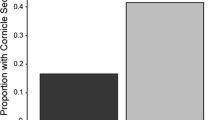Abstract
Insect natural enemies use several environmental cues for host/prey finding, and adjust their foraging behavior according to these signals. In insects, such cues are mainly chemical, derived from the host plant or the prey itself. The aphid alarm pheromone, (E)-β-farnesene (EBF), is believed to be such a cue, because several aphid enemies are able to perceive EBF and show attractant behavior. These studies are, however, based mainly on electroantennogram or olfactometer assays, and often use unnaturally high pheromone concentrations. It is, therefore, unclear if EBF is used to locate prey in the field when only naturally released amounts are present. We monitored the frequencies and durations of plant visits by aphid natural enemies in the field using long-duration camera observations. By placing pheromone releasers emitting no, natural or exaggerated amounts of EBF next to small colonies of pea aphids (Acyrthosiphon pisum), we analyzed if EBF presence altered long-range foraging behavior of natural enemies. Thirteen potential groups of aphid natural enemies were observed in 720 hr of analyzed video data. There was no effect of EBF on the number of predator visits to an aphid colony, or on predator patch residence times. The number of plant visits increased at exaggerated EBF amounts but not at natural EBF levels. We conclude that while there may be potential for use of high EBF concentrations for agricultural pest management strategies, an ecological role of EBF as a kairomone in a natural context is doubtful.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When searching for a host or herbivore prey (henceforth prey), predatory and parasitic insects generally follow a hierarchical behavioral pattern (Vinson 1976). The first step is the long-range localization of a habitat where prey might be present. The second step is short-range localization of the prey itself within the chosen habitat. To do this, many insect natural enemies utilize chemical cues for both steps of prey localization (Cardé and Bell 1995; Fellows et al. 2005). Potential chemical cues that predators and parasitoids can exploit generally derive from chemicals used for communication. Such semiochemicals may serve various purposes, and their classification thus depends on the ecological context. Pheromones, such as alarm pheromones, released by herbivorous insects often can be perceived by natural enemies, which use them in prey localization; i.e., they can be classified as kairomones in this specific interaction (Hatano et al. 2008b; Kost 2008).
In many aphid species (Hemiptera: Aphididae), (E)-β-farnesene (EBF) is the only alarm pheromone component (Francis et al. 2005a). Although it has the benefit of warning colony members of impending danger, EBF bears the potential cost of attracting additional natural enemies, since a vast variety of aphid predators and parasitoids are believed to use EBF as a host/prey finding kairomone (Hatano et al. 2008b). Electroantennogram (EAG) studies and olfactometer assays demonstrate that several aphid natural enemies are capable of perceiving EBF or are attracted to it, such as the ladybird species Adalia bipunctata L. (Francis et al. 2004), Coccinella septempunctata L. (Al Abassi et al. 2000; Ninkovic et al. 2001), Coleomegilla maculata (DeGeer) (Zhu et al. 1999), Harmonia axyridis Pallas (Verheggen et al. 2007) and Hippodamia convergens Guérin-Méneville (Acar et al. 2001), the green lacewing Chrysoperla carnea (Say) (Zhu et al. 1999, 2005), the hoverfly Episyrphus balteatus DeGeer (Francis et al. 2005b; Verheggen et al. 2008a), and the parasitoid wasps Aphidius ervi Haliday (Heuskin et al. 2012), Aphidius uzbekistanicus Luzhetzki, and Praon volucre (Haliday) (Micha and Wyss 1996).
The evidence that EBF operates as a kairomone for these natural enemies under field conditions is, however, not very strong. The amounts of EBF released by aphids after an attack can range from <1 ng up to 50 ng (Joachim et al. 2013; Schwartzberg et al. 2008). These signals are not amplified by other colony members (Hatano et al. 2008a; Verheggen et al. 2008b). Most studies that show kairomonal effects of EBF have been carried out under laboratory conditions and use alarm pheromone amounts much higher than those naturally released. For example, using an olfactometer, Acar et al. (2001) showed that adults of the convergent ladybird, H. convergens, perceive EBF and orientate their search to the source of emission, when 860 μg pure EBF were presented. In that study, the concentration was around 17,200 times higher than the maximum amount that generally is released by a single aphid. Zhu et al. (1999) demonstrated that laboratory studies on kairomonal effects that use very high amounts of chemical might not correctly predict ecological relevance under natural field conditions. Adults of both the ladybird C. maculata and the green lacewing C. carnea perceived EBF, as shown by EAG analysis, with the response increasing as the amount of EBF increased from 1 to 1000 μg. However, even the lowest concentration used was at least 20 times greater than the maximum amount released by attacked aphids. Moreover, when sticky traps with an EBF dispenser (50 mg pure EBF) were placed in an alfalfa field, there was no difference between control and EBF treatments in the number of natural enemies caught. Therefore, to understand the ecological relevance of (E)-β-farnesene as a kairomone in natural predator–prey interactions, it is important to investigate the effect of naturally occurring amounts in a natural setting.
