 cm) and annealed at 400 °C for 2 h to reduce internal thermal stress. Glass samples in Table 1 were prepared by traditional technique as the following equation: Na2CO3+ Fe2O3 + PbO+ Al2O3 + 2(NH4)2HPO4 \(\frac{{\Delta \,at\,450{\text{ }}^\circ C}}{{ - (4NH3 + 6H2O + CO2)}} \to\)[Na2O+Fe2O3+PbO+ P2O5+ Al2O3] \(\xrightarrow{{\Delta 1150^\circ C}}\) glasses \(\xrightarrow{{{\text{annealing at 400}}^\circ C}}\) samples. The density is determined using Archimedes Code. Molar volume is established by\(Vm =M/ \rho .\) The pulse-echo method was used to obtain longitudinal (\({v}_{L}\)) and shear (\({v}_{T}\)) ultrasonic velocities at room temperature. A digital ultrasonic flaw detector (KARL DEUTSCH Echograph model 1085) was used in conjunction with x-cut and y-cut transducers (KARL DEUTSCH) operating at a fundamental frequency of 4 MHz. The ultrasonic velocity measurement has a \(\pm\)10 m/s uncertainty. Elastic moduli were determined as: longitudinal \(L=\rho {v}_{l}^{2}\), transverse \(G=\rho {v}_{t}^{2}\), Young’s \(Y=\left(1+\sigma \right)2G\),& bulk \(K=L-\left(\frac{4}{3}\right)G\). Using the model in refs. [19, 21], it is possible to estimate the elastic moduli of the glasses based on packing density \(Vi\) and dissociation energy \(Gi\): \(Vi=\left(\frac{3\pi }{4}\right){N}_{A} (m\text{R}{}_{\text{A}}{}^{3}+ n \text{R}{}_{\text{O} }{}^{3}) {m}^{3}.{mol}^{-1},\) \(Gi=\left(\frac{1}{{V}_{m}}\right){\sum }_{i}GiXi\). Longitudinal \(L=K+\left(\frac{4}{3}\right)G,\) transverse \(G=30*\left(\frac{{V}_{i}^{2}{G}_{i}}{{V}_{i}}\right)\) Young’s \(Y=8.36{V}_{i }{G}_{i}\), bulk \(K=10{V}_{i}^{2}{G}_{i}\). Poisson’s ratio \(\sigma {\text{ = }}\frac{{\text{1}}}{{\text{2}}}{\text{ - }}\left( {\frac{1}{{{\text{7}}{\text{.2* }}Vi}}} \right)\). Impedance; \(Z= {v}_{L}\rho .\) Hardness; \(\text{H}=\frac{\left(1-2\sigma \right)\text{Y}}{6\left(1+\sigma \right)}\). Debye: \({\theta }_{D}= \frac{h}{k}{\left(\frac{9{N}_{A}}{4\pi {V}_{m}}\right)}^{\frac{1}{3}}{M}_{s}\). Average velocities\({M}_{s}= \frac{1}{3}{\left(\left(\frac{1}{{v}_{l}^{3}}\right)+\left(\frac{2}{{v}_{t}^{3}}\right)\right)}^{-\frac{1}{3}}\). Packing density & Oxygen molar volume: \({V}_{o}=\left(\frac{M}{\rho }\right) \left(\frac{1}{\sum xini}\right)\), \(OPD=\left(\frac{1000 C}{Vm}\right)\left(\frac{Mol}{L}\right)\), Fractal bond connectivity (\(d\))= \(4 (G/K)\). Phy-X / PSD, by Sakar et al. [29] can compute several shielding factors at (0 MeV-15 MeV). Beer-Lambert law as written in Eq in mathematical form: \(\mu =- \frac{ln \frac{I}{{I}_{o}}}{x} ,\)Where µ represents the linear attenuation coefficient (cm-1) I0 and I, respectively, show unattenuated and attenuated photon intensities. Mass attenuation coefficient (MAC) was calculated as follows: \(\left(\frac{\mu }{{\uprho }}\right)=\sum _{i}{w}_{i }{\left(\frac{\mu }{\rho }\right)}_{i}.\) Mean free path (MFP), tenth and half-value layer (TVL), (HVL) considered as \(MEP=\left(\frac{1}{\mu }\right),\) (TVL) (HVL) are calculated by: \(\text{T}\text{V}\text{L}= \left(\frac{\text{l}\text{n}10}{{\upmu }}\right) ,\) \(\text{H}\text{V}\text{L}=\left(\frac{\text{l}\text{n}2}{{\upmu }}\right).