Abstract
A polymeric ligand (BFG), containing glycine moiety was synthesized by the polycondensation reaction of bisphenol-A and formaldehyde with amino acid (glycine) in alkaline medium. The polymer–metal complexes were synthesized with transition metal ions. The polymer and its metal complexes were characterized by elemental analysis and other spectroscopic techniques. The analytical data revealed that the coordination polymers of Cr(III), Co(II), and Ni(II) were coordinated with two water molecules, which are further supported by FTIR spectra and TGA data. The amino acid was found to act as bidentate ligand toward metal ions via the nitrogen of the NH group and carboxyl oxygen of the respective amino acid. The in-vitro preliminary antimicrobial activities of all the synthesized polymers were investigated against some bacteria and fungi. The polymer–metal complexes showed excellent antimicrobial activities against both types of microorganisms. Interestingly the polymeric ligand was found antimicrobial in nature but less effective as compared the polymer–metal complexes. On the basis of the antimicrobial behavior, these polymers hold potential in their application as antifungal and antifouling coating materials in medical devices as well as antimicrobial packaging material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last two decades, several antibacterial polymers have been synthesized by immobilization of low molecular weight antibacterial agents to polymers. As compared to conventional low molecular weight biocides, polymeric agents have the advantages of enhanced antibacterial activity, efficiency, selectivity, reduced residual toxicity, and prolonged stability [1–3]. They can significantly reduce the loss of antimicrobial activity associated with volatilization, photolytic decomposition, dissolution and permeation-migration [4]. A number of in vivo studies have indicated that biologically active compounds become more bacteriostatic and carcinogenic upon chelation [5]. The interaction of transition metal ions with amino acids is of immense biological importance [6–8]. Polymer–metal complexes play an important role in biological applications namely healthcare products, hospital and dental equipment, food packaging, and storage material [9–12]. They also find application in chemical industries such as oil sterilization, hydrometallurgy, nuclear chemistry, air/water purification systems, biofouling coatings of ship hulls, shower walls and many kinds of tubing to minimize the problems of biofouling [13–16].
Two approaches are generally employed for the synthesis of antimicrobial polymers [17]. The first approach involves the introduction of the antibacterial agents to monomers, followed by their polymerization. This method has the advantage that the monomers can be polymerized with several other co-monomers and the composition can be varied easily. The second approach involves the linking of the antibacterial agents directly onto preformed functional polymers. Amino carboxylic acid can bond to the metal atoms in different ways by making use of both the amino nitrogen and the carboxyl oxygen atoms for coordination under neutral and alkaline conditions. Literature survey reveals that at alkaline pH, the amino acid can coordinate with the metal through the amino nitrogen atom as well as through the carboxyl oxygen atoms [18–19].
This study reports the synthesis of a new antimicrobial agent, prepared by the condensation of bisphenol-A, formaldehyde with glycine. The polymer-metal complexes were synthesized using the transition metal ions and were characterized by elemental analysis, spectral studies (FT-IR, UV–visible, 1H-NMR), magnetic susceptibility measurements and thermal techniques. The synthesized ligands as well as polychelates were also tested for preliminary antimicrobial activity against E. coli, S. aureus, B. subtillis, A. flavus, C. albicans, and A. niger by agar well diffusion method.
Experimental
Materials
Bisphenol-A (G.S.Chemicals), Formaldehyde 37–41% (S.D. fine chemical), glycine, sodium hydroxide pellets (Merck India), metal(II) acetates and metal(III) chlorides (Merck India) were commercially available and were used as received. Methanol, ethanol, diethyl ether, dimethylesulfoxide (DMSO), dimethylformamide (DMF) were purified by standard procedures prior to use.