In this paper, we investigated the significance of the aphid alarm pheromone, (E)-β-farnesene, as a long-range attractant for aphid natural enemies in the field. By observing predator dynamics under natural conditions, using long-duration camera monitoring, we addressed the following question: Does the emission of EBF pose a risk for aphids by acting as a kairomone that attracts aphid predators or parasitoids under field conditions?
Methods and Materials
General Experimental Conditions
The red BP clone of the pea aphid, Acyrthosiphon pisum Harris, originally collected in Bayreuth, Germany (Kunert et al. 2005), were reared on 3-wk-old broad bean plants, Vicia faba L., variety The Sutton (Nickerson-Zwaan, UK), in 10 cm diam. plastic pots. To prevent escape of aphids, plants were covered with air-permeable cellophane bags (Unipack GmbH, Germany). Aphids were kept in a climate chamber under constant environmental conditions (20 °C, 65 % RH, photoperiod: 16:8 h L:D). A split-brood design was employed according to Kunert et al. (2005) to control for any effect of previous rearing conditions on predator attraction. A different aphid line was used for all treatments of each replicate.
(E)-β-Farnesene Slow Releasers
EBF slow releasers were established following Heuskin et al. (2012) with slight modifications. In brief, to protect EBF from fast degradation (Kourtchev et al. 2009), the chemical was encapsulated within an alginate matrix (beads), which slowly releases pheromone (Heuskin et al. 2012). We established three EBF treatments: 1) no EBF (control), 2) natural EBF amounts, 3) exaggerated EBF amounts. The following recipe was used for the matrix: 4 ml sodium alginate solution (1.5 % w/v), 0.9 ml sunflower oil, and 0.075 mg α-tocopherol (Sigma Aldrich, Schnelldorf, Germany). Based on preliminary trials, we then added 0, 0.02, or 0.05 g (E)-β-farnesene. The compounds were mixed (Ultra Turrax IKA T18 basic) at 24.000 rpm for 30 sec. The homogenous emulsion then was extruded by syringe (Braun, Omnifix, 10 ml Leur Lock Solo) with a fine disposable hypodermic needle (Braun, Sterican Gr. 1, 0.90 × 40 mm) in a calcium solution (0.5 M) stirred at 200 rpm with a magnetic stir bar. The distance between the needle and the calcium solution was fixed to 20 cm. The spherical EBF alginate beads (fresh weight, 7.61 ± 0.12 mg; dry weight, 1.80 ± 0.05 mg, N = 10, calculated from 5 beads) were matured in solution for 48 hr and then dried on filter paper.
The slow releasers were produced once, shortly before the experiment, stored in 25 ml Falcon Tubes at 4–6 °C, and then used in all replicates. The releasers finished their 24 hr drying process on July 15th 2013 and the experiment started on July 17th 2013 with the first replicate.
Monitoring Emission Rates of the Slow Releasers
We tested EBF release rate of the alginate beads after production of the beads, before the experiment started. Because the releasers were produced only once, while the experiment was run over a period of 5 wk during which new beads from stock were used each time a new replicate was started (see below), we tested EBF emission of the releasers during this 5 wk period. EBF emission of the beads was measured using a zNose™ 4300 (Electronic Sensor Technology, USA). The surface acoustic wave (SAW) detector of the zNose™ was set to 40 °C. To calibrate the zNose™, a dilution series was created by dissolving EBF (Bedoukian Research Inc., Danbury, CT, USA) in methanol (Carl Roth Germany, 99.8 %) at concentrations of 0.25, 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, and 10.0 μg.ml−1. An aliquot (0.5 μl) of each diluted sample was injected directly by syringe into the LUER inlet of the zNose™ while the instrument was sampling (10 sec., trapping the total amount of the injected solution). Volatized samples were eluted under programmed conditions. Each concentration was tested at least five times. EBF was identified by comparison to a synthetic standard. Regression analysis showed that the response of the SAW detector to EBF changed in a linear fashion. The calibration curve was described by y(x) = 1620.4 x, where y = response of the SAW detector (Hz) and x = amount of EBF (ng) (R 2 = 0.877, P < 0.001, N = 48).