\) The effective atomic number (Zeff) is predictable by \({Z}_{eff}=\left(\frac{{\sigma }_{a}}{{\sigma }_{e}}\right)\) where (σa) is the atomic cross-section, \({{\upsigma }}_{a} ={\sigma }_{m}\frac{1}{{\sum }_{i}{n}_{i}}={\left(\frac{\mu }{\rho }\right)}_{target}/{N}_{A}{\sum }_{i}\frac{{w}_{i}}{{A}_{i}}\), and σe is the electronic cross-section, \({\sigma }_{e} =\frac{1}{N} \sum _{i}{\left(\frac{\mu }{\rho }\right)}_{i}\frac{{f}_{i}{wA}_{i}}{{z}_{i}}\). Equivalent atomic number Zeq is predictable by \(Zeq= \frac{\text{Z}1(\text{l}\text{o}\text{g}\text{R}2-\text{l}\text{o}\text{g}\text{R})+\text{Z}2(\text{l}\text{o}\text{g}\text{R}-\text{l}\text{o}\text{g}\text{R}1)}{\text{l}\text{o}\text{g}\text{R}2-\text{l}\text{o}\text{g}\text{R}1}\). Electron density (Neff) is predictable by \({\text{N}}_{\text{eff}}={\text{N}} \frac{{\text{Z}}_{\text{eff}}}{{\sum }_{\text{i}}{{\text{F}}}_{{\text{i}}}{\text{A}}_{\text{i}}}\). Effective removal cross-section (ΣR), is predictable as:\(\left(\frac{{{\Sigma }}_{\text{R}}}{{\uprho }}\right)= \sum _{\text{i}}{{\text{w}}_{\text{i}}\left(\frac{{{\Sigma }}_{\text{R}}}{{\uprho }}\right)}_{\text{i}}\) and\(\text{R} = \sum _{\text{i}}{ {{\uprho }}_{\text{i}} \left(\frac{\text{R}}{{\uprho }}\right)}_{\text{i}}\)
3 Results and Discussion
3.1 Physical Properties
The XRD of the glass system is illustrated in Fig. 1. the amorphous state of all glasses is detected. Figure 2 illustrates the molar volume (\({V}_{m}\)) and glass density (ρ) as Fe2O3 concentration increases. The results demonstrate that ρ is increasing, while \({V}_{m}\) decreases. Several factors, such as density and molecular mass, have a direct impact on the (ρ) and (\({V}_{m}\)). The density of Na2O and Fe2O3, (2.27, 5.25), and molecular weights (61.69, 159.688). The atomic radii of [Fe (0.83oA) & Na (1.8oA)] [30,31,32,33,34,35]. Fe-ions concentration was calculated as\(Ni=\left(\frac{6.023\times {10}^{23}x mol fraction of cation\times valency of cation}{Vm}\right)\). It is well-known that Fe3+concentration increments due to the decreasing of \({V}_{m}\). Inter-ionic distance (\({R}_{i}\)) calculated as \({R}_{i}= {\left(\frac{1}{\text{c}\text{o}\text{n}\text{t}\text{e}\text{n}\text{t} \text{o}\text{f} \text{P}\text{b}}\right)}^{\frac{1}{3}}\), it well-known that \({R}_{i}\) declined, this decline is associated with reductions of \({V}_{m}\). P – P separation (dP-P) could be estimated by \((\text{d}\text{P}-\text{P}) = {\left(\frac{{V}_{m}^{B}}{N }\right)}^{\frac{1}{3}}\) and \({V}_{m}^{B}\text{ =}\frac{Vm}{2\left(1- 2\text{X}\text{n}\right)}\). The values of \((\text{d}\text{P}-\text{P})\) increased due to the decrease of \({V}_{m}\). Radius Polaron rp & distance inter-nuclear ri are predictable by \(rp=\frac{1}{2}\) \({\left(\frac{\pi }{6 N }\right)}^{\frac{1}{3}}\), \(ri={\left(\frac{1}{N }\right)}^{\frac{1}{3}}.\) It is mentioned that, with the increases of Fe3+ these values become smaller, due to reduction of \({V}_{m}\). Table 2 documents these estimations. The variations in \({V}_{o}\) and \(OPD\) are exemplified in Fig. 3. The \({V}_{o}\) values are decreased, while \(OPD\) increased.
