Abstract
The polymer metal complexes of transition metal ions Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) with a new polymeric Schiff base containing formaldehyde and piperazine moieties have been synthesized by the condensation and characterized by elemental analyses, infrared spectra, electronic spectra, magnetic susceptibility measurements and thermogravimetric analyses (TGA). The results of the electronic spectra and magnetic moments indicate that the polymer–metal complexes of Mn(II), Co(II) and Ni(II) have octahedral geometry, while the complexes of Cu(II) and Zn(II) show square planar and tetrahedral geometry, respectively. The analyses of the thermal curves of all the polymer metal complexes show better thermal stability than the polymeric Schiff base. All compounds show excellent antibacterial as well as antifungal activity against three types of bacteria and two types of fungi. The antimicrobial activities were determined by using the agar well diffusion method with 100 μg/mL concentrations of polymer metal complex.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A major challenge facing academic and industrial communities is the relationship between modern societies and the environment that requires reinventing the manufacture and use of materials. Synthetic methodologies nowadays should be designed to use and generate substances that possess little or no toxicity to humans and the environment. For example, polymeric Schiff bases belong to a widely used group of organic intermediates important for production of specialty chemicals; e.g. pharmaceuticals, rubber additives [1] and amino protective groups in organic synthesis [2–5]. They also have use as liquid crystals [6], and in analytical [7, 8], medicinal [9, 10] and polymer chemistry [11]. These bases have been the subject of numerous studies after the pioneering investigations of D’Alelio et al. [12, 13]. They were synthesized by polycondensation of dialdehydes or diketones with an aliphatic or aromatic diamine to give high molecular weight polymers. However, their rigidity caused poor solubility in organic solvents and limited their practical applications [14].
Polymeric Schiff bases usually show basic properties owing to the presence of a C=N linkage in backbone. Interest has been focused on polymeric Schiff bases owing to their thermal stability, potential semiconducting, conducting and non-linear optical properties [15–17].
Coordination polymers derived from polymeric Schiff bases have also been extensively studied [18, 19]. It is known that reaction of metal ions with organic ligands produces coordination species that have enhanced thermal stability and often improved chemical resistance [20–23]. The nature of a coordination polymer depends on the metal ion, the donor atoms, the structure of the ligand and the metal–ligand interaction. With increasing knowledge of the properties of functional groups, the nature of donor atoms and the central metal ion, ligands with more selective chelating groups; i.e., imines or azomethines, have been used for polymer–metal complex formation studies.
These facts have propagated our interest in synthesizing new materials with antimicrobial and thermal resistant property. This report deals with the synthesis and characterization of a monomeric Schiff base by condensation of salicylaldehyde with o-aminophenol. The polymeric Schiff base is obtained by the reaction of the monomeric Schiff base with formaldehyde and piperazine. The resulting polymeric Schiff bases readily form polymeric metal complexes with Mn(II), Co(II), Ni(II), Cu(II) and Zn(II). The coordination behavior of the Schiff base towards transition metal ions was investigated by IR, 1H-NMR, 13C-NMR, UV–visible spectroscopy, magnetic moment measurements and thermal studies. The antimicrobial activity of the polymeric Schiff base and its polymeric metal complexes against the bacteria and fungi; i.e., E. coli, B. subtillis, S. aureus, P. aeruginosa, S. typhi (bacteria) and T. longifusus, C. albicans, M. canis, F. solani and A. niger (yeast), using the agar well-diffusion method is reported.
2 Experimental Section
2.1 Materials and Bacterial Strains
Salicylaldehyde, o-aminophenol, piperazine, formaldehyde (37% aqueous solution), hydrochloric acid, Mn(CH3COO)2·4H2O, Co(CH3COO)2·4H2O, Ni(CH3COO)2·4H2O, Cu(CH3COO)2·H2O and Zn(CH3COO)2·2H2O (S. D. Fine Chem. Ltd., Mumbai, India) were used without further purification. Solvents such as dimethylformamide (DMF), dimethylsulfoxide (DMSO), ethyl alcohol, methanol and acetone (Qualigens India) were purified by standard procedures before use. Agar purchased was purchased from Difco Laboratories. Bacteria strains used for antimicrobial activity tests included E. coli, B. subtillis, S. aureus, P. aeruginosa, S. typhi (bacteria) and T. longifusus, C. albicans, M. canis, F. solani and A. niger (yeast) were kept at −80 °C in freezer (Culture collection of Microbiology Laboratory, Department of Microbiology, A.M.U. Aligarh).