To test the release rate of the beads, three beads were randomly chosen from stock for each of the two EBF treatments, before the experiment and in each week of the 5 wk experimental period. Each bead was placed individually in a 4 ml vial connected to the zNose™ by inserting a stainless steel inlet needle (Hamilton, 50 mm) through a rubber septum (CS Chromatographie Service, Langwehe, Germany) in the lid of the vial. An air-inlet was created by inserting a hypodermic needle (0.9 × 40 mm; B. Braun Melsungen AG, Melsungen, Germany) in the septum, allowing influx of fresh air during sampling. Within one 10 sec sample, the complete air in the vial was inhaled by the zNose™. Thus, the electronic nose detected all alarm pheromone in the headspace. The release rate was observed for 1 h in 3 min intervals, with a total of 20 headspace samples.
At the start of the experiment (July 15th), mean release rate of the EBF dispensers was 80.02 ± 25.18 ng.h−1 for the natural EBF (N = 3), and 1494.57 ± 153.03 ng.h−1 in the exaggerated EBF amount treatments (N = 3). After 5 wk, at the end of video monitoring (August 19th), the emission rate was 128.01 ± 16.14 ng.h−1 for the natural (N = 3) and 628.69 ± 38.92 ng.h−1 for the exaggerated EBF amount treatments (N = 3), respectively. Thus, while the release rate of the exaggerated EBF treatment decreased over time, the release rate of the natural EBF treatment was relatively stable over the 5 wk period (ESM 1). In order to account for the decrease in EBF emission rate of the exaggerated EBF amount releasers, we adjusted the number of beads placed on plants: in weeks 1 and 2, one bead each was placed on each plant for both treatments. After 2 wk, we placed two exaggerated amount beads in the exaggerated treatment, while we continued to use only one bead for the plants in the natural EBF amount treatment.
Experimental Setup
Four aphids were glued by their hind legs, using UHU All Purpose Adhesive (UHU GmbH & Co. KG, Germany), close to the mid-vein of the underside of a leaf from the bottom leaf pair of 2-3-wk-old Vicia faba plants (2–4 leaf pairs), directly before the experiment started. Aphids were still alive and predominantly continued feeding after being glued to the plant. The leaf was held upright for camera observation by two small wooden sticks and a thin pliable wire. Since preliminary experiments revealed the possibility of plant destruction by mice (Myodes glareolus, Microtus arvalis), plant pots were placed in water containers (diam. 25 cm) embedded into the soil at ground level. To include ground foraging predators, such as ants, carabid beetles or spiders, six wooden sticks connected the plant pot with the outer rim of the water container, i.e., it was connected to the surrounding environment (ESM 2).
The camera-based observations were conducted on fallow grassland outside the city of Freising, Germany (latitude, longitude: 48.405716, 11.688162; vegetation of the grassland: 26.1 % Taraxacum, 17.5 % Trifolium, 11.1 % Symphytum, 8.9 % Achillea, 36.4 % others). The experiment was carried out between July 17th and August 22nd 2013 (19 rounds of observation, 24 hr each). Mean daily temperature was 19.1 °C and mean daily max. 25.9 °C. The field site was divided into a 2 × 9 grid; each cell was 10 m × 10 m. The distance from the grid to the margin of the grassland was at least 3 m on all sides, thus excluding edge effects. Six plants were placed in the grassland for each round of observation, two replicates of each of the three treatments: no EBF (control), natural EBF amount, and exaggerated EBF amount. Plants were always placed in the center of a grid cell, such that the minimum distance between plants was 10 m. The grid cell for a plant was determined randomly, and treatment position was randomized after each round of observation. The corresponding EBF slow releaser bead was placed next to the glued aphids (ESM 2). For the control, an alginate bead containing no EBF was used.