Synthesis
Synthesis of polymeric ligand
Aliquot of bisphenol-A (2.28 g, 0.01 mol) was dissolved in DMSO (5–7 mL) and heated until it was completely dissolved. Formaldehyde (37% aqueous solution) (1.5 mL, 0.02 mol)) was added and the pH was adjusted to 9 with sodium hydroxide. The reaction mixture was stirred on a magnetic stirrer and heated at 45–50 °C for 4 h. The resulting mixture obtained was colorless. Glycine (0.75 g, 0.01 mol) dissolved in water (10 mL) was added drop wise to the reaction mixture and stirred at 60 °C for 3 h. A colorless liquid was formed which was digested at 35 °C for 6 h. The ligand was precipitated out as a colorless solid when poured into water. It was then filtered and washed several times with water followed by diethyl ether and dried in vacuum desiccator. The polymeric ligand was found to be soluble in polar solvents DMSO and DMF, and insoluble in water, ethanol, methanol, chloroform, carbon tetrachloride, benzene. Yield 62%, 1H NMR (DMSO, δ, 300 MHz) 2.57 (s, 1H, NH–CH2), 9.30 (s, 1H, Ar–OH), 4.45 (s, 1H, –CH2–OH), 3.62 (s, 2H, O–CH 2 –Ar), 3.53 (s, 2H, –NH–CH 2 –Ar), 3.87 (s, 2H, –NH–CH 2 ), 1.5 (s, 3H,–CH3), 7.11–7.98 (s, 6H, Ar), Mn-11945, Anal. Calc. C-74.05, H-6.57, N-2.47 found C-74.01, H-6.52, N-2.41.
Synthesis of metal polychelates
Polychelates of BFG ligand were prepared by using equimolar ratio (1:1) of BFG and metal salts. The preparation of Zn(II) polychelate was carried out with (5.68 g, 0.01 mol) of BFG and Zn(II) salt (2.19 g, 0.01 mol) dissolved in hot DMSO (30 mL). Both the solutions were mixed and the resulting mixture was heated on a boiling water bath for 2 h. A white colored product was obtained, which was filtered off, washed several times with water and finally with diethyl ether and dried in a vacuum desiccator over calcium chloride at room temperature (30 °C). Similar procedure was adopted for the synthesis of Cr(III), Mn(II), Co(II), and Ni(II). The percent yield of each polychelate was found to be in range of 60–80%. All the metal polychelates were found to be soluble in polar solvents, namely DMSO and DMF, and insoluble in water, ethanol, methanol, chloroform, carbontetrachloride and benzene.
Characterization
Elemental analysis
Elemental analysis of C, H, and N of the metal polychelate were carried out using elemental analyzer system GmbH Vario ELIII. The percentage of metals in the metal polychelates was determined by complexometric titration with ethylenediaminetetraacetic acid (EDTA) after decomposition with fuming HNO3.
Spectroscopic analysis
The FT-IR spectra were recorded on a Perkin Elmer IR spectrophotometer (Model 621) using KBr discs in the range 4000–500 cm−1.The 1H NMR spectra were recorded on a Bruker spectrospin DPX-30 MHz. The number-average molecular weight (Mn) of the polymeric ligand was determined by 1H NMR end-group analysis [20]. The ultra violet–visible (UV–Vis) spectra were taken on a Perkin Elmer Lambda (EZ-201) spectrophotometer in solution form.
Magnetic measurements
Magnetic susceptibility of the powder samples was measured on a vibrating sample magnetometer (model 155).
Thermal analysis
The thermal behavior of the metal polychelates was recorded with TGA analyzer (Perkin Elmer Pyris Diamond) at a heating rate of 10 °C/min in nitrogen atmosphere.