2.2 Synthesis of Monomeric Schiff Base of o-Aminophenol
For the preparation of monomeric Schiff base of o-aminophenol, a solution of o-aminophenol (1.1 gm) in ethanol (20 mL) was added into a magnetically stirred solution of salicylaldehyde (1.22 mL) in hot ethanol (30 mL). The mixture was refluxed for 2 h. After allowing the solution to cool at room temperature, the solvent was evaporated to give an orange solid product. The product was filtered, washed rapidly with cold distilled water and ethanol and dried in a vacuum desiccator over calcium chloride; Yield 70%, m.p. 135 °C.
2.3 Synthesis of Polymeric Schiff Base (SAPFP)
The polymeric Schiff base was prepared by taking a mixture of the monomeric Schiff base (2.14 gm) and formaldehyde (1.5 mL) in a 250 mL three-necked round bottom flask equipped with a thermometer, condenser and a magnetic stirrer. Aqueous NaOH (2 mL, 40%) was added to this reaction mixture and the temperature was increased to 70 °C for 1 h with continuous stirring. Piperazine (0.86 gm) in ethanol (20 mL) was added and the mixture was stirred again for ~3 h at 100 °C. The progress of reaction was monitored by thin layer chromatography. The reaction mixture was cooled and precipitated with 50/50 (v/v) water/acetone. The polymeric Schiff base (brown precipitate) was filtered, washed with distilled water, dried, recrystallised from ethanol and dried in a vacuum desiccator over CaCl2; Yield, 73%.
2.4 Syntheses of Polymer Metal Complexes of SAPFP
All the polymer metal complexes were prepared by the reaction of the polymeric Schiff base with metal salts. For example, SAPFP-Mn(II) was prepared by the addition of a hot solution of Mn(CH3COO)2·4H2O (2.45 gm) in DMF (25 mL) to the hot solution of the polymeric Schiff base (2.99 gm) in the same solvent (25 mL). The resulting mixture was stirred and refluxed. SAPFP-Mn(II) was precipitated and collected by filtration, washed with acetone and dried in a vacuum desiccator over calcium chloride; Yield, 70%. A Similar procedure was adopted for the synthesis of the other polymer metal complexes (Scheme 1).
2.5 Measurements
The IR spectra were recorded on a Perkin-Elmer infrared spectrometer model 621 by using KBr pellets. The 1H-NMR spectra were recorded on a JOEL-FX-100 FT NMR instrument in dimethylsulfoxide (DMSO) solution with tetramethylsilane as an internal standard. The elemental analyses of carbon, hydrogen and nitrogen were carried out on a Perkin-Elmer model-2400 elemental analyzer (CDRI Lucknow). The metals were determined by complexometric titration against EDTA after decomposing with concentrated nitric acid. The solubility of polymeric Schiff base and its polymer metal complexes were checked at room temperature in different solvents. The thermal stability of polymeric Schiff base and its polymer metal complexes were evaluated with a TA analyzer 2000 at a heating rate of 20 °C/min under nitrogen. The electronic spectra of the polymer metal complexes were recorded on a Perkin-Elmer Lambda-201 and magnetic susceptibility measurements were carried out with vibrating sample magnetometer. The antimicrobial activity of the polymeric Schiff base and the polymer metal complexes are reported against the bacteria and fungi.
