Abstract
Coordination polymers were prepared by the condensation reaction of salicylaldehyde and thiosemicarbazide with formaldehyde, transition metal acetates Mn(II), Co(II), Ni(II), Cu(II) and Zn(II). Structural and spectral properties have been studied by elemental, spectral (FT-IR, 1H NMR, and UV–Vis), and thermogravimetric analysis. The geometry of the chelated coordination polymers was confirmed by magnetic susceptibility measurements and UV–Visible spectroscopy. Antimicrobial screening was done against microbes such as Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus, Staphylococcus typhi, Candida albicans, Microsporum canis, Aspergillus niger. In vitro antimicrobial activity was determined by the Agar Well Diffusion method and result show that all the coordination polymers exhibited better antimicrobial activity than their parent polymeric Schiff base. Coordination polymers were found to be more stable than their corresponding ligand as deduced by on thermogravimetric analysis. Because of antimicrobial and thermal behavior, these coordination polymers have broad range of applications as thermally resistant as well as antimicrobial-biocidal and antifouling coating materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coordination polymers provide a great opportunity for obtaining materials with desired properties by using designed monomers with different combinations and functionalities. The properties of polymeric material are due to its structure. Metal coordination, selection of ligand, topology and geometry etc. are responsible for various physicochemical properties. Organic polymers when used on the surface as adhesives or coatings, microorganism causes rupturing of these coatings through bonding and disbonding. But when metal chelating polymers are used, it protects the adhered surface due to its biocidal behavior and thus deterioration can be avoided. Besides biocidal behavior, the thermal stability of these organic polymers is also enhanced by metal insertion into the polymeric backbone. Active hydroxyl and azomethine groups in Schiff base polymers containing conjugated bonds have been studied [1]. Polymeric Schiff bases usually show basic properties owing to the presence of a C=N linkage in backbone. Interest has been focused on preparing composites and graphite materials resistant to high temperatures, photoresistors, thermostabilizers, epoxide oligomers, block copolymers, antistatic and flame resistant materials [2–6]. Schiff bases polymers demonstrate antimicrobial activity against various bacteria, yeast, and fungi [7, 8].
Metal incorporation into the polymers also possesses wide application as catalysts, impregnants, textile sizers, aqueous thickeners, adhesives, resins, and in the biomedical field [9–12]. Nitrogen and sulphur atoms play a key role in metal coordination at active sites of numerous ligands. Important class of nitrogen and sulphur atom donor ligands are thiosemicarbazones, particularly for transition metals [13, 14]. Remarkable biological activities have been exhibited by thiosemicarbazone complexes ranging from anti-fungal, anti-bacterial, anti-inflammatory, anti-viral to anti-tumour, activities [15–19].
A lot of inclination towards the complexation of thiosemicarbazones with iron, has been reported because of its pharmaceutical application [20] as potential antitumor agents, with aromatic thiosemicarbazones, copper complexes affected the mechanism of leukemic transformations by inhibiting the replication and triggering apoptotic processes [21]. At present, as for as biological activities are concerned, much study on transition metal complexes of thiosemicarbazones has been done. Keeping all the above facts in our mind, here we record the synthesis, characterization, thermal and biological activity of newly developed thiosemicarbazide based polymeric ligand and its coordination polymers. All synthesized polymeric compounds were characterized by elemental analysis, various spectral techniques like 1H NMR, FT-IR, UV–Visible spectra and in addition to antimicrobial activity, thermal stability of all polymeric compounds have also been discussed.
2 Experimental
2.1 Materials and Microbial Strains
Salicylaldehyde, thiosemicarbazide, ethanol, formaldehyde (37–41 %) (S.D. Fine), DMF, DMSO, acetone, sodium hydroxide (Merck). Barbituric acid, transition metal acetates (Qualingens): Manganese(II) acetate tetrahydrate [Mn(CH3COO)2·4H2O], Cobalt(II) acetate tetrahydrate [Co(CH3COO)2·4H2O], Nickel(II) acetate tetrahydrate [Ni(CH3COO)2·4H2O], Copper(II) acetate monohydrate [Cu(CH3COO)2·H2O], Zinc(II) acetate dihydrate [Zn(CH3COO)2·2H2O] were used without further purification. Elemental analysis of C, H and N of all polymeric compounds was carried out using elemental analyzer system GmbH Vario ELIII. All microorganisms were provided by the culture collection center of Microbiology Laboratory, Department of Microbiology (A.M.U. Aligarh).