Antibacterial assessment
All the antimicrobial tests were carried out by the Department of agriculture and Microbiology, Aligarh Muslim University, Aligarh. The antimicrobial activity of the polymeric ligand and its polymer–metal complexes were tested against different microorganisms in DMSO as a solvent. The sample concentration was 50 μg mL−1 for antibacterial and antifungal studies, respectively. Bacterial strains were nourished in a nutrient broth and yeasts in a malt-extract broth and incubated for 24 and 48 h, respectively. According to the agar-diffusion method, bacteria were incubated on Muller-Hinton agar and yeast on Sabouraud dextrose agar. The wells were dug in the media with the help of a sterile steel borer and then 0.1 mL of each sample was introduced in the corresponding well. Other wells were supplemented with solvent (DMSO) for positive control and standard drug, viz. kanamycin (antibacterial) and miconazole (antifungal), for negative control. The resulting zones of inhibition on the plates were measured in millimeters.
Results and discussion
The structure of BFG prepared by the procedure mentioned in the experimental section is shown in Scheme 1. The polymeric ligand was a colorless solid, while the coordination polymers were colored solid materials, soluble in DMSO and DMF but insoluble in common organic solvents. The molecular weight of the coordination polymers could not be determined by GPC due to their insoluble nature in common organic solvents. The analytical data of the polymeric ligand (BFG) with its polymer–metal complexes are given in Table 1, and are in good agreement with 1:1 molar metal to polymeric ligand ratio. The analytical data revealed that the coordination polymers of Cr(III), Co(II), and Ni(II) coordinated with two molecules of water with each metal ion, which is also corroborated by the FT-IR and TGA analyses discussed in the proceeding sections.
FT-IR analysis
The assignments of FT-IR bands of ligand BFG as well as its polymer–metal complexes are summarized in Table 2. The presence of the methylene group was confirmed by the appearance of a strong band in the region of 2940–2840 cm−1 that can be correlated to the νCH2 sym and asym stretching mode [21]. The band at 1,448–1,464 cm−1 revealed the C–N stretching vibration which confirmed the reaction between salicyl alcohol and amino acid. The comparison of FT-IR spectrum of the parent ligand BFG with that of its each metal chelates revealed the absence of broad and strong band in the region of 3,453 cm−1 due to νOH vibration as the oxygen of this –OH group formed a bond with the metal ion. The characteristic bands appearing in the region 1,600–1,570 cm−1 were assigned to the asymmetric stretching vibration of the coordinated carboxylate group. Furthermore, the band responsible for the symmetric stretching vibration of the coordinated carboxylate ion appeared in the range 1,410–1,380 cm−1; Δν(COO−) ~200 cm−1 indicating the unidenticity of the carboxylate group [22–24]. A medium band at the 2,854 cm−1 of the ligand was assigned to –NH group, which was found to shift toward lower frequency in the complexes. This confirmed the involvement of this group in coordination [25]. The appearance of a strong band in the region 990–995 cm−1 and 760–758 cm−1 in the complexes was assigned to the rocking and wagging modes of the coordinated water, whereas the free ligand, Mn(II) and Zn(II) metal complexes did not exhibit this band. This substantiates the presence of coordinated water molecule in the other complexes. The appearance of new bands in the region 530–545 cm−1 can be attributed to ν(M–N) while the stretching frequency bands in the 418–430 cm−1 region were correlated to ν(M–O), which confirmed the coordination through nitrogen and oxygen [26]. The FT-IR data confirmed that the nitrogen and carboxyl oxygen atoms are found to be involved in coordination with the metal ion in complexes.