2.6 Biological Activity
The Schiff base and its corresponding polymer metal complexes were screened in vitro for antibacterial activity against E. coli, B. subtillis, S. aureus, P. aeruginosa, S. typhi and for antifungal activity against T. longifusus, C. albicans, M. canis, F. solani and A. niger using the agar well-diffusion method. The wells were dug in the media with the help of a sterile metallic borer with centers of at least 24 mm. The recommended concentration (100 μL/mL) of the test sample (1 mg mL−1 in DMSO) was introduced into the corresponding wells. Other wells supplemented with DMSO and reference antibacterial drugs served as negative and positive controls, respectively. The plates were incubated immediately at 37 °C for 20 h. The activity was determined by measuring the diameter (mm) of zones showing complete inhibition. Kanamycine was used as a standard drug for antibacterial activity and Miconazole for antifungal activity.
3 Results and Discussion
3.1 Composition and Chemistry
The preparation of polymeric Schiff base was carried out in two steps. First, the monomeric Schiff base was prepared by the reaction of salicylaldehyde with o-aminophenol in 1:1 molar ratio. Second, the polymeric Schiff base was prepared by condensation of the monomeric Schiff base with formaldehyde and piperazine in 1:2:1 molar ratio in the presence of NaOH. The products are soluble in DMF, DMSO and insoluble in common polar organic solvents, such as ethanol, methanol, chloroform, water and acetone. They are stable at room temperature and are non-hygroscopic The desired polymer metal complexes, which have a 1:1 stoichiometry, were obtained in quantitative yield by the interaction of an equimolar amount of the metal(II) salts with polymeric Schiff base in DMF; all the polymer metal complexes are colored, stable at room temperature and non-hygroscopic. They are soluble in DMF and DMSO but insoluble in distilled water, acetone and common organic solvents.
The geometry of the newly synthesized compounds were elucidated based on their elemental analysis, magnetic susceptibility measurements and spectral data. The stoichiometry of the Schiff base and its polymer metal complexes were confirmed by elemental analysis. The metal/ligand ratio was found to be 1:1 estimated by determining the metal and ligand content of the polymer metal complexes (Table 1). Elemental analysis of the polymeric Schiff base and its complexes of Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) are in good agreement with the proposed formulas, which suggest the presence of coordinated water molecules in case of the Mn(II), Co(II) and Ni(II) polymer complexes. The Cu(II) and Zn(II) polymer complexes do not have coordinated water, which was further supported by IR.
4 Characterization
4.1 FTIR Spectra
The important IR bands and assignments of the monomeric Schiff base and the polymer metal complexes are given in Table 2. The absorption bands at 1,663 and 1,654 cm−1 in the monomeric Schiff base and SAPFP are attributed to the presence of azomethine group (HC=N). The band due to the azomethine group of the Schiff base is shifted to lower a frequency by 53–38 cm−1 in all polymer metal complexes, which indicates coordination of azomethine nitrogen to metal ion [24]. A broad band at 3,420–3,330 cm−1 for both the monomeric Schiff base and SAPFP is due to the phenolic–OH group and disappears in the spectra of the polymer metal complexes and suggests the deprotonation of the phenolic-OH group by the metal ion [25]. All the polymer metal complexes of SAPFP display strong bands from 1,554 to 1,510 cm−1, which are ascribed to aromatic ring stretching. Two absorption bands that appear at 2,870–2,810 cm−1 are due to νCH asymmetric and symmetric stretching; and, the frequency bands that appear at 1,445–1,400 cm−1 are due to νCH2 bending mode. The aromatic νC–H out-of-plane bending vibration is observed from 770 to 750 cm−1 [26] and the aliphatic C–N band appears at 1,020 cm−1. The appearance of two new bands at 550–520 and 470–430 cm−1 in all the polymer metal complexes are due to the coordination of metal ions with the phenolic-oxygen (M–O) and azomethine nitrogen (M–N), respectively [27, 28]. Polymer metal complexes of Mn(II), Co(II) and Ni(II) show absorption band from 3,410 to 3,400 cm−1, which is assigned to the water molecules within the coordination sphere and as lattice water. This band is not found in the spectra of polymer metal complexes of Cu(II) and Zn(II).