Six cameras (SONY ® CUT-HR-SHAD-day/night auto-iris-camera), capable of night recording using infrared lights mounted on wooden boards (20 cm× 20 cm) were used. Videos were recorded using mini long-term recording devices (1 K TosiVision, Gmyrek Elektronik GmbH, Germany) and saved on SDHC-cards (32 GB, Class 2, SanDisk, USA). Each camera was powered by a 12 V battery (Banner Energy Bull, 956 01, K5/60 Ah K20/80 Ah K100/90 Ah), and each plant was observed by one camera. Lens distance to the object was 25 cm. Cameras were arranged to depict the entire plant and the water container. Due to this setup, there was, however, a dead spot behind the plants.
Observations started between 7 am and 10 am and lasted for 24 hr. The number of aphids left on each plant was counted after each replicate.
The final video material was analyzed manually using the VLC media player 2.0.3 (www.videolan.org) with individual adjustment of video effects. Because the primary aim of this experiment was to check for the long-range attractancy of EBF, we quantified visits of natural enemies for two image sections. First, at the patch level; i.e., the spatial area within a radius of 10 cm around an observational plant. Any individual entering a patch was counted even if it subsequently did not contact the plant. The second spatial level of analysis was the target level; i.e., the underside of a leaf where the aphids and the slow releaser were placed, not including the petiole.
The following variables were analyzed: patch entrance time, time of day at which an aphid natural enemy entered a patch; patch residence time, duration of a stay of an aphid natural enemy within a patch; target entrance time, time of day at which an aphid natural enemy entered a target; and target residence time, the duration of an aphid natural enemy stay on a target leaf. In cases in which a natural enemy stayed within a patch but entered and exited a target more than once, times were summed for the target residence time. When a predator entered a patch away from the camera (i.e., in the dead spot behind the plant), the first appearance on camera was noted as the patch entrance time.
Statistical Analysis
Data were analyzed using R software 3.0.1 (www.r-project.org). All data are presented as mean ± standard error (SE).
The effects of treatments on the observed variables (e.g., mean number of predator taxon group visits, patch/target visits, and patch/target residence times) were analyzed using Generalized linear models (GLM) with model simplification; i.e., stepwise backward selection of independent variables to obtain the minimal adequate model. Data were transformed if necessary. When F-values are given for GLMs with count data, a quasi-Poisson distribution, rather than Poisson distribution, was assumed (residual deviance greater than degrees of freedom, see Crawley 2007, pp. 530/542). For analyzing the absence/presence (0/1) of aphid natural enemies, a binomial distribution was assumed.
Results
Video Material Assessed
Overall, 43.0 % of video observations had to be discarded; of these 61.2 % due to camera system failure, 24.5 % because of snail frass on plants, and 14.3 % for other reasons. From the suitable video material (65 out of 114 video observations during 19 rounds of observation, hence 1560 hr), 13 consecutive rounds of observation were analyzed with a total of N = 10 replicates for each treatment, and a total of 720 hr observation time (3 treatments × 10 replicates × 24 hr; i.e., in total, 30 plants were observed over a period of 24 hr).
Identification of Aphid Natural Enemies
The video system generally allowed good classification of arthropods to family and sometimes to the species level. While large- or medium-size Hymenoptera, such as wasps (Vespidae) or bees (Apidae), Diptera, such as hover, house, or horse flies (Syrphidae, Muscidae, Tabanidae), Hemiptera, such as stink bugs (Pentatomidae), or Coleoptera, such as ladybirds (Coccinellidae), allowed for identification to family, genera, or even species level. Medium-size arthropods, such as spiders (Arachnidae) or leafhoppers (Cicadellidae) allowed for identification to family level only. The identification of small arthropods, in general, and small natural enemy groups in particular, such as parasitoids, was sometimes problematic, as the experimental setup was optimized to depict the entire plant with only minor magnification. The majority of small arthropods belonged to Collembola (springtails) and the suborder Nematocera (lower Diptera, in particular Simuliidae or Sciaridae). Hence they were non-target taxa; i.e., not known to prey on aphids. In total, 622 individuals of 13 potential aphid natural enemy groups were identified. Frequent patch visitors included Araneae (spiders), Formicidae (ants), and Polistinae (polestine wasps), but also adult Syrphidae (hoverflies). The list of rare aphid natural enemies comprised Opiliones (harvestmen), Carabidae (ground beetles), Coccinellidae larvae, and Coccinellidae adults (ladybird beetles), Staphylinidae (rove beetles), Forficulidae (earwigs), Syrphidae larvae (hoverflies), Chrysopidae larvae (lacewings), as well as parasitoid wasps (henceforth parasitoids) (Fig. 1). For Syrphidae, most individuals were identified to species level, but because some could not, we included Syrphidae at the family level (c.f., Meyhofer 2001). Identification of 11 individuals was uncertain; nine were probably hymenopteran parasitoids and two were ants.