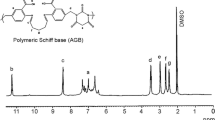
1H NMR spectra
The 1H-NMR spectra of amino acid based ligand (BFG) and their Zn(II) metal complexes were recorded in DMSO with tetramethysilane as internal reference. In Fig. 1 and Fig. 2, the 1H NMR spectra of polymeric ligand and its metal complex showed a peak for the NH protons of the –CH2–NH–CH2– group at 2.57 ppm, which confirmed the reaction between salicyl alcohol and amino acid. This peak was observed to shift downfield in case of Zn(II) polychelate and appeared at 2.57–2.69 ppm, which further supported the participation of this group in chelation. The phenolic protons showed a resonance signal at 9.30 ppm in BFG. In the Zn(II) complexes the intensity of O–H proton peak completely disappeared due to the coordination of oxygen with the metal ion after deprotonation, which is also confirmed by FT-IR spectra of the same. The appearance of peak at 4.45 ppm can be correlated to the hydroxyl proton of terminal CH2OH group and confirms the reaction of bisphenol-A with formaldehyde [27]. The methylene groups of BFG and BFG–Zn(II) exhibited signals at 3.53–3.59 and 3.62–3.65 ppm due to Ar–CH2–NH– and –O–CH2–Ar, respectively, while the methylene proton of glycine moiety in BFG and BFG–Zn(II) complexes appeared at 3.82 and 3.85 ppm, respectively [28, 29]. The peaks for protons of dimethyl groups of bisphenol-A and the protons of aromatic rings were observed at 1.5 and 7.11–7.98 ppm, respectively. The 1H-NMR analysis revealed that the metal coordinated with carboxyl oxygen and –NH group formed covalent bond with phenolic oxygen after complexation. The Mn of the polymer chelates was determined using end group analysis and is depicted in Table 1. The Mn value was found to be in the range of 19,000–20,000. Highest Mn value was found incase of BFG–Zn(II) while BFG–Cr(III) showed the lowest value among all the polymer chelates. The presence of high Mn value also confirmed the formation of high molecular weight coordination polymers.
Electronic spectra and magnetic property
The electronic spectra of the synthesized polymers were taken in DMSO solution (Fig. 3). The various crystal field parameters (10 Dq), Racah interelectronic repulsion parameter (B), nephelauxetic ratio (β), and covalency parameter (β0) were calculated using known equations and the values are given in Table 3. The magnetic moment of BFG–Cr(III) was 3.18 B.M. and suggested the presence of three unpaired electrons. The electronic spectrum of this complex exhibited three absorption bands at 17,755, 26,210 and 37,410 cm−1, which were assigned to the 4T2g(F) ← 4A2g(F)(ν1), 4T1g(F) ← 4A2g(ν2), and 4T1g(P) ← 4A2g(ν3) transitions, respectively, suggesting the octahedral geometry [30]. The BFG-Mn(II) polychelate had a magnetic moment of 5.43 B.M. due to five unpaired electrons and showed two bands at 23,790 and 15,600 cm−1, due to 4E ← 6A1(ν1) and charge transfer transitions, respectively, suggesting tetrahedral geometry which is also supported by the TGA. The Co(II) complex with BFG showed three bands at 9,150, 18,095, and 19,600 cm−1, assigned to the transitions 4T2g(P) ← 4T1g(F)(ν1), 4A2g(F) ← 4T1g(F)(ν2), and 4T1g(P) ← 4T1g(F)(ν3), respectively. The calculated values of Dq, B, β, and β0 suggesting that there is an octahedral geometry around the Co(II) ion. The magnetic susceptibility of Co(II) complexes is 5.57 B.M. The BFG–Ni(II) complex showed three bands at 10,500, 18,000, and 24,000 cm−1, assigned to the 3T2g(F) ← 3A2g(F)(ν1), 3T1g(F) ← 3A2g(F)(ν2), and 3T1g(P) ← 3A2g(F)(ν3) transitions, respectively. The ν1:ν2 value for the present compound was 1.71 and this corresponds to the usual range (1.60–1.82) reported for Ni(II) complexes having an octahedral geometry of the Ni(II) ion. BFG–Zn(II) is a d10 system, it has diamagnetic nature, coupled with elemental analysis and thermal decomposition analysis suggested a tetrahedral environment of chelating ligands around the Zn(II) metal.