2.2 Synthesis of Monomeric Schiff Base
Monomeric Schiff base, N,N′-bis (Salicylidene) thiosemicarbazide Schiff base (yield 72 %) and other polymeric compounds have been synthesized and synthetic method has been illustrated as supporting information.
2.3 Synthesis of Polymeric Schiff Base (STFB)
Polymeric Schiff base was synthesized by adding formaldehyde (0.02 mol, 1.5 cm3) into monomeric Schiff base (0.01 mol, 2.99 gm) in molar ratio of (2:1) Polymeric Schiff base STFB was obtained 70 % yield.
2.4 Synthesis of Coordination Polymers
Coordination polymers of [Mn(II), Co(II), Ni(II), Cu(II) and Zn(II)] were prepared by using equimolar ratio (1:1) of polymeric ligand (AGP) and metal(II) acetates. The polychelate of manganese [STFB-Mn(II)] 81 % yield. The above procedure was adopted for the synthesis of other coordination polymers and yields are given in Table 1.
3 Antimicrobial Assessment
In vitro antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus, Staphylococcus typhi, Candida albicans, Microsporum canis, Aspergillus niger, was adopted for measuring the effectiveness of the synthesized polymeric Schiff base (STFB) and its corresponding coordination polymers. Wells were dug in media with the help of a sterile metallic borer with centers of at least 25 mm. Recommended concentration (100 μl mL−1) of the test sample [1 mg mL−1 in dimethylsulphoxide (DMSO)] was introduced in the corresponding wells. Other wells supplemented with DMSO and reference antimicrobial drugs served as negative and positive controls, respectively. Plates were incubated immediately at 37 °C for 20 h. Activity was determined by measuring the diameter (millimeters) of zones showing complete inhibition. Kanamycin was used as a standard drug for antibacterial activity and, Miconazole for antifungal activity.
4 Measurements
Elemental analysis was carried out using elemental analyzer system GmbH Vario ELIII. Percentage of metals in the coordination polymers was determined by complexometric titration with ethylenediaminetetraacetic acid (EDTA) after decomposition with fuming HNO3. FT-IR spectra were recorded on a Perkin Elmer IR spectrophotometer (Model 621) using KBr discs in the range (4,000–400 cm−1). 1H NMR spectra were recorded on a JEOL-GSX 300 MHz FX 1000 FT NMR spectrometer. Ultra violet–visible (UV–Vis) spectra were taken on a Perkin Elmer Lambda (EZ-201) spectrophotometer in solution form. Magnetic susceptibility of the powder samples were measured on a vibrating sample magnetometer (Model 155). Comparative thermal behavior of polymeric Schiff base (STFB) and its coordination polymers were recorded with TG analyzer (Perkin Elmer Pyris Diamond) at a heating rate of 20 °C min−1 in nitrogen atmosphere.
5 Results and Discussion
5.1 Composition and Chemistry
Ligand was prepared in two steps by the polycondensation reaction. Firstly, monomeric Schiff base was prepared by the reaction of salicylaldehyde with thiosemicarbazide in 2:1 molar ratio according to Scheme 1. In the second step, polymeric Schiff base was prepared by the condensation of monomeric Schiff base with formaldehyde according to Scheme 2 in slight basic medium. These compounds were soluble in DMF, DMSO and insoluble in common polar organic solvents, such as ethanol, methanol, acetone and water. These are stable at room temperature and are non hygroscopic. The desired coordination polymers having the stoichiometry ratio 1:1 were obtained in quantitative yields by the interaction of an equimolar amount of metal(II) acetates with polymeric Schiff base STFB in DMF as shown in Scheme 3. Elemental analysis data of all polymers show that experimentally determined percentage values of C, H, N, S and M were also in very good agreement with calculated values, results being depicted in Table 1. A slight deviation in elemental analysis may be due to the polymeric nature of the compounds, as the value of end groups are not taken into account for theoretical reasons.