3.2 Mechanical Properties
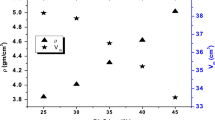
Figure 4 exemplifies the velocities (vL, vT ) of glasses. With an increase in Fe2O3 content, each velocity is increased and documented in Table 3 [36,37,38,39,40,41,42,43]. Arrange vL between 5252, 5370 m/s and vT 3067, 3136 m/s. When Fe2O3 is increased, the network glass modifier increases, explaining the observed variations in (vL, vT ) of the glasses. This oxide causes bond lengths to shorten and ultrasonic velocities to increase by increasing the stretching force constantly. Furthermore, the increased in vL and vT of the investigated glasses can be linked to the higher bond strength of Fe–O than Na–O.
Figures 5 and 6 show elastic moduli with Fe2O3 content (experimentally and theoretically). Elastic modulus values decreased with the reduction of Fe2O3 content. This behavior is attributed to an increment in coordination number as Fe2O3 rises, in addition to the increase in the average force and cross-link density. As Na2O replaces Fe2O3, \({V}_{m}\) reduces, and the ρ rises, attempting to make the glass matrices extra compact. The above results are listed in Table 3. This could be because the elastic moduli are dependent on the density values, which are increased when Fe2O3 is added to the glasses, compacting the glass structure, and increasing network rigidity.
Mechanical values (Vi), (Gi), (H), (αP), (Z), (OPD), (Vo), and (σ) are listed in Table 4. (Vi), (H), (αP), (Z), (OPD), (d), and (σ) is increased with Fe2O3, whereas (Gi) decrements. The content of the network modifier (NWM) is linked to an enhancement in the glass structure’s rigidity. The addition of Fe2O3 also increased crosslink density and sample compactness due to the current glassy samples’ small variability in configuration. The fractal bond connectivity (\(d\)) is an important parameter that connects the elastic properties of the glasses to their structures. The values of (\(d\)) around 3. The structure of the glassy samples will be converted into a 3D network as the Fe2O3 content increases.
3.3 Radiation Properties
In terms of γ- shielding, a lead phosphosilicate glass system doped with Fe2O3 has been explored [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. The MAC of the glass under consideration is the first criterion to be explained. Figure 7 illustrates the MAC of the glasses and estimated by \(\left(\frac{\mu }{{\uprho }}\right)=\sum _{i}{w}_{i }{\left(\frac{\mu }{\rho }\right)}_{i}.\)Table 5 presents values of MAC. The reducing order of the samples is (G1) > (G2) > (G3) > (G4) > (G5), because of the atomic number variance between Fe and Na, this is not a significant increase. It is demonstrated that the MAC of the glass system increased at low energy and then stayed constant at higher energy [32, 34, 36,37,38, 41,42,43]. MAC trend with energy is the same as observable and is monitored by the major procedures of common gamma-ray interaction as photoelectric (PE) effect, Compton effect (CE), and pair production (PP) effect. MAC of glasses under-investigated compared to other samples are presented in Table 6.
Figure 8 shows the Zeff of studied samples with γ-energy. The glass with the code G1 possesses the maximum score, whereas G5 possesses the minimum. The trend order of the samples is (G1) Zeff > (G2) Zeff > (G3) Zeff > (G4) Zeff > (G5) Zeff. The obtained results for Zeff values are shown in Table 7. The rate of -radiation attenuation for the glasses is accelerated as a result of the addition of Fe2O3 to the glasses [32, 34, 36,37,38, 41,42,43]. Figure 9 represents the (Neff) with energy. (Neff) has the same behaviour as Zeff [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60].
The (Zeq) values of prepared samples were represented in Fig. 10. It is illustrated that (Zeq) increased with energy and with the replacement of Na2O with Fe2O3. The (Zeq) values are reduced with energy increased and with the Fe2O3 content because of the Compton scattering interaction. The highest (Zeq) value is 1 MeV for prepared samples. The obtained results for Zeff values are shown in Table 8 [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60].