Thermal analysis
The results of the thermal analysis of BFG ligand and its metal polychelates are depicted in Table 4. The thermal degradation in parent polymeric ligand is initiated with a very slight decrease in weight loss of about 3.44 wt% in the temperature range of 24–100 °C This can be correlated to the loss of entrapped moisture in the ligand. The thermal degradation in the temperature range of 300–400 °C can be attributed to the decomposition of a more labile aliphatic bridge [–HN–CH2–COO–] present between the two salicyl alcohol units. Around 73 wt% weight loss is observed at 400 °C which reached about 85 wt% at 750–800 °C. The gradual weight loss below 150 °C in all of the polymeric chelates was correlated to the removal of uncoordinated water, whereas weight loss obtained in Cr(III), Co(II), Ni(II) complexes in the temperature range 150–200 °C was due to the loss of coordinated water molecules [31]. The Mn(II) and Zn(II) complexes did not reveal any weight loss up in this region which suggests the absence of coordinated water molecule. After the loss of coordinated water molecules, all of the polymer metal complexes showed gradual mass loss but the rate of decomposition was quite rapid between 200 and 400 °C. This may be due to the decomposition of the uncoordinated part of the complexes, while the coordinated part of all the polymer metal complexes decomposes after 400 °C, followed by the formation of metal oxides (Cr2O3, MnO, CoO, NiO, ZnO). The results of thermogravimetric analysis revealed that the BFG–Zn(II) is thermally more stable than the Cr(III), Mn(II),Co(II), and Ni(II) complexes. The order of stability on the basis of thermal residual weight at 800 °C can be given in the following order BFG–Zn(II) > BFG–Ni(II) > BFG–Co(II) > BFG–Mn(II) > BFG–Cr(III) > BFG. All the polymers showed good heat resistant characteristics than the parent polymeric ligand due to the coordination of metal ions.
Biological evaluation
The fungicidal and bacteriological activities of polymeric ligand and its corresponding metal polychelates were determined against three bacteria and three fungi. All the synthesized polymers showed varying activity against all the bacteria and fungi shown in Table 5. For BFG-Zn(II) polychelate, the highest zone of inhibition values, i.e., 22 and 20 mm were measured in E. coli and S. aureus, respectively. The BFG–Ni(II) polychelate had the highest inhibition zone value 19 mm against B. subtelillis while BFG–Cr(III) showed the lowest antibacterial activity as compared with the other coordination polymers. The BFG–Mn(II) and BFG–Co (II) polychelates had intermediate inhibition zone values. The data revealed better antibacterial activity than their parent ligand. The BFG–Zn(II) complex showed the highest while the BFG–Cr(III) complex showed the lowest antimicrobial activity. The inhibition effect of the complexes was found to be comparable to the standard drug (kanamycin).
The antifungal activities of the synthesized polymers were tested against three fungi—A. flavus, C. albicans, and A. niger by the agar well diffusion method. The highest inhibition zones, i.e., 18, 20, and 16 mm were measured in BFG–Zn(II) complex against A. flavus, C. albicans, and A. niger, respectively. When BFG was screened against A. flavus, C. albicans and A. niger, the zones of inhibition were found to be 10, 6, and 8 mm, respectively. Against A. flavus the polychelates of Co(II) and Cr(III) showed the same inhibition zone of 12 mm, while Mn(II) and Cr(III) revealed similar inhibition effect of 15 mm against A. niger. The results of these investigations revealed that all the synthesized polymers showed good antimicrobial activity.
It has been commonly observed that the polymers containing amino acid cause membrane linkage, perhaps by interfering with the change of the phosphate on phospholipids of the membranes [32]. It was noticed that the antimicrobial activity of the ligand increased after chelation because chelation usually reduces the polarity of the central metal ion by partial sharing of its positive charge with the donor groups [33].This process increases the lipophilic nature of the central metal ion, which in turns favors its permeation to the lipid layer of the membrane. Other factors, viz. stability constant, molar conductivity, solubility and magnetic moment, are also responsible for increasing the antimicrobial activity of the complexes [34].