4.2 1H-NMR Spectra
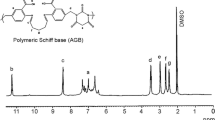
The 1H-NMR spectra of SAPFP and the polymer metal complex of Zn(II) are shown in Figs. 1 and 2. The azomethine proton of the polymeric Schiff base was recorded at 8.00 ppm. The aromatic protons show multiple resonance signals between 7.94 and 6.37 ppm for SAPFP and its Zn(II) complex [29, 30]. The resonance of the phenolic-OH group at 10.24 ppm is observed due to hydroxy proton of salicylaldehyde moiety [31]; and, the signal at 8.90 ppm is attributed to the hydroxyl proton of o-aminophenol moiety. The methylene protons of the Ar–CH2–N moiety appear in the 3.85–2.90 ppm region [32, 33] and the broad absorptions from 5.00 to 4.7 ppm is ascribed to the hydrogen-bonded terminal methylol group (Ar–CH2–OH). The presence of the piperazine moiety is confirmed by the signals at 2.68–2.49 ppm. In the 1H-NMR spectra of the Zn(II)–polymer metal complex, the OH resonance, which was observed in the polymeric Schiff base, disappears and signals for the azomethine (CH=N) group is shifted after complexation, which indicates involvement of the phenolic oxygen [34] and azomethine nitrogen in coordination.
4.3 Electronic Spectra and Magnetic Properties
The electronic spectra and magnetic properties of SAPFP metal complexes were recorded at room temperature in DMSO-d6. The transitions and assignments are given in Table 3. The magnetic moment of SAPFP-Mn(II) is 5.30 B.M., which suggest the presence of five unpaired electrons. The electronic spectrum of SAPFP-Mn(II) exhibits three bands at 17110, 21860 and 22995 cm−1, which correspond to 4T1g(G) ← 6A1g(F), 4T2g(G) ← 6A1g(F) and 4A1g(G) ← 6A1g(F) transitions, respectively. The crystal field parameter (10Dq) is 7,843 cm−1, the Racah parameter (B) is 713 cm−1 and the nephelauxetic parameter (β) is 0.74, which indicates the covalent nature of the compound [35].
The SAPFP-Co(II) has a magnetic moment of 3.81 B.M. and shows three bands at 8960, 15890 and 20350 cm−1 assigned to 4T2g(F) ← 4T1g(F), 4A2g(F) ← 4T1g(F) and 4T1g(P) ← 4T1g(F), respectively, and indicates an octahedral environment for Co(II) [36]. The crystal field parameters of the compound are 10Dq = 9,340 cm−1, B = 747 cm−1 and β = 0.77.
An octahedral SAPFP-Ni(II) is expected to be paramagnetic owing to the two unpaired d-electrons. The experimental magnetic moment is 2.91 B.M. The electronic spectrum showed three bands at 8980, 16075 and 24894 cm−1, which are assigned to 3T2g(F) ← 3A2g(F), 3T1g(F) ← 3A2g(F) and 3T1g(P) ← 3A2g(F), respectively; 10Dq = 9,450 cm−1, B = 945 cm−1 and β = 0.88, which is consistent with an octahedral geometry about Ni(II) [37]. The above data accounts for the occupation of two out of six octahedral coordinating sites by H2O.
The polymer metal complex SAPFP-Cu(II) exhibits two bands at 15,300 and 24,210 cm−1 due to 2A1g ← 2B1g(F) and charge transfer transitions, respectively, and indicate a square-planar geometry. The magnetic moment of SAPFP-Cu(II) is 1.84 BM, which is in accordance with square planar geometry [38]. The diamagnetic nature and the absence of d–d transition in Zn(II) are consistent with a tetrahedral geometry.
4.4 Thermogravimetric Analysis
The thermal decomposition of all the compounds was studied by the thermogravimetry (Fig. 3; Table 4). The thermal stability of all the polymer metal complexes is higher than that of the parent ligand and did not decompose easily at high temperature. The TGA confirms the presence of water molecules in all the metal complexes except Cu(II) and Zn(II) [39]. Removal of water from the Mn(II)-, Co(II)- and Ni(II)–polymer metal complexes of was complete by 130 °C. Decomposition of the polymer metal complexes is initially very slow and fast above 400 °C. Decomposition of all the coordination polymers is complete at 800 °C. The char yield at 800 °C for the polymer metal complexes is 15–22%, which is due mostly to the nonvolatile metal oxides.