(TVL), and (HVL) of prepared samples are shown in Fig. 11,&12. The shielding capacity of glasses possessing lowering HVL is the greatest. In this regard, of all the synthesized glasses, G5 appeared to be the most effective at shielding against gamma ionizing radiation. The trend order of the samples is (G1) HVL > (G2) HVL > (G3) HVL > (G4) HVL > (G5) HVL. Table 9 summarises the HVL results. TVL& HVL possess the same trend as the synthesized samples [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60].
Figure 13 depicts the change in MFP. MFP values increased as photon energy increased. Additionally, MFP decreased as Fe2O3 content increased. This information reveals that as the photon’s energy increases, it becomes capable of transmitting the prepared sample on purpose. For the best attenuation capabilities, G5 glass is recommended. It is well known that MEP and shielding have a relationship, with lower MFP values indicating better shielding material.
Figure 14 shows the (ΣR) of fabricated samples. At lower energy, it is detected that (ΣR) increment with energy. At higher energies, glass samples show minor deviations as (ΣR) decreases. Figure 15 depicts the Fast neutron removal cross-section (FNRCS).
of the glass system. It should be noted that as the Fe2O3 content increment, the FNRCS values increment [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. In terms of FNRCS, the glasses are arranged in the following order: G5 > G4 > G3 > G2 > G1. The Fe2O3 content of the glasses supports this conclusion.
4 Conclusions
The melt quenching procedure was used to manufacture a set of phosphate glasses with the following formulation: \(x20\)PbO – 45P2O5 − 15Al2O3– (\(20-x\)) Na2O- \(x\) Fe2O3 ( \(0\le x\le 2).\)The mechanical and radiation characteristics of these glasses were examined. The density, ultrasonic velocity, and elastic moduli are incremented as the Fe2O3expands.Experimental results and calculated elastic moduli values using the Makishima-Mackenzie theoretical model are deemed acceptable. The parameters like \(MAC, LAC, TVL, HVL, MFP\), and \(\sum R\)are computed to estimate the capabilities of glasses doped with Fe2O3 to shield radiation. The results show that as the Fe2O3 content increases, the values of \(MAC\)and \(\sum R\) decrement.The G5 glass sample shields photons and neutrons better than other glass samples, according to this investigation. The parameters’ analysis revealed that the glasses under consideration are strong candidates for radiation shielding. Finally, the increase of Fe2O3 in prepared glasses increase γ-radiation attenuation rate.
Availability of Data and Material
My manuscript and associated personal data.
References
A.M. Abdelghany, H.A. ElBatal, J. Mol. Struct. (2014) doi:https://doi.org/10.1016/j.molstruc.2014.03.032
A.M. Abdelghany, G. El-Damrawi, A.H. Oraby, M.A. Madshal, J. Non-cryst. Solids (2018) doi:https://doi.org/10.1016/j.jnoncrysol.2018.07.022
H.A. ElBatal, A.M. Abdelghany, F.H. ElBatal, K. ElBadry, F.A. Moustaffa, Phys. B: Condens. Matter (2011) doi:https://doi.org/10.1016/j.physb.2011.06.074