Conclusions
The BFG ligand and its metal complexes were prepared in good yield and characterized by various techniques. It has been observed that the attachment of the metal ion in the polymeric backbone enhances thermal as well as antimicrobial activity. The antimicrobial activity of Zn(II) coordinated polymer was found to be greater than that of other metal-coordinated polymers. Owing to its effective antimicrobial activity, BFG-Zn(II) may be used as antifungal and antifouling coating materials for various projects such as medical instruments and the bottoms of ships.
References
Li G, Shen J, Zhu Y (2000) A study of pyridinium-type functional polymers. III. Preparation and characterization of insoluble pyridinium-type polymers. J Appl Polym Sci 78(3):668–675
Talaro K, Talaro A (1993) In foundations in microbiology. WCB, Dubuque, p 286
Sauvet G, Dupont S, Kazmierski K, Chojnowski J (2000) Biocidal polymers active by contact. V. Synthesis of polysiloxanes with biocidal activity. J Appl Polym Sci 75(8):1005–1012
Temiz A, Togay SO, Sener A, Guven G, Rzaev ZMO, Piskin E (2006) Antimicrobial poly(N-vinyl-2-pyrrolidone-alt-maleic anhydride)/poly(ethylene imine) macrocomplexes. J Appl Polym Sci 102(106):5841–5847
Saxena AK (1987) Organotin compounds: toxicology and biomedicinal applications. Appl Organo Chem 1(1):39–56
Jones AD, Williams DR (1971) Thermodynamic considerations in co-ordination. Part IX. Heat capacity investigations into complex formation between some lanthanide(III) ions and histidine. J Chem Soc A 3159–3162
Clifford P, Singh S, Stjernsward J, Klein G (1967) Long-term survival of patients with Burkitt's lymphoma: an assessment of treatment and other factors which may relate to survival. Cancer Res 27(12):2578–2615
Stock JA (1970) Chemotherapy of cancer. Chem Br 6(1):11–16
Valentine JS, Gralla B (2002) Advances in protein chemistry: copper containing molecules (Advances in protein chemistry). Elsevier Science, USA, p 60
Bolto BA (1980) Novel water treatment processes which utilize polymers. J Macromol Sci Chem A 14:107–120
Schmuckler G (1965) Chelating resins—their analytical properties and applications. Talanta 12:281–285
Ukey VV, Juneja HD (2006) Synthetic and spectroscopic studies of chelate polymers involving azelaoyl bis-N-phenyl hydroxamic acid with transition metal ions. J Appl Polym Sci 99(1):273–278
Kenawy ER, Abdel-Hay FI, El-Magd AA, Mahmoud Y (2005) Biologically active polymers: Modification and anti-microbial activity of chitosan derivatives. J Bioact Compat Polym 20:95–111
Kenawy ER, Abdel-Hay FI, El-Shanshoury AR, El-Newehy MH (2002) Biologically active polymers. V. Synthesis and antimicrobial activity of modified poly(glycidyl methacrylate-co-2-hydroxyethyl methacrylate) derivatives with quaternary ammonium and phosphonium salts. J Polym Sci Part A Poly Chem 40:2384-2393
Worley SD (1996) Biocidal polymers. Trends Polym Sci 4:364–370
Louie AY, Meade TJ (1999) Metal complexes as enzyme inhibitors. Chem Rev 99:2711–2734
Wang R, Liu H, Carducci MD, Jin T, Zheng C, Zheng Z (2001) Lanthanide coordination with α-amino acids under near physiological pH conditions: polymetallic complexes containing the cubane-like [Ln4(μ3-OH)4]8+ cluster core. Inorg Chem 40(12):2743–2750
Mercier N, Riou A (2004) An organic–inorganic hybrid perovskite containing copper paddle-wheel clusters linking perovskite layers: [Cu(O2C–(CH2)3–NH3)2]PbBr4. Chem Commun 7:844–845
Dan M (2004) A mixed valent iron glycinate with the kagome structure. J Mol Struct 706(1–3):127–131
Liu K-J (1968) NMR studies of polymer solutions VI: molecular weight determination of poly(ethylene glycol) by NMR analysis of near end group. Makromolekulare Chemie 116(1):146–151
Nishat N, Parveen S, Dhyani S, Asma, Ahamad T (2009) Synthesis, characterization and thermal and antimicrobial studies of newly developed transition metal poly-chelates derived from polymeric schiff base. J Appl Polym Sci 113:1671–1679
EL-Gahami MA, Khafagy ZA, Ali AMM, Ismail NM (2004) Thermal, spectroscopic, cyclic voltammetric, and biological activity studies of cobalt(II), nickel(II), and copper(II) complexes of dicarboxylic amino acids and 8-hydroxyquinoline. J Inorg Organomet Polym 14(2):117–129
Wang G, Chang JC (1991) Synthesis and characterization of copper(II) and zinc(II) complexes of Schiff bases derived from amino acids and 2,4-dihydroxybenzaldehyde. Synth React Inorg Met Org Chem 21(5):897−902
Khan MMT, Kureshy RI, Khan NH (1991) Synthesis and characterization of Ru(III) chiral schiff base complexes derived from salicylaldehyde and L-aminoacids. Tetrahed Asymm 2:1015–1020
Roy SM, Juneja HD, Munshi KN (2001) Synthetic and thermal studies of polymeric chelates of some bis-biurets with first transition series metals. J Therm Anal Calorim 65(1):197–203
Mart H (2005) Oligo-ortho-chloroazomethinephenol and its metal complexes: synthesis, characterization, antimicrobial and thermal properties. J Macromol Sci A Pure Appl Chem 42:1197–1206
Canpolat E, Kaya M (2004) The synthesis and characterization of N-{2-[(1,4-Dioxaspiro[4.5]-Dec-2-Ylmethyl)amino]ethyl}-N’-hydroxy-2-(hydroxyimino)ethanimidamide and some of its transition metal complexes. J Coord Chem 57(1):25–32
Ahamad T, Kumar V, Nishat N (2006) Synthesis, characterization and antimicrobial activity of transition metal chelated thiourea-formaldehyde resin. Polym Int 55:1398–1406
Kemp W (1996) Proton NMR spectra in NMR in chemistry, 1st edn. Macmillian Education, London, p 57
Nakamoto K (1968) Infrared spectra of inorganic and coordination compounds, 2nd edn. Wiely, New York, p 167
Bhave NS, Aswar AS (1991) Thermal and electrical studies of some polychelates. Colloid Polym Sci 269:547–552
Ahmad T, Kumar V, Praveen S, Nishat N (2007) In vitro antibacterial and antifungalassay of poly-(ethylene oxamide-N-N’-diacetate) and its polymer metal complexes. Appl Organomet Chem 21:1013–1021
Bottei RS, Frangman JT (1996) Thermal and spectral study of some divalent metal chelates of 2,5-dihydroxy-p-benzoquinone. J Inorg Nucl Chem 28(5):1259–1264
Singh H, Yadav LDS, Mishra SBS (1981) Studies on some antifungal transition metal chelates of N-(5-phenyl-1, 3, 4-thiadiazol-2-yl) dithiocarbamic acid. J Inorg Nucl Chem 43(7):1701–1704
Acknowledgement
We thank the “Third World Academy of Science, Italy” for UV–visible spectrophotometer EZ201 through research grant no. 00-047 RG/CHE/AS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishat, N., Dhyani, S., Hasnain, S. et al. Development of antimicrobial aminoacid-modified bisphenol-A formaldehyde resin and its transition-metal complexes. Polym. Bull. 64, 523–536 (2010). https://doi.org/10.1007/s00289-009-0154-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-009-0154-8