4.5 Antimicrobial Activity
The polymeric Schiff base and its polymer metal complexes exhibited varying degrees of inhibiton on the growth of the bacterial/fungal strains (Tables 5, 6). The antimicrobial data are shown in Fig. 4a and b. All the polymeric compounds were screened for their antibacterial activity against E. coli, B. subtillis, S. aureus, P. aeruginosa and S. typhi and antifungal activity against T. longifusus, C. albicans, M. canis, F. solani and C. glaberata. All the compounds show good antibacterial activity; significant antifungal activity is observed against most of the strains. It is evident from the data that the antimicrobial activity of all the polymeric compounds is increased on coordination. This enhancement in the activity may be rationalized based on structures possessing an additional CH=N bond. Moreover, chelation/coordination reduces the polarity of the metal ion by partial sharing of the metal’s positive charge with the donor group and possible π-electron delocalization within the chelate ring. This increases the lipophilic nature of the central metal atom, which, in turn, favors greater penetration through the bacterial wall of the microorganism.
It has also been observed [40–43] that the solubility, conductivity and dipole moment are influenced by the presence of metal ions; these could be the significant factors responsible for increasing the hydrophobic character and liposolubility of the molecule, hence enhancing the biological activity. The results of antifungal and antibacterial screening indicate that the Cu(II)–polymer complexes exhibit a higher activity than the other polymer metal complexes. The result may be due generally to the higher stability constants of Cu(II) complexes.
5 Conclusions
Polymer metal complexes are prepared by the reaction of polymeric Schiff base with metal acetates in 1:1 molar ratio and characterized by analytical, magnetic, spectral and thermal data. All the polymers showed excellent antimicrobial activities against E. coli, B. subtillis, S. aureus, P. aeruginosa and S. typhi, T. longifusus, C. albicans, M. canis, F. solani and C. glaberata. The polymer metal complexes show higher antibacterial activity than the polymeric Schiff base. Polymer metal complex of Cu(II) show higher antibacterial activity and higher thermal stability.
References
V. Macho, M. Kralik, J. Hudec, J. Cingelova, J. Mol. Catal. A Chem. 209, 69 (2004)
P. Bey, J.P. Vevert, Tetrahedron Lett. 18, 1455 (1977)
R.A. Lucas, D.F. Dickel, M.J. Dziemian, B.L. Hensle, H.B. Mcphillarney, J. Am. Chem.Soc. 82, 5688 (1960)
G.W.J. Fleet, I. Fleming, J. Chem. Soc. C. Organic 1969, 1758–1763 (1969)
B. Bezas, L. Zervas, J. Am. Chem. Soc. 83, 719 (1961)
J.P. Adams, J. Chem. Soc. Perkin Trans. 1, 125 (2000)
R.W. Layer, Chem. Rev. 63, 489 (1963)
A. Abbaspour, A.R. Esmaeilbeig, A.A. Jarrahpour, B. Khajeh, R. Kia, Talanta 58, 397 (2002)
A.A. Jarrahpour, M. Motamedifar, K. Pakshir, N. Hadi, M. Zarei, Molecules 9, 815 (2004)
V. Alexander, Chem. Rev. 95, 273 (1995)
M. Higuchi, K. Yamamoto, Org. Lett. 1, 1881 (1999)
G.F. D’Alelio, J.V. Crivello, R.K. Schoenig, J.F. Huemmer, J. Macromol. Sci. Chem A1, 1169–1249 (1979)
G.F. D’Alelio, J.V. Crivello, T.R. Dehner, R.K. Schoenig, J. Macromol. Sci. Chem. A1(7), 1331–1364 (1967)
O. Thomas, O. Inganas, M.R. Andersson, Macromolecules 31, 2676–2678 (1998)
A.D. Delman, A.A. Stein, B.B. Simms, J. Macromol. Sci. Chem. A1, 147–178 (1967)
S. Kanda, H.A. Pohl, in Organic Semiconducting Polymers, Chap. 3, ed. by J.E. Katon (Marcel Dekker, New York, 1970)
P.N. Prasad, D.R. Ulrich, Nonlinear Optical and Electroactive Polymers (Plenum Press, New York, 1988)
C.S. Marvel, N. Tarkoy, J. Am. Chem. Soc. 80, 832–835 (1958)
H. Reitzle, W. Sawodny, Inorg. Chim. Acta 103, 53–55 (1985)
H.A. Goodwin, J.C. Bailar, J. Am. Chem. Soc. 83, 2467–2471 (1961)
W. Sawodny, M. Reiderer, E. Urban, Inorg. Chim. Acta 29, 63–65 (1978)
M.N. Patel, M.M. Patel, P.E. Cassidy, J.W. Fitch, Inorg. Chim. Acta 118, 33–35 (1986)
M.N. Patel, S.H. Patil, J. Macromol. Sci. Chem. A18, 521–533 (1982)
G.C. Bassler, R.M. Silverstein, Spectrophotometric Identification of Organic Compounds, 3rd edn. (Wiley, New York, 1992), p. 111
A. Syamal, M.M. Bari Niazi, J. Ind. Chem. Soc. A 23, 163 (1984)
L.J. Bellamy, The Infrared Spectra of Complex Molecules, 2nd edn. (Chapman and Hall, London, 1980), p. 299
S.C. Gagieva, T.A. Sukhova, D.V. Savinov, V.A. Optov, N.M. Bravaya, Y.M. Belokon, B.M.B. Bulychev, J. Appl. Polym. Sci. 95, 1040 (2005)
K. Syamasundar, M. Adharvanachary, J. Ind. Chem. Soc. 73, 32 (2001)
D. Bailey, D. Trirrell, C. Pinazzi, O. Voges, Macromolecules 11, 312 (1978)
J. Luston, Z. Managek, R. Palovcil, J. Macromol. Sci. Chem. A9, 1413 (1975)
N.S. Youssef, K.H. Hegab, A.E. Eid, Synth. React. Inorg. Met. Org. Chem. 33, 1647 (2003)
J.R. Dyer, Application of Absorption Spectroscopy of Organic Compounds, 2nd edn. (Prentice-Hall of India Pvt Ltd., New Delhi, 1972), p. 33
R.M. Silverstein, G.C. Bassler, Spectrometric Identification of Organic Compounds, 2nd edn. (Wiley, New York, 1967), p. 80
V.K. Sharma, S. Srivastava, Ind. J. Chem. 45A, 1368 (2006)
A.B.P. Lever, Inorganic Electronic Spectroscopy (Elsevier, Amsterdam, 1968), p. 317
S. Chandra, K. Gupta, Ind. J. Chem. 40A, 775 (2001)
A.N.M. Kasim, D. Venkappayya, G.V. Prabhu, J. Ind. Chem. Soc. 76, 67 (1999)
R.L. Carlin, Transition Metal Chemitry (Marcel Decker, New York, 1965), p. 1
H. Irving, R.J.P. Williams, J. Chem. Soc. (Resumed) 1953, 3192–3210 (1953)
Z.H. Chohan, A. Munawar, C.T. Supuran, Met Based Drugs 8, 137 (2001)
Z.H. Chohan, C.T. Supuran, Main Group Met. Chem. 24, 399 (2001)
Z.H. Chohan, H. Pervez, A. Rauf, C.T. Supuran, Met Based Drugs 8, 287 (2002)
M.U. Hassan, Z.H. Chohan, C.T. Supuran, Main Group Met. Chem. 25, 291 (2002)
Acknowledgment
Shadma Parveen wishes to acknowledge the Council of Scientific and Industrial Research (CSIR, New Delhi, India) for granting Senior Research Fellowship (SRF) vide grant no. 9/466(0097)2K8-EMR-I.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishat, N., Khan, S.A., Rasool, R. et al. Synthesis, Spectral Characterization and Biocidal Activity of Thermally Stable Polymeric Schiff Base and Its Polymer Metal Complexes. J Inorg Organomet Polym 21, 673–681 (2011). https://doi.org/10.1007/s10904-011-9457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-011-9457-y