A.F.A. El-Rehim, A.M. Ali, H.Y. Zahran, I.S. Yahia, K.S. Shaaban, J. Inorg. Organomet. Polym. (2021) doi:https://doi.org/10.1007/s10904-020-01799-w
Y.B. Saddeek, K.A. Aly, K.S. Shaaban, A.M. Ali, M.M. Alqhtani, A.M. Alshehri, M.A. Sayed, E.A. Abdel, Wahab, J. Non-cryst. Solids (2018) doi:https://doi.org/10.1016/j.jnoncrysol.2018.06.002
K.S. Shaaban, Y.B. Saddeek, Silicon (2017) doi:https://doi.org/10.1007/s12633-017-9558-5
E.A.A. Wahab, A.M. Al-Baradi, M.A. Sayed, A.M. Ali, S.A. Makhlouf, K.S. Shaaban, Silicon (2022) doi:https://doi.org/10.1007/s12633-021-01652-w
E.A. Abdel Wahab, A.A. El-Maaref, K. Shaaban, J. Börcsök, M. Abdelawwad, Opt. Mater. (2021) doi:https://doi.org/10.1016/j.optmat.2020.110638
A.A. El-Maaref, S. Badr, K. Shaaban, E.A. Abdel Wahab, M.M. ElOkr, J. Rare Earths (2019) doi:https://doi.org/10.1016/j.jre.2018.06.006
A.M. Fayad, K.S. Shaaban, W.M. Abd-Allah, M. Ouis, J. Inorg. Organomet. Polym. (2020) doi:https://doi.org/10.1007/s10904-020-01641-3
K.S. Shaaban, A.M. Al-Baradi, A.M. Ali, Silicon (2022) doi:https://doi.org/10.1007/s12633-022-01702-x
K.S. Shaaban, Z.A. Alrowaili, A.M. Al-Baradi, A.M. Ali, E.A.A. Wahab, M.S. Al-Buriahi, Silicon (2021) doi:https://doi.org/10.1007/s12633-021-01441-5
K.S. Shaaban, E.A.A. Wahab, E.R. Shaaban, E.S. Yousef, S.A. Mahmoud, Opt. Quant. Electron. (2020) doi:https://doi.org/10.1007/s11082-020-2191-3
K.S. Shaaban, E.S. Yousef, S.A. Mahmoud, E.A.A. Wahab, E.R. Shaaban, J. Inorg. Organomet. Polym. (2020) doi:https://doi.org/10.1007/s10904-020-01574-x
K.S. Shaaban, H.Y. Zahran, I.S. Yahia, H.I. Elsaeedy, E.R. Shaaban, S.A. Makhlouf, E.A.A. Wahab, E.S. Yousef, Appl. Phys. A (2020) doi:https://doi.org/10.1007/s00339-020-03982-9
N. Sharmin, F. Gu, I. Ahmed, A.J. Parsons, J. tissue Eng. (2017) doi:https://doi.org/10.1177/2041731417744454
A.H. Almuqrin, K.A. Mahmoud, E.A.A. Wahab, M.S.I. Koubisy, M.I. Sayyed, K.S. Shaaban, Eur. Phys. J. Plus (2021) doi:https://doi.org/10.1140/epjp/s13360-021-01564-z
S. Alomairy, M.S. Al-Buriahi, E.A. Abdel Wahab, C. Sriwunkum, K. Shaaban, Ceram. Int. (2021) doi:https://doi.org/10.1016/j.ceramint.2021.03.045
A. El-Taher, A.M. Ali, Y.B. Saddeek, R. Elsaman, H. Algarni, K. Shaaban, T.Z. Amer, Radiat. Phys. Chem. (2019) doi:https://doi.org/10.1016/j.radphyschem.2019.108403
M.A. Sayed, A.M. Ali, A.F. Abd El-Rehim, E.A. Abdel Wahab, K.S. Shaaban, J. Elec Materi (2021) doi:https://doi.org/10.1007/s11664-021-08921-9
K.S. Shaaban, E.S. Yousef, E.A. Abdel Wahab, E.R. Shaaban, S.A. Mahmoud, J. of Materi Eng. and Perform. (2020) doi:https://doi.org/10.1007/s11665-020-04969-6
H.H. Somaily, K.S. Shaaban, S.A. Makhlouf, H. Algarni, H.H. Hegazy, E.A.A. Wahab, E.R. Shaaban, J. Inorg. Organomet. Polym. (2021) doi:https://doi.org/10.1007/s10904-020-01650-2
E.A. Abdel Wahab, K.S. Shaaban, S. Alomairy, M.S. Al-Buriahi, Eur. Phys. J. Plus (2021) doi:https://doi.org/10.1140/epjp/s13360-021-01572-z
P. Das, S. Ganguly, M. Bose, D. Ray, S. Ghosh, S. Mondal, V.K. Aswal, A.K. Das, S. Banerjee, N.C. Das, New. J. Chem. (2019) doi:https://doi.org/10.1039/c8nj06308g
E.A. Abdel Wahab, K.S. Shaaban, Appl. Phys. A (2021) doi:https://doi.org/10.1007/s00339-021-05062-y
B. Albarzan, A.H. Almuqrin, M.S. Koubisy, E.A. Wahab, K.A. Mahmoud, K. Shaaban, M.I. Sayyed, Prog. Nucl. Energy (2021) doi:https://doi.org/10.1016/j.pnucene.2021.103931
M.S. Al-Buriahi, Z.A. Alrowaili, I. Kebaili, A.M. Al-Baradi, E.A.A. Wahab, I.O. Olarinoye, C. Sriwunkum, K.S. Shaaban, Phys. Scr. (2021) doi:https://doi.org/10.1088/1402-4896/ac4121
K.S. Shaaban, A.M. Al-Baradi, Z.A. Alrowaili, A.M. Ali, M.S. Al-Buriahi, E.A.A. Wahab, J. Mater. Sci: Mater. Electron. (2021) doi:https://doi.org/10.1007/s10854-021-07158-w
K.S. Shaaban, S. Alomairy, M.S. Al-Buriahi, J. Mater. Sci: Mater. Electron. (2021) doi:https://doi.org/10.1007/s10854-021-05885-8
A.A. El-Maaref, R.M. El-Agmy, K.S. Shaaban, E.A. Abdel, Wahab, Eur. Phys. J. Plus (2021) doi:https://doi.org/10.1140/epjp/s13360-021-01798-x
A.A. El-Maaref, E.A. Wahab, K.S. Shaaban, R.M. El-Agmy, Solid State Sci. (2021) doi:https://doi.org/10.1016/j.solidstatesciences.2021.106539
A.F.A. El-Rehim, K.S. Shaaban, H.Y. Zahran, I.S. Yahia, A.M. Ali, M.M.A. Halaka, S.A. Makhlouf, E.A.A. Wahab, E.R. Shaaban, J. Inorg. Organomet. Polym. (2021) doi:https://doi.org/10.1007/s10904-020-01708-1
K.S. Shaaban, S.M. Abo-naf, A.M. Abd Elnaeim, M.E.M. Hassouna, Appl. Phys. A (2017) doi:https://doi.org/10.1007/s00339-017-1052-9
K. Shaaban, E.S. Yousef, Optik (2020) doi:https://doi.org/10.1016/j.ijleo.2019.163976
E.A.A. Wahab, A.M. Aboraia, A.M.E. Shafey, K.S. Shaaban, A.V. Soldatov, Opt. Quant. Electron. (2021) doi:https://doi.org/10.1007/s11082-021-03164-8
M.A. Azooz, Y.B. Saddeek, K.A. Aly, K.S. Shaaban, H.M. Mokhtar, J. Inorg. Organomet. Polym. (2019) doi:https://doi.org/10.1007/s10904-019-01130-2
Y.B. Saddeek, K.A. Aly, K.S. Shaaban, A.M. Ali, M.A. Sayed, Silicon (2019) doi:https://doi.org/10.1007/s12633-018-9912-2
K. Shaaban, Y.B. Saddeek, K. Aly, Ceram. Int. (2018) doi:https://doi.org/10.1016/j.ceramint.2017.11.175
K.S. Shaaban, S.M. Abo-naf, M.E.M. Hassouna, K.S. Shaaban, S.M. Abo-naf, Silicon (2019) doi:https://doi.org/10.1007/s12633-016-9519-4
K.S. Shaaban, A.M. Ali, Y.B. Saddeek, K.A. Aly, A. Dahshan, S.A. Amin, Silicon (2019) doi:https://doi.org/10.1007/s12633-018-0004-0
K.S. Shaaban, Y.B. Saddeek, M.A. Sayed, I.S. Yahia, K.S. Shaaban, Silicon (2018) doi:https://doi.org/10.1007/s12633-017-9709-8
K.S. Shaaban, M.A. Sayed, Y.B. Saddeek, I.S. Yahia, K.S. Shaaban, Silicon (2019) doi:https://doi.org/10.1007/s12633-016-9465-1
E.A.A. Wahab, K.S. Shaaban, Mater. Res. Express (2018) doi:https://doi.org/10.1088/2053-1591/aaaee8
E.A. Abdel Wahab, M. Koubisy, M.I. Sayyed, K.A. Mahmoud, A.F. Zatsepin, S.A. Makhlouf, K. Shaaban, J. Non-cryst. Solids (2021) doi:https://doi.org/10.1016/j.jnoncrysol.2020.120509
A.S. Abouhaswa, M.I. Sayyed, A.S. Altowyan, Y. Al-Hadeethi, K.A. Mahmoud, Opt. Mater. (2020) doi:https://doi.org/10.1016/j.optmat.2020.109981
A.S. Abouhaswa, M.I. Sayyed, A.S. Altowyan, Y. Al-Hadeethi, K.A. Mahmoud, J. Non-cryst. Solids (2020) doi:https://doi.org/10.1016/j.jnoncrysol.2020.120134