Predator Visits to Plants
On average, 3.2 ± 0.3 (range: 1–4) of the 13 enemy groups visited a patch over 24 hr in control treatments, not different (GLM: χ 2 = 0.52, d.f. = 2, P = 0.773, N = 10/treatment) from the 3.5 ± 0.4 (range: 2–5) groups in the natural EBF amount and 3.8 ± 0.4 (range: 2–5) in the exaggerated EBF amount treatments.
Across all groups, the number of individual patch visits over 24 hr tended to be greatest in the exaggerated EBF treatment (26.7 ± 3.6 visits, range: 13–42), but this did not differ from the control (9.0 ± 3.6 visits, range: 3–38) or natural EBF amount treatments (17.6 ± 2.6 visits, range: 7–32; GLM: F = 3.21, d.f. = 2, P = 0.058, N = 10/treatment).
When the natural enemy group was included in the analysis as an independent variable, the total number of patch visits within 24 hr was highest in the exaggerated EBF treatment (GLM: F = 9.87, d.f. = 2, P < 0.001, Fig. 2A). Adult syrphid flies were the most abundant patch visitors (GLM: F = 128.12, d.f. = 12, P < 0.001). The interaction between EBF treatment and enemy taxon group was not significant (GLM: F = 1.53, d.f. = 24, P = 0.056).
Visits of aphid natural enemies to a 10 cm radius around a plant (patch) on which four aphids were fixed along with slow releasers that emitted no (control), natural, or exaggerated (E)-β-farnesene (EBF) amounts. a Number of individuals per group over a 24 hr observation period. b Patch residence time for different aphid natural enemy groups. Data are presented in mean + SE. * = Difference among treatments within a group of natural enemies, as tested by GLM
Total patch residence time (i.e., the time all aphid natural enemies spent within a patch within 24 hr) was 15,550 ± 8444 sec. (range: 127–82,211 sec., N = 10) for the control, 6209 ± 2445 sec. (range 680–24,216 sec., N = 10) for the natural EBF amount, and 13,772 ± 8512 sec. (range: 337–87,622 sec., N = 10) for the exaggerated EBF amount treatments, not different among treatments (GLM: F = 0.09, d.f. = 2, P = 0.918, N = 10/treatment, data ln-transformed).
The mean patch residence times (i.e., the mean time a predator group spent within a patch per day) of the 13 aphid natural enemy groups was 561 ± 328 sec. (range: 0–32,627 sec., N = 130) for the control, 290 ± 162 sec. (range: 1–17,645 sec., N = 130) for the natural EBF amount, and 832 ± 668 sec. (range: 1–86,251 sec., N = 130) for the exaggerated EBF amount treatments, and was not influenced by treatment (GLM: F = 0.38, d.f. = 2, P = 0.683) or enemy group (GLM: F = 1.36, d.f. = 12, P = 0.182, Fig. 2B). There also was no interaction between the enemy groups and the EBF treatment on mean patch residence time (GLM: F = 0.93, d.f. = 24, P = 0.562).
Predator Visits of Leaf with Fixed Aphids and Slow Release Dispenser
Natural enemies rarely entered the target (i.e., the leaf with the four fixed aphids and alginate beads). In only 40 cases (6.43 % of the 622 visits), did natural enemies visit a leaf with the fixed aphids after entering a patch. The total number of target visits by all aphid natural enemies within 24 hr was 1.90 ± 0.53 (range: 0–5, N = 10) for the control, 1.00 ± 0.39 (range: 0–4, N = 10) for natural EBF amount, and 1.10 ± 0.35 (range: 0–3, N = 10) for exaggerated EBF amount treatments; there was no difference among treatments (GLM: F = 1.27, d.f. = 2, P = 0.298, N = 10/treatment).
The total target residence time (i.e., the time all aphid natural enemies spent on a target leaf within 24 hr) was 4037 ± 3318 sec. (range: 0–33,584 sec., N = 10) for the control, 1384 ± 1148 sec. (range 0–11,638 sec., N = 10) for the natural EBF amount, and 175 ± 114 sec. (range: 0–1,164 sec., N = 10) for the exaggerated EBF amount treatments, not significantly different among treatments (GLM: F = 1.18, d.f. = 2, P = 0.322, N = 10/treatment, data sqrt-transformed).
The mean target residence time of the 13 aphid natural enemy groups was 169 ± 132 sec. (range: 0–16,786 sec., N = 10) for the control, 106 ± 89 sec. (range: 0–11,543 sec., N = 10) for the natural EBF amount, and only 6 ± 4 sec. (range: 0–388 sec., N = 10) for the exaggerated EBF amount treatments; due to the high variation, the differences were not significant (GLM: F = 0.82, d.f. = 2, P = 0.442). The target residence time, however, was affected by the enemy group visiting the target (GLM: F = 2.39, d.f. = 12, P = 0.006), with representatives of the Araneae having the longest visits (10,622 ± 3,852 sec., N = 3). There was no interaction between enemy taxon group and EBF treatment on the mean target residence time (GLM: F = 0.75, d.f. = 24, P = 0.800).
Predator Activity – Diurnal Pattern
Most of the predator activity occurred in the early morning and late afternoon (Fig. 3). Taxa differed in the time of day they were active (Table 1, ESM 3). While spiders mainly visited a patch during the night and early morning, individuals of the subfamily Polistinae visited plants in the late morning and early afternoon. The most common plant visitor, adult syrphid flies, had activity peaks in the early morning and late afternoon.
Activity density of aphid natural enemies at the patch level as a function of time of day. The solid line is a kernel-density estimate of enemy activity for all (E)-β-farnesene treatments combined. The short vertical lines above the x-axis indicate time-points when a predator entered the patch. The grey dashed vertical lines indicate the mean sunrise and sunset during the observation period. N = 622
Prey Consumption
In total, 11 out of the 120 fixed aphids (4 aphids × 3 treatments × 10 replicates) were partly or fully consumed at the end of the experiment: seven in the no-EBF treatment and two in each of the two treatments with EBF. However, not all of these predation events were due to the focal aphid natural enemies. Mice were observed to consume four aphids on two different plants, and two aphids were eaten by a slug. Thus, only five aphids were consumed by typical aphid predators (spiders).
Discussion
Nordlund and Lewis (1976) defined kairomone as a “substance, produced, acquired by, or released as a result of the activities of an organism, which, when it contacts an individual of another species in the natural context (our emphasis), evokes in the receiver a behavioral or physiological reaction adaptively favorable to the receiver but not to the emitter”. When discussing the function of a substance, it is important to consider it with respect to the implicit quantitative qualification of this definition, because substances may cause a reaction when presented in unnaturally high concentrations, but not at natural concentrations, and hence have no ecological relevance (Byers 1988). Unnaturally high concentrations of a substance may not only cause a reaction not observed under natural conditions (i.e., an artifact), but also may cause a reaction opposite to that at natural concentrations. For example, Roelofs (1978) showed that in different moth species the behavioral response to a sex pheromone can shift from attraction at natural concentrations to inhibition at unnaturally high concentrations. Thus, before a substance is described as a semiochemical, knowledge about natural release rates is required (Byers 1988). For the aphid alarm pheromone, (E)-β-farnesene, this knowledge has only recently become available, with non-invasive rapid gas chromatography making it possible to measure EBF in the headspace of attacked aphids (Schwartzberg et al. 2008). These studies showed that the amounts of EBF released are small, in the range of nanograms, and lower than the amount of EBF stored in an aphid’s body (Joachim et al. 2013). As a consequence, many behavioral studies on the ecological effects of EBF have used very high amounts, sometimes greater than 1000 fold the natural amount. It is, therefore, important to verify the results of such studies, as the supposed ecological effect may be an artifact of the experimental design. The opposite also may be true: that there are effects of EBF on ecological interactions that occur only and, therefore, only can be detected when naturally occurring amounts of EBF are used. Our study suggests that aphid natural enemies do not use EBF as a kairomone for host/prey localization under natural conditions in the field.
Aphids are important plant pests and, when considering a kairomonal effect of EBF, a context with unnaturally high pheromone concentrations may still be interesting, as specific behaviors may be induced, such as aphid feeding disruption or attraction of natural enemies, which could be exploited for biological pest management strategies. Such results, however, have to be distinguished from the natural scenario that occurs in the field. For example, to understand costs and benefits of alarm signaling, only observations at natural EBF release rates should be used. Given that pea aphids generally emit alarm pheromone amounts from <1 ng up to 50 ng after an attack (Joachim et al. 2013; Schwartzberg et al. 2008), the levels of EBF released by our natural level EBF slow releasers resembled the amounts of about two to three attacks by a natural predator per hour causing release of the maximum amount, a realistic scenario. In contrast, in the exaggerated amount treatment, the quantity of EBF released corresponds to around 30 attacks within an hour, of high dose EBF emissions, an unlikely scenario because pea aphid colonies disperse after such frequent attacks and generally do not always emit maximal EBF amounts (Joachim et al. 2013; Minoretti and Weisser 2000). Thus, in our experiment, any attraction of natural enemies in the natural EBF treatment could have been considered to be consistent with a kairomone function of EBF, while the treatment with exaggerated EBF amounts, in contrast. tested for overdose effects.
In our study, the presence of EBF had no influence on almost any of the measurements, such as total and mean number of visits or patch residence times, for any of the observed aphid natural enemy groups. The only exception was the number of patch visits in which aphid enemies showed increased visits to plants at the exaggerated EBF amount compared to the control and natural EBF amount. This is not surprising, since it already has been demonstrated that application of exaggerated EBF amounts (e.g., 1 mg in dispensers or a release of 0.45 μg h−1 by slow releasers) can attract additional predators, such as syrphid flies, ladybirds, and parasitoid wasps (Aphidiidae) in agricultural fields, and can decrease the infestation pressure on a crop (Alhmedi et al. 2010; Cui et al. 2012). However, the use of EBF alone to control aphid populations without the presence of aphid enemies will not lead to a decrease in infestation rates on plants (Calabrese and Sorensen 1978). Interestingly, larvae of syrphid flies visited plants only with EBF, with a significant difference between the control and EBF treatments. While Francis et al. (2005b) described an attractant effect of EBF for larvae of E. balteatus at levels of 2 μg, our study suggests that syrphid fly larvae, which normally do not move very far in the field, also are attracted to naturally occurring amounts in the field. This can, however, not be confirmed for adult syrphid flies, for which the number of visits tended to be higher in the presence of exaggerated EBF amounts than for the control and natural EBF treatments.
Behavioral observations of Almohamad et al. (2008) and Verheggen et al. (2008a) showed that females of E. balteatus responded to 40 μg EBF. The differences in the effects of exaggerated EBF amounts on the attraction of natural enemies between this and other studies (that also have reported conflicting results) may be due to a variety of reasons. First, the concentration applied in the field (i.e., how overdosed the dispensers are) could be important. For example, our exaggerated EBF amount was even less than the amount used by others, e.g., Heuskin et al. (2012). Second, the environmental context is likely to be important; e.g., the surrounding vegetation, the time of the year, the climatic region, and possibly also predator diversity. That environment is important is underlined by the study of Meyhofer (2001), who used video observations to follow the composition, abundance, and activity of aphid natural enemy groups over the day. In his study, many more ladybirds, carabid beetles, and lacewings, but fewer syrphid flies, were observed than in our study. Nevertheless, this study gives no support to the idea that using an exaggerated amount of EBF to control aphids in the field will be successful.
There are several reasons why it may not be adaptive for aphid predators to use EBF as a kairomone. First, the presence of EBF in the headspace of an aphid colony indicates a predation event, which implies that the aphid colony has been disturbed; i.e., aphids may be walking, have dropped off the plant, or are otherwise scattered over the plant (e.g., Kislow and Edwards 1972; Minoretti and Weisser 2000; Wohlers 1981). Hence, there may be no aphids left for consumption, especially when the colony was initially small. Second, the presence of EBF in the headspace indicates the presence of a competitor or even an intraguild predator on the plant. The foraging success may, therefore, be low on the plant, and there is an additional mortality risk for the foraging predator due to intra-guild predation. In addition to these disadvantages, there also may be physiological constraints: EBF is emitted only in very low quantities and is not amplified by the colony (Hatano et al. 2008a; Joachim et al. 2013; Schwartzberg et al. 2011; Verheggen et al. 2008b), yet EBF degenerates when in contact with air (Kourtchev et al. 2009). Little is known about how EBF disperses within the plant and how far it may be detected by a predator and by conspecifics. More information is needed on the movement of EBF molecules emitted by an attacked aphid, and the rate of decay with distance.
Instead of using EBF as a kairomone or long-range attractant, other chemical cues are likely to be more beneficial under natural conditions, since aphid natural enemies, as do all other insect predators and parasitoids, face the ‘reliability-detectability’ problem (Vet and Dicke 1992): While volatile cues emitted by prey, in this case EBF, are often present only in low, inconspicuous concentrations, plant-derived volatile compounds are produced in greater quantities and can be detected more easily. There are several studies that demonstrate that plants change their emission profile under herbivory and that aphid parasitoids are attracted by such volatile cues (Du et al. 1998; Guerrieri et al. 1999; Powell et al. 1998). For aphid predators, laboratory studies often have failed to demonstrate this (Hatano et al. 2008b). However, as they are believed to visit plants frequently and feed on nectar or pollen, as also observed in our study for ants and syrphid flies, the chance of visiting a plant with aphid infestation through random search is still high.
In summary, video monitoring of aphid colonies in the field exposed to different levels of aphid alarm pheromone showed that EBF released by aphids is unlikely to play a major ecological role with respect to natural enemy attraction in the field. While there seems to be an attractant effect of exaggerated EBF amounts on some aphid predators under specific environmental conditions, which could be exploited in biological control strategies, we are reluctant to conclude that EBF is a kairomone in the aphid-natural enemy interaction.
References
Acar EB, Medina JC, Lee ML, Booth GM (2001) Olfactory behavior of convergent lady beetles (Coleoptera: Coccinellidae) to alarm pheromone of green peach aphid (Hemiptera : Aphididae). Can Entomol 133:389–397
Al Abassi S, Birkett MA, Pettersson J, Pickett JA, Wadhams LJ, Woodcock CM (2000) Response of the seven-spot ladybird to an aphid alarm pheromone and an alarm pheromone inhibitor is mediated by paired olfactory cells. J Chem Ecol 26:1765–1771
Alhmedi A, Haubruge E, Francis F (2010) Identification of limonene as a potential kairomone of the harlequin ladybird Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 107:541–548
Almohamad R, Verheggen F, Francis F, Haubruge E (2008) Impact of aphid colony size and associated induced plant volatiles on searching and oviposition behaviour of a predatory hoverfly. B J Entomol 10:17–26
Byers JA (1988) Novel diffusion-dilution method for release of semiochemicals: testing pheromone component ratios on western pine beetle. J Chem Ecol 14:199–212
Calabrese EJ, Sorensen AJ (1978) Dispersal and recolonization by Myzus persicae following aphid alarm pheromone exposure. Ann Entomol Soc Am 71:181–182
Cardé RT, Bell WJ (1995) Chemical Ecology of Insects 2. Chapman & Hall, New York
Crawley MJ (2007) The R book. John Wiley & Sons Ltd., Chichester
Cui LL, Francis F, Heuskin S, Lognay G, Liu YJ, Dong J, Chen JL, Song XM, Liu Y (2012) The functional significance of E-β-farnesene: does it influence the populations of aphid natural enemies in the fields? Biol Control 60:108–112
Du YJ, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM (1998) Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol 24:1355–1368
Fellows MDE, Van Alphen JJM, Jervis MA (2005) Foraging behaviour. In: Jervis MA (ed) Insects as natural enemies: A practical perspective. Springer, Dordrecht, pp 1–71
Francis F, Lognay G, Haubruge E (2004) Olfactory responses to aphid and host plant volatile releases: (E)-β-farnesene an effective kairomone for the predator Adalia bipunctata. J Chem Ecol 30:741–755
Francis F, Vandermoten S, Verheggen F, Lognay G, Haubruge E (2005a) Is the (E)-β-farnesene only volatile terpenoid in aphids? J Appl Entomol 129:6–11
Francis FD, Martin T, Lognay G, Haubruge E (2005b) Role of (E)-β-farnesene in systematