Abstract
In glasses with the composition 45P2O5–45PbO2−\((10-x)\) CdO–\(x\) MoO3, \(x\) = (0 ≤ \(x\) ≤ 5 mol%), melt quenching technique was used to fabricate these samples. The structural characteristics of the proposed glasses were determined using X-ray diffraction. Fabricated glasses were found to have a higher density and refractive index. The spectroscopic characteristics of fabricated glasses were evaluated using UV–VIs. Energy bandgap increases as the content of MoO3 increases, while Urbach energy decreases. Phy-X/PSD has been used to investigate radiation shielding properties. Mass attenuation coefficient values are positively affected by increases in the MoO3 ratio. G1 has the maximum half-value layer value, whereas G5 has the minimum. The exposure and energy absorption build-up factor (EBF and EABF) values decreased slightly as the MoO3 content increased. As a result, among all other glasses, G5 glasses have the best shielding capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
MoO3 is not a glass-forming oxide on its own, but it can combine with other glass-forming oxides as P2O5 to form glasses. Recently, several glass systems involving P2O5 and MoO3 were investigated [1,2,3,4]. IR spectroscopy has been extensively used for structural characterization of MoO3–P2O5 glasses [5]. Selvaraj and Rao [3] investigated PbO–MoO3–P2O5 glasses. Because of PbO's ability to act as a modifier with the structural units of PbO6, adding PbO to phosphate glass increases the glass's stability against devitrification and causes the glass to become chemically inactive [6].
Incorporating transition metal oxide (TMO) to glass improves electronic characteristics by allowing electrons to transfer between valence states. The physical and mechanical characteristics of the glass are improved dramatically by CdO. The physical and chemical properties of glass systems with oxidation states 4 or 6 (CdO4 or CdO6) are altered by CdO [6,7,8,9].
Many industries, such as medical physics and nuclear research, can benefit from nuclear technologies. The interaction of X-ray radiation and a neutron with the material is critical in a variety of radiation technologies and applications. Therefore, radiation shielding has gotten a lot of press. The appropriate precursors for radiation shielding could be specific glass compositions. Glass materials have a range of excellent compositional properties that make them suitable for these applications [10,11,12,13].
Over the last few decades, researchers have investigated several types of glasses for use as alternative shield materials in a variety of nuclear applications. In extension to their ability to absorb high-energy photons, they have a variety of intriguing physical characteristics like transparency, ease of preparation, and stability when exposed to an external field.
The authors of this article investigate the use of fabricated glasses in radiation shielding applications. These glasses have excellent optical characteristics and can be used as radiation shields. Some physical and radiation shielding features were evaluated to achieve this goal.
2 Materials and methods
The chemical composition of the materials used to fabricate glass samples is shown in Table 1. In the following reaction, five lead, phosphate-based glass samples were manufactured. PbO2 + CdO + MoO3 + 2(NH4)2HPO4 \(\frac{\Delta }{-(4{\text{NH}}_{3}+3{\text{H}}_{2}{\text{O}})} \,\to\,\)( PbO2 + CdO + MoO3 + P2O5) \(\,\to\, \text{glass samples}\). The classic melt quenching method was used to produce the glasses, which took 45 min at 1100 °C. To relieve thermal stress, the fabricated glasses were annealed at 400 °C for 2 h and then cooled gradually.
XRD patterns on a Rigaku-Top XRD were used to check the condition of the glasses. The spectrophotometer type (JASCO V-670) recorded UV–VIS–NIR spectra in the range of 2700–200 nm. The study's goal was achieved by estimating all effective parameters that judge the shielding effectiveness of the prepared glasses using the online version of the Phy-X/PSD software. These glasses were studied for their (MAC), (LAC), and (HVL). We calculated and discussed EBF and EABF.
3 Results and discussion
3.1 Physical investigation
Figure 1 displays the XRD for the glass samples. There were no peaks discovered; instead, a broad hump in the XRD confirmed that the glass samples were amorphous [14, 15].
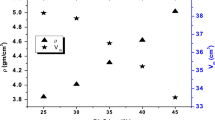
Figure 2 shows ρ & \({V}_{\text{m}}\) values of the fabricated samples. As the amount of MoO3 in the sample increased, the density increased. This is because MoO3 (143.938 g mole−1) has a higher density and molecular weight than CdO (128.41 g mole−1). The value of \({V}_{\text{m}}\) declined as MoO3 increased, as displayed in Fig. 2. This could be due to changes in the glass structure, such as a reduction in bond length between the glass network's structure [16, 17].
3.2 Optical measurements
Figure 3 demonstrates the variation in UV–Vis absorbance of fabricated glasses in the 200–1200 nm range. As a result, the growth of BO is attributed to MoO3. The absorbance coefficient (α) of the prepared glasses [18,19,20,21,22,23,24] is displayed in Fig. 4.
Energy bandgap \({E}_{\text{opt}.}\) was estimated using absorption UV–Vis regions by (\(\alpha \cdot h\nu {)}^{1/2}=B(h\nu -{E}_{\text{opt}.})\) , B is a constant that is not affected by energy. To calculate the indirect \({E}_{\text{opt}.}\) from the intercept, plot (\(\alpha \cdot h\nu {)}^{1/2}\) against \(h\nu\) as is shown in Fig. 5. Because oxygen bridges (BO) are established, which bind excited electrons more strongly than non-bridging oxygen, (NBO) \({E}_{\text{opt.}}\) increases with the increase in MoO3 as shown in Table 2. As shown in Fig. 6 Urbach energy has been calculated as \({\propto }_{0 }{\text{exp}} \left(\frac{h\upsilon }{{E}_{\text{u}}}\right)\) . Figure 7 depicts the \({E}_{\text{opt.}}\) and \({E}_{\text{u}}\) values.
According to these findings, increasing the amount of MoO3 has a significant impact on the \({E}_{\text{opt.}}\) and \({E}_{\text{u}}\). The values of \({E}_{\text{opt.}}\) and \({E}_{\text{u}}\) are presented in Table 2.
The refractive index of fabricated glass was determined by using the following formula: \({n}_{\text{D}}= \frac{{(1- R)}^{2}+{k}^{2}}{{(1+ R)}^{2}+ {k}^{2}}\), k = αλ/4π. \({n}_{\text{D}}\) of fabricated glasses is shown in Fig. 8. \({n}_{\text{D}}\) of these glasses increases as density rises, as previously stated.
The following formulas were used to calculate molar polarization \({R}_{\text{m}}\), polarizability \({\propto }_{0}^{2-}\), and optical basicity \(\Lambda\) of glasses: \({R}_{\text{m}}=\langle {n}^{2}-1|{n}^{2}+2\rangle Vm, {\propto }_{m}=\left(3|4\pi N\right){R}_{\text{m}},\) and \({\propto }_{0}^{2-}=\frac{\left[\frac{Vm}{2.52}\left(\frac{{n}^{2 }-1}{{n}^{2}+2}\right)-\sum {\propto }_{cat}\right]}{{n}_{\text{o}}^{2-}}\) and \(\Lambda = 1.67\left(1- \frac{1}{{\propto }_{0}^{2-}}\right)\). Figures 9, 10, and 11 show the \({R}_{\text{m}}\), \({\propto }_{0}^{2-}\), and \(\Lambda\) of these glasses, respectively. As the \({n}_{\text{D}}\) increases these variables increase. Therefore, \({n}_{\text{D}}\) and \({R}_{\text{m}}\), \({\propto }_{0}^{2-}\), and \(\Lambda\) have a similar trend.
The dispersion of Eo and Ed were estimated by Wemple and Didomenico [25] as \({n}^{2} - 1= \frac{{E}_{0 } {E}_{\text{d}}}{{E}_{0 }^{2}-{ E}^{2}}\). As shown in Fig. 12, the plotting of (n2−1)−1 with (hυ)2 Eo and Ed was calculated from the slope and intercept. A list of these values can be found in Table 2. With the increase in Mo3+, Eo and Ed have been slightly increased. The optical energy Eopt represents \(E_{\text{opt}}=\frac{{E}_{\text{d}}}{2}\). Refractive Static index at an infinite wavelength (no) was estimated by \({n}_{0 }= \sqrt{1 +\frac{{E}_{\text{d}}}{{E}_{0}}}\) and the static dielectric \({\varepsilon }_{\infty }={n}_{0}^{2}\). The oscillator's wavelength (λo) and strength (So) were calculated using the following formula: \({n}^{2} - 1= \frac{{S}_{0 } {\lambda }_{0}^{2}}{1-(\frac{{\lambda }_{0}}{\lambda } )2}\). Table 2 lists the available items.
3.3 Shielding properties
In this study, the radiation attenuation capacities of five different glass samples were assessed. Figure 13 depicts the MAC of the fabricated glasses as a function of photon energy. Furthermore, the MAC trend with photon energy in the range in MeV is readily apparent (0.015 to 15). As shown in Fig. 13, the MAC values decrease as photon energy increases. With an increase in the MoO3 ratio, it is more obvious that the MAC values decrease. In the intermediate photon energy zone, there is a stable or slightly changing behavior, while in the maximum photon energy zone, there is an increase in the MAC values. The photoelectric effect, Compton effect, and pair production effect, and cross-sections in the low, intermediate, and high energy zones, respectively, can be discussed [2, 26,27,28,29,30,31,32].
Figure 14 shows the LAC plotted. The photon interaction probability per unit length of the absorber material or medium, measured in cm−1, was illustrated by LAC. As shown in Fig. 14, the LAC values decrease as photon energy increases and increase with the increase of MoO3. This increase is due to an increase in the density of the glasses. As a result, the LAC values increased, indicating an inverse trend for glasses.
The HVL parameter is inversely proportional to LAC and defines the shield, absorber, or medium thickness that attenuates or absorbs 50% of the incident intensity. The sample with the lowest HVL has the greatest shielding capacity. As a result of this behavior, G1 is predicted to have the maximum HVL, while G5 will have the minimum. Among all fabricated glasses, G5 appeared to be the greatest material for shielding. As shown in Fig. 15, the trend (G1) > (G2) > (G3) > (G4) > (G5) ensured the best shielding capacity for fabricated glasses among all other glasses.
Finally, exposure and energy absorption build-up factors are the final parameters that demonstrate the effectiveness of the tested glasses (EBF and EABF). Figures 16 and 17 show the plots of both parameters. With the substitution of CdO for MoO3, the two parameters have a slight difference. The EBF and EABF were slightly reduced when the MoO5 molar ratios were increased from 0 to 5. This finding could be linked to or attributed to the investigated substance's effective atomic number. Furthermore, by increasing the MFP from 1 to 40 for all synthesized glasses, the two parameters introduced an increase in their values. The gamma photon energy has an impact on these parameters as well. As a result, the EBF and EABF variance behavior is primarily influenced by the three main interaction mechanisms discussed in the MAC section.
4 Conclusions
For optical and radiation shielding applications, the current study prepared and characterized lead cadmium phosphate glasses doped with different concentrations of MoO3. We replaced CdO with MoO3 during this study, which increased density and \({n}_{\text{D}}\) but a decrease in molar volume. UV–VIs spectroscopy has been used to evaluate the optical characteristics of fabricated glasses. \({E}_{\text{opt.}}\) values for the investigated glasses have increased while the \({E}_{\text{u}}\) values have decreased. As the \({n}_{\text{D}}\) increase \({R}_{\text{m}}\), \({\propto }_{0}^{2-}\), and \(\Lambda\) were increased. The values of fabricated sample dispersion parameters related to \({n}_{\text{D}}\) were determined. Phy-X/PSD was used to investigate the radiation shielding properties. MAC values improve as the MoO3 ratio rises. G1 has the maximum HVL value, whereas G5 has the minimum. The exposure and energy absorption build-up factor (EBF and EABF) values decreased slightly as the MoO3 content increased. As a result, among all other glasses, G5 glasses have the best shielding capacity.
References
J. Šubčík, L. Koudelka, P. Mošner, L. Montagne, B. Revel, I. Gregora, Structure and properties of MoO3-containing zinc borophosphate glasses. J. Non-Cryst. Solids 355, 970–975 (2009). https://doi.org/10.1016/j.jnoncrysol.2009.04.017
K.S. Shaaban, H.Y. Zahran, I.S. Yahia, H.I. Elsaeedy, E.R. Shaaban, S.A. Makhlouf, E.A.A. Wahab, E.S. Yousef, Mechanical and radiation-shielding properties of B2O3–P2O5–Li2O–MoO3 glasses. Appl. Phys. A (2020). https://doi.org/10.1007/s00339-020-03982-9
M.A. Sayed, A.M. Ali, A.F. Abd El-Rehim et al., Dispersion parameters, polarizability, and basicity of lithium phosphate glasses. J. Electron. Mater. 50, 3116–3128 (2021). https://doi.org/10.1007/s11664-021-08921-9
S.R. Yousefi, M. Ghanbari, O. Amiri, Z. Marzhoseyni, P. Mehdizadeh, M. Hajizadeh-Oghaz, M. Salavati-Niasari, Dy2BaCuO5/Ba4DyCu3O9.09 S-scheme heterojunction nanocomposite with enhanced photocatalytic and antibacterial activities. J. Am. Ceram. Soc. 104, 2952–2965 (2021). https://doi.org/10.1111/jace.17696
U. Selvaraj, K.J. Rao, Role of lead in lead phosphomolybdate glasses and a model of structural units. J. Non-Cryst. Solids 104, 300–315 (1988). https://doi.org/10.1016/0022-3093(88)90401-2
E.A. Abdel Wahab, A.A. El-Maaref, K.S. Shaaban, J. Börcsök, M. Abdelawwad, Lithium cadmium phosphate glasses doped Sm3+ as a host material for near-IR laser applications. Opt. Mater. 111, 110638 (2021). https://doi.org/10.1016/j.optmat.2020.110638
S.R. Yousefi, H.A. Alshamsi, O. Amiri, M. Salavati-Niasari, Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J. Mol. Liq. 337, 116405 (2021). https://doi.org/10.1016/j.molliq.2021.116405
S.R. Yousefi, A. Sobhani, H.A. Alshamsi, M. Salavati-Niasari, Green sonochemical synthesis of BaDy2NiO5/Dy2O3 and BaDy2NiO5/NiO nanocomposites in the presence of core almond as a capping agent and their application as photocatalysts for the removal of organic dyes in water. RSC Adv. 11, 11500–11512 (2021). https://doi.org/10.1039/d0ra10288a
K.S. Shaaban, M.S.I. Koubisy, H.Y. Zahran, I.S. Yahia, Spectroscopic properties, electronic polarizability, and optical basicity of titanium-cadmium tellurite glasses doped with different amounts of lanthanum. J. Inorg. Organomet. Polym. Mater. 30, 4999–5008 (2020). https://doi.org/10.1007/s10904-020-01640-4
S.R. Yousefi, O. Amiri, M. Salavati-Niasari, Control sonochemical parameter to prepare pure Zn0.35Fe2.65O4 nanostructures and study their photocatalytic activity. Ultrason. Sonochem. 58, 104619 (2019). https://doi.org/10.1016/j.ultsonch.2019.104619
S.R. Yousefi, M. Masjedi-Arani, M.S. Morassaei, M. Salavati-Niasari, H. Moayedi, Hydrothermal synthesis of DyMn2O5/Ba3Mn2O8 nanocomposite as a potential hydrogen storage material. Int. J. Hydrog. Energy 44(43), 24005–24016 (2019). https://doi.org/10.1016/j.ijhydene.2019.07.113
S.R. Yousefi, A. Sobhani, M. Salavati-Niasari, A new nanocomposite superionic system (CdHgI4/HgI2): synthesis, characterization and experimental investigation. Adv. Powder Technol. 28(4), 1258–1262 (2017). https://doi.org/10.1016/j.apt.2017.02.013
S.R. Yousefi, D. Ghanbari, M. Salavati-Niasari, M. Hassanpour, Photo-degradation of organic dyes: simple chemical synthesis of Ni (OH)2 nanoparticles, Ni/Ni (OH)2 and Ni/NiO magnetic nanocomposites. J. Mater. Sci.: Mater. Electron. 27(2), 1244–1253 (2016)
K.S. Shaaban, I. Boukhris, I. Kebaili, M.S. Al-Buriahi, Spectroscopic and attenuation shielding studies on B2O3-SiO2-LiF-ZnO-TiO2 glasses. SILICON (2021). https://doi.org/10.1007/s12633-021-01080-w
K.S. Shaaban, A.M. Al-Baradi, E.A.A. Wahab, The impact of Y2O3 on physical and optical characteristics, polarizability, optical basicity, and dispersion parameters of B2O3–SiO2–Bi2O3–TiO2 glasses. SILICON (2021). https://doi.org/10.1007/s12633-021-01309-8
K.H. Mahmoud, A.S. Alsubaie, E.A.A. Wahab, F.M. Abdel-Rahim, K.S. Shaaban, Research on the effects of yttrium on bismuth titanate borosilicate glass system. SILICON (2021). https://doi.org/10.1007/s12633-021-01125-0
A.F.A. El-Rehim, K.S. Shaaban, Influence of La2O3 content on the structural, mechanical, and radiation-shielding properties of sodium fluoro lead barium borate glasses. J. Mater. Sci.: Mater. Electron. 32, 4651–4671 (2021). https://doi.org/10.1007/s10854-020-05204-7
K.S. Shaaban, S. Alomairy, M.S. Al-Buriahi, Optical, thermal and radiation shielding properties of B2O3–NaF–PbO–BaO–La2O3 glasses. J. Mater. Sci.: Mater. Electron. (2021). https://doi.org/10.1007/s10854-021-05885-8
A.A. El-Maaref, E.A.A. Wahab, K.S. Shaaban, R.M. El-Agmy, Enhancement of spectroscopic parameters of Er3+-doped cadmium lithium gadolinium silicate glasses as an active medium for lasers and optical amplifiers in the NIR-region. Solid State Sci. 113, 106539 (2021). https://doi.org/10.1016/j.solidstatesciences.2021.106539
A.F.A. El-Rehim, A.M. Ali, H.Y. Zahran, I.S. Yahia, K.S. Shaaban, Spectroscopic, structural, thermal, and mechanical properties of B2O3-CeO2-PbO2 glasses. J. Inorg. Organomet. Polym. Mater. 31, 1774–1786 (2021). https://doi.org/10.1007/s10904-020-01799-w
S. Alomairy, A.M. Aboraia, E.R. Shaaban, K.S. Shaaban, Comparative studies on spectroscopic and crystallization properties of Al2O3 -Li2O- B2O3-TiO2 glasses. Braz. J. Phys. 51, 1237–1248 (2021). https://doi.org/10.1007/s13538-021-00928-1
A.M. Al-Baradi, A.F.A. El-Rehim, Z.A. Alrowaili, M.S. Al-Buriahi, K.S. Shaaban, FT-IR and gamma shielding characteristics of 22SiO2–23Bi2O3–37B2O3–13TiO2–(5–x) LiF- x BaO glasses. SILICON (2021). https://doi.org/10.1007/s12633-021-01481-x
E.A.A. Wahab, A.M. Aboraia, A.M.E. Shafey, K.S. Shaaban, A.V. Soldatov, The effect of ZrO2 on the linear and non-linear optical properties of sodium silicate glass. Opt. Quant. Electron. (2021). https://doi.org/10.1007/s11082-021-03164-8
E.A. Abdel Wahab, K.S. Shaaban, S. Alomairy, M.S. Al-Buriahi, Electronegativity and optical basicity of glasses containing Na/Pb/B and their high performance for radiation applications: role of ZrO2 nanoparticles. Eur. Phys. J. Plus (2021). https://doi.org/10.1140/epjp/s13360-021-01572-z
S.H. Wemple, M. DiDomenico Jr., Behavior of the electronic dielectric constant in covalent and ionic materials. Phys. Rev. B 3, 1338–1351 (1971). https://doi.org/10.1103/PhysRevB.3.1338
E. Şakar, Ö.F. Özpolat, B. Alım, M.I. Sayyed, M. Kurudirek, PhyX/PSD: development of a user-friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 166, 108496 (2020). https://doi.org/10.1016/j.radphyschem
M.A. Alothman, Z.A. Alrowaili, J.S. Alzahrani, E.A.A. Wahab, I.O. Olarinoye, C. Sriwunkum, K.S. Shaaban, M.S. Al-Buriahi, Significant influence of MoO3 content on synthesis, mechanical, and radiation shielding properties of B2O3-Pb3O4-Al2O3 glasses. J. Alloys Compd. 882, 160625 (2021). https://doi.org/10.1016/j.jallcom.2021.160625
S. Alomairy, Z.A. Alrowaili, I. Kebaili, E.A.A. Wahab, C. Mutuwong, M.S. Al-Buriahi, K.S. Shaaban, Synthesis of Pb3O4-SiO2-ZnO-WO3 glasses and their fundamental properties for gamma shielding applications. SILICON (2021). https://doi.org/10.1007/s12633-021-01347-2
B. Albarzan, A.H. Almuqrin, M.S. Koubisy, E.A. Abdel Wahab, K.A. Mahmoud, Kh.S. Shaaban, M.I. Sayyed, Effect of Fe2O3 doping on structural, FTIR and radiation shielding characteristics of aluminium-lead-borate glasses. Prog. Nucl. Energy 141, 103931 (2021). https://doi.org/10.1016/j.pnucene.2021.103931
A.F.A. El-Rehim, H.Y. Zahran, I.S. Yahia, S.A. Makhlouf, K.S. Shaaban, Radiation, crystallization, and physical properties of cadmium borate glasses. SILICON 13, 2289–2307 (2021). https://doi.org/10.1007/s12633-020-00798-3
A.F.A. El-Rehim, H.Y. Zahran, I.S. Yahia, A.M. Ali, K.S. Shaaban, Physical, radiation shielding and crystallization properties of Na2O–Bi2O3–MoO3–B2O3–SiO2–Fe2O3 Glasses. SILICON (2020). https://doi.org/10.1007/s12633-020-00827-1
A.M. Fayad, K.S. Shaaban, W.M. Abd-Allah, M. Ouis, Structural and optical study of CoO doping in borophosphate host glass and effect of gamma irradiation. J. Inorg. Organomet. Polym. Mater. 30, 5042–5052 (2020). https://doi.org/10.1007/s10904-020-01641-3
Acknowledgements
We would like to thank Taif University Research Supporting Project number (TURSP-2020/24), Taif University, Taif, Saudi Arabia. Moreover, the authors express their gratitude to the Deanship of Scientific Research at King Khalid University for funding this work through research groups program under Grant Number R.G.P. 2/137/42.
Author information
Authors and Affiliations
Contributions
All the authors have accepted full responsibility for the content of this manuscript and have given their approval to its submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests.
Consent to participate & publication
The authors consent to participate & publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaaban, K.S., Al-Baradi, A.M. & Ali, A.M. Physical, optical, and advanced radiation absorption characteristics of cadmium lead phosphate glasses containing MoO3. J Mater Sci: Mater Electron 33, 3297–3305 (2022). https://doi.org/10.1007/s10854-021-07530-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07530-w