A.M. Al-Baradi, A.F.A. El-Rehim, Z.A. Alrowaili, M.S. Al-Buriahi, K.S. Shaaban, Silicon (2021) doi:https://doi.org/10.1007/s12633-021-01481-x
S. Alomairy, A.M. Aboraia, E.R. Shaaban, K.S. Shaaban, Braz J. Phys. (2021) doi:https://doi.org/10.1007/s13538-021-00928-1
M.A. Alothman, Z.A. Alrowaili, J.S. Alzahrani, E.A. Wahab, I.O. Olarinoye, C. Sriwunkum, K. Shaaban, M.S. Al-Buriahi, J. Alloys Compd. (2021) doi:https://doi.org/10.1016/j.jallcom.2021.160625
Z.A. Alrowaili, A.M. Al-Baradi, M.A. Sayed, A. Mossad Ali, E.A. Abdel Wahab, M.S. Al-Buriahi, K. Shaaban, Optik (2022) doi:https://doi.org/10.1016/j.ijleo.2021.168259
Z.A. Alrowaili, A.M. Ali, A.M. Al-Baradi, M.S. Al-Buriahi, E.A.A. Wahab, K.S. Shaaban, Opt. Quant. Electron. (2022) doi:https://doi.org/10.1007/s11082-021-03447-0
A.A. El-Rehim, H.Y. Zahran, I.S. Yahia, S.A. Makhlouf, K.S. Shaaban, Silicon (2021) doi:https://doi.org/10.1007/s12633-020-00798-3
A.F.A. El-Rehim, K.S. Shaaban, J. Mater. Sci: Mater. Electron. (2021) doi:https://doi.org/10.1007/s10854-020-05204-7
A.F.A. El-Rehim, H.Y. Zahran, I.S. Yahia, A.M. Ali, K.S. Shaaban, Silicon (2022) doi:https://doi.org/10.1007/s12633-020-00827-1
M.S.I. Koubisy, K.S. Shaaban, E.A.A. Wahab, M.I. Sayyed, K.A. Mahmoud, Eur. Phys. J. Plus (2021) doi:https://doi.org/10.1140/epjp/s13360-021-01125-4
Y.S. Rammah, F.I. El-Agawany, K.A. Mahmoud, R. El-Mallawany, E. Ilik, G. Kilic, J. Mater. Sci: Mater. Electron. (2020) doi:https://doi.org/10.1007/s10854-020-03440-5
Y.S. Rammah, F.I. El-Agawany, E.A. Wahab, M.M. Hessien, K. Shaaban, Radiat. Phys. Chem. (2022) doi:https://doi.org/10.1016/j.radphyschem.2021.109956
Y.S. Rammah, K.A. Mahmoud, E. Kavaz, A. Kumar, F.I. El-Agawany, Ceram. Int. (2020) doi:https://doi.org/10.1016/j.ceramint.2020.04.018
Y.B. Saddeek, K. Shaaban, R. Elsaman, A. El-Taher, T.Z. Amer, Radiat. Phys. Chem. (2018) doi:https://doi.org/10.1016/j.radphyschem.2018.04.028
K.S. Shaaban, A.M. Al-Baradi, A.M. Ali, J. Mater. Sci: Mater. Electron. (2022) doi:https://doi.org/10.1007/s10854-021-07530-w
Acknowledgements
The authors express their gratitude to princess Nourah bint Abdulrahman University, Researchers Supporting Project (Grant No. PNURSP2022R32) Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. A.F. Abd El-Rehim extends his appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a research groups program under grant number R.G.P. 1/277/42.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All of the authors have taken full responsibility for the content of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
The manuscript has not been published elsewhere.
Declaration of Competing Interest
There are no known competing financial interests among the authors.
Consent to Participate
The author’s consent to participate & publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Maaref, A.A., Alotaibi, B.M., Alharbi, N. et al. Effect of Fe2O3 as an Aggregate Replacement on Mechanical, and Gamma/ Neutron Radiation Shielding Properties of Phosphoaluminate Glasses. J Inorg Organomet Polym 32, 3117–3127 (2022). https://doi.org/10.1007/s10904-022-02345-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02345-6