Elemental analysis data was in agreement with the proposed formula of polymers and confirmed octahedral geometry for [STFB-Mn(II), STFB-Co(II) and STFB-Ni(II)] while square planar geometry for STFB-Cu(II) and tetrahedral environment around STFB-Zn(II).
5.2 FT-IR Spectra
FTIR spectra of polymeric Schiff base and its coordination polymers with their assignments are given in the Table 2 and Fig. 1. Phenolic –OH stretching for the absorption frequency of polymeric Schiff base STFB appeared at 3,422 cm−1. These very broad bands disappear in the spectra of all coordination polymers, suggesting the involvement of hydroxyl oxygen in bond formation [22]. In the ligand, band at 1,246 cm−1 for STFB correspond to phenolic (C–OH) group appeared but in the coordination polymers these bands shifted to lower frequency by 17–23 cm−1, indicating the coordination of metal to oxygen [23]. Peaks for azomethine group were observed at 1,620 cm−1 for the ligand (STFB), while these bands shifted to lower frequency by 8–25 cm−1 in coordination polymers, thus indicating the coordination of metal to nitrogen. Lowering in the IR frequency indicates the coordination of metal through the azomethine nitrogen [24]. Coordination polymers exhibited broad band in the region of 3,330–3,166 cm−1 suggesting the presence of coordinated or absorbed H2O molecule [25]. Peaks at 1,590–1,487 cm−1are attributed to aromatic C=C stretch, C=S sharp signal also appeared at 835–833 cm−1 which shows no change in the frequency of STFB and STFB-M(II) which confirming that sulphur is not involved in bond formation. No peak is observed at 2,600–2,400 cm1 which shows that thiosemicarbazide remains in its thio-keto form [26].
Coordinated water shows medium to weak intensity bands at 965–942 cm−1 (rocking), 755–743 cm−1(wagging) in spectra of STFB-Mn(II), STFB-Co(II) and STFB-Ni(II) polychelates but not observed in the spectra of STFB-Cu(II) and STFB-Zn(II) polychelates. This difference suggests the presence of coordinated water molecule in Mn(II), Co(II) and Ni(II) polychelates. Bands at 567–518 cm−1 indicate M–N bonding while M–O band appears at 678–646 cm−1 [27].
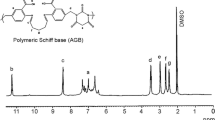
5.3 1H NMR Spectra
1H NMR spectra of Schiff base, polymeric Schiff base and its Zn(II) polychelate was carried out in DMSO-d6 at room temperature using TMS as internal standard is shown in Fig. 2. Aromatic protons show multiple resonance signals between 6.40 and 8.12 ppm for Schiff base, STFB and STFB-Zn(II) [28]. The signals observed in Schiff base have slight deviation as compared to polymeric Schiff base. Methylene proton of Ph–CH2–N– group show a sharp resonance signal at 3.50 ppm in STFB, due to the polymerization of salicylaldehyde-formaldehyde unit with barbituric acid moiety, whereas the resonance signals found at 3.15 ppm in STFB, indicate the presence of methylene group (–CH2–) of barbituric acid. Results of 1H NMR spectra reveal that barbituric acid moiety is attached to the polymeric Schiff base with methylene group of formaldehyde. Phenolic OH signals are observed in case of, Schiff base and polymeric Schiff base STFB at 5.00–5.10 ppm [29], while in coordination polymers of Zn(II), signals for phenolic proton disappeared which suggested the coordination of phenolic group to the metal. Formation of Ar–O–M and a significant shifting in all other peaks was observed due to the drifting of electrons towards the metal centre. Azomethine linkage appears at 8.32 ppm [30] in polymeric Schiff base STFB, which show the shifting in coordination polymers interpreting the participation of nitrogen of azomethine in M–N bond formation.
5.4 Electronic Spectra
Electronic spectra of all the synthesized polymers, STFB and STFB-M(II) were recorded at room temperature using DMSO as a solvent and transitions with assignments obtained by fitting the observed spectrum to the Tanabe-Sugano diagram, are tabulated in Table 3. Electronic spectrum of STFB-Mn(II) exhibited three bands at 14,810, 18,139 and 23,134 cm−1 which corresponds to 4T1g(G) ← 6A1g(F),4T2g(G) ← 6A1g(F) and 4A1g(G) ← 6A1g(F) transitions, respectively. Magnetic moment of STFB-Mn(II) was found 5.65 B.M., suggesting the presence of five unpaired electrons. The above data were used to calculate the crystal field parameter (10Dq), Racah parameter (B) and Nephelauxetic effect (β) and the covalency parameter (β %) values. The values of ligand field parameters 10Dq, B and β are listed in table indicating the covalent nature of compound and suggest the octahedral environment around the Mn(II) ion [31].
STFB-Co(II) has a magnetic moment of 4.60 B.M., [32] and showed three bands at 9,865, 16,674, 21,170 cm−1 which were assigned to 4T2g(F) ← 4T1g(F), 4A2g(F) ← 4T1g(F) and 4T1g(P) ← 4T1g(F) transitions, respectively and the value of 10Dq, B and β indicate an octahedral environment around Co(II) ion [33].
The octahedral STFB-Ni(II) was expected to be paramagnetic owing to two unpaired d-electrons, and the experimental magnetic moment was found 2.83 B.M. [34]. The electronic spectrum showed three bands at 8,545, 13,970, 22,920 cm−1 were assigned to 3T2g(F) ← 3A2g(F), 3T1g(F) ← 3A2g(F) and 3T1g(P) ← 3A2g(F) transitions for STFB-Ni(II). Crystal field parameters were in favor of octahedral geometry for STFB-Ni(II) [35]. The above discussion very strongly indicates an octahedral geometry around the central metal ion in the coordination polymers of Mn(II), Co(II), Ni(II). It accounts for the occupation of two coordinating sites by H2O out of six in making the octahedral environment.
Metal polychelate STFB-Cu(II) exhibited two bands at 17,120 and 26,123 cm−1 due to 2A1g ← 2B1g(F) and charge transfer, respectively, which indicate square-planar geometry and the magnetic moment values of STFB-Cu(II) is found 1.75 B.M., in accordance with square planar geometry [36]. The diamagnetic nature and absence of d–d transition in Zn(II) represent tetrahedral geometry.
5.5 Thermogravimetric Analysis
TGA thermogram of all synthesized polymers and data are tabulated in Table 4 and Fig. 3. Thermal data revealed that the thermal stability of coordination polymers is higher than that of the parent ligand and did not decomposed easily even at high temperature. TGA of coordination polymers shows no sharp weight loss was observed in the TGA curves of polymeric Schiff base (STFB) indicating their polymeric nature. In Mn(II), Co(II) and Ni(II) coordination polymers of STFB, thermogram curve show a 5–14 % weight loss corresponding to two water molecules up to 150–180 °C [37]. IR studies also support the presence of water molecules in these coordination polymers. In case of STFB-Cu(II) and STFB-Zn(II) coordination polymers, weight loss correspond to the loss of solvent or absorbed water molecules up to 130 °C while for STFB a steady and regular loss of weight was observed and at 300 °C the weight loss was about 50 %. Maximum rate of decomposition for the ligand was at 700 °C and almost entire ligand was lost by 800 °C, whereas in coordination polymers of STFB the weight loss was 86–93.5 %. This suggests that all coordination polymers show higher thermal stability than their corresponding polymeric ligand STFB, due to chelation. Another factor responsible for increased thermal stability of the coordination polymers is increase in molecular weight due to joining of two different polymer chains. Thermal stability of the polymeric compounds of STFB was in the order STFB-Cu(II) > STFB-Mn(II) > STFB-Ni(II) > STFB-Co(II) > STFB-Zn(II) > STFB. The greater stability of Cu(II) metal polychelate compared with other coordination polymers is in agreement with the spectrochemical series [38], according to which Cu(II) metal polychelate is always more stable than other coordination polymers.
5.6 Antimicrobial Activity
Polymeric Schiff base STFB and its coordination polymers individually exhibited varying degrees of inhibitory effects on growth of bacterial and fungal strains tested by agar well diffusion method [39]. Results represented in Fig. 4a, b which shows the newly synthesized STFB and its Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes posses good biological activity. The results of these investigations indicated that the synthesized polymers showed good antimicrobial activity. The polymer metal complexes show moderate to high biocidal action compared to the ligand against most of the strains. These polymers show better antibacterial activity compared to polymers reported by Hasnain et al. [26] and Nishat et al. [27], while the antifungal activities were better in latter cases.
Investigation revealed that the antimicrobial activity of polymeric Schiff base compound increases after metal chelation, as chelation reduces the polarity of central metal ion by partial sharing of positive charge with donor groups [40]. This process increases the lipophilic nature of central metal ion [41], which in turn favors its permeation to the lipid layer of membrane.
This enhanced biological activity can be rationalized to their structures possessing an additional C=N bond and chelation. It has also been observed [42] that solubility, conductivity, thermal stability, and dipole moment are influenced by the presence of metal ions. Cu(II) has stronger interaction with N and O donor atoms by which lipophilic nature is enhanced. The results of antifungal and antibacterial screening indicate that coordination polymers of Cu(II) possess best activity compared to other coordination polymers.
6 Conclusions
Polymeric Schiff base STFB and its coordination polymers were prepared in good yield and characterized by various microanalytical, magnetic, spectroscopic and thermal techniques. Thermal stability and antimicrobial behavior of all polymers were analyzed by using some bacteria and fungi. All polymeric compounds were soluble in DMF and DMSO, but insoluble in common organic solvents, giving opportunity to use these materials as solvent resistant coating materials. The incorporation of metal ion in organic backbone enhances thermal as well as antimicrobial activity. STFB-Cu(II) can be used for medical and biomaterial applications requiring thermal sterilization owing to stability at high temperatures while STFB-Cu(II) may be used as antimicrobial and antifouling coating for various projects.
References
S.C. Suh, S.C. Shim, Synth. Met. 114, 91–95 (2000)
K.I. Aly, A.A. Khalaf, J. Appl. Polym. Sci. 77, 1218–1229 (2000)
M. Grigoras, C.O. Catanescu, J. Macromol. Sci. C 44, 131–173 (2004)
R.H. Baughman, J.L. Bredas, R.R. Chance, R.L. Elsenbaumer, L.W. Shacklette, Chem. Rev. 82, 209–222 (1982)
F.R. Diaz, J. Moreno, L.H. Tagle, G.A. East, D. Radic, Synth. Met. 100, 187–193 (1999)
A.V. Ragimov, B.A. Mamedov, S.G. Gasanova, Polym. Int. 43, 343–346 (1997)
I. Kaya, H.Ö. Demir, A.R. Vilayetoglu, Synth. Met. 126, 183–191 (2002)
I. Kaya, A.R. Vilayetoglu, H. Topak, J. Appl. Polym. Sci. 85, 2004–2013 (2002)
H. Matsuda, K. Kanaoka, J. Appl. Polym. Sci. 30, 1229–1239 (1985)
N. Nishat, R. Rasool, S.A. Khan, S. Parveen, J. Coord. Chem. 64, 4054–4065 (2011)
H. Matsuda, J. Appl. Polym. Sci. 22, 3371–3386 (1978)
J.H. Silver, A.P. Hart, E.C. Williams, S.L. Cooper, S. Charef, D. Labarre, M. Jozsfowivz, Biomaterials 13(6), 339–344 (1992)
J.S. Chandra, Y.A.S.J.P. Kumari, P.N.V.V.L.P. Rani, Y. Sunandamm, Ind. J. Adv. Chem. Sci. 2(1), 32–37 (2013)
S.B. Padhye, G.B. Kauffmann, Coord. Chem. Rev. 63, 127–160 (1985)
P. Bindu, M.R.P. Kurup, T.R. Satyakeerty, Polyhedron 18, 321–331 (1999)
J.P. Scovill, D.L. Klayman, C.F. Franchino, J. Med. Chem. 10, 1261–1264 (1982)
A.K. Nandi, S. Chaudhri, S.K. Mazumdah, S. Ghosh, J. Chem. Soc. Perkin Trans. 2(11), 1729–1733 (1984)
M.A. Ali, D.A. Chowdhary, M. Naziruddin, Polyhedron 5, 595–598 (1984)
M.E. Hossain, M.N. Alam, J. Begum, M.A. Ali, M. Nazimuddin, F.E. Smith, R.C. Hynes, Inorg. Chim. Act. 249, 207–213 (1996)
M. Baldini, M.B. Ferrari, F. Bisceglle, P.P.D. Agllo, G. Pelos, S. Pinelli, P. Tarasconi, J. Inorg. Chem. 43, 7170–7179 (2004)
R.H.U. Borges, E. Paniago, H. Beraldo, J. Inorg. Biochem. 65, 267–275 (1997)
N. Chantarasiri, T. Damrongkosit, W. Jangwong, D. Sridaeng, S. Suebphan, Eur. Polym. J. 40, 1867–1874 (2004)
S. Saydam, E. Yilmaz, Spectrochim. Acta A 63, 506–510 (2006)
M. Joseph, M. Kuriakose, M.R.P. Kurup, E. Suresh, A. Kishore, S.G. Bhat, Polyhedron 25, 61–70 (2006)
H.H. Freedman, J. Am. Chem. Soc. 83, 2900–2905 (1961)
S. Hasnain, M. Zulfequar, N. Nishat, J. Coord. Chem. 64(6), 952–964 (2011)
N. Nishat, M. Zulfequar, Asma, S. Hasnain, J. Coord. Chem. 63(7), 1273–1281 (2010)
N. Nishat, R. Rasool, S. Parveen, Manisha, S.A. Khan, Int. J. Polym. Mat. 61, 41–56 (2012)
N. Nishat, S. Dhyani, S. Hasnain, Manisha, Polym. Bull. 64, 523–536 (2010)
N. Nishat, Asma, S. Dhyani, J. Coord. Chem. 62(18), 3003–3011 (2009)
F. Cotton, G. Wilkinson, C. Murillo, M. Bochmann, Advanced inorganic chemistry, 6th edn. (Wiley, New York, 1999)
N. Nishat, S. Hasnain, Manisha, Asma, J. Appl. Polym. Sci. 124, 3971–3979 (2012)
A.B.P. Lever, Inorganic electronic spectroscopy, 2nd edn. (Elsevier, Amsterdam, 1984)
F.A. Cotton, G. Wilkinson, Advanced inorganic chemistry (Wiley, New York, 1962)
E. Konig, Structure and bonding (Springer, Berlin, 1971)
N. Nishat, S. Parveen, S. Dhyani, Asma, J. Coord. Chem. 62(7), 1091–1099 (2009)
S.Y. Jang, Y.H. Ha, S.W. Ko, W. Lee, J. Lee, S. Kim, Y.W. Kim, W.K. Lee, H.J. Ha, Bioorg. Med. Chem. Lett. 14, 3881–3883 (2004)
I.V. Szmercsanyi, A. Szilagyi, J. Therm. Anal. 18(2), 235–246 (1980)
N. Nishat, S. Hasnain, S. Dhyani, Asma, J. Coord. Chem. 63(21), 3859–3870 (2010)
N. Raman, A. Kulandaisamy, A. Shunmugasundaram, K. Jeyasubramanian, Trans. Met. Chem. 26, 131–135 (2001)
N. Nishat, S.A. Khan, R. Rasool, S. Parveen, J. Inorg. Organomet. Polym. 21, 673–681 (2011)
R. Rasool, S. Hasnain, N. Nishat, Des. Monomers Polym. 17(3), 217–226 (2014)
Acknowledgments
One of the authors (Raza Rasool) is thankful to University Grants Commission (New Delhi) for financial support in the form of the Basic Scientific Research Fellowship. The authors express their sincere thanks to ‘‘The Third World Academy of Sciences, Italy’’ for UV–Visible spectrophotometer EZ-201 (Perkin-Elmer) through research Grant Scheme No. 00-047 RG/CHE/AS.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rasool, R., Hasnain, S. & Nishat, N. Coordination Polymers: Preparation, Physicochemical Characterization, Thermal and Biological Evaluation of Thiosemicarbazide Polychelates. J Inorg Organomet Polym 25, 763–771 (2015). https://doi.org/10.1007/s10904-014-0156-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-014-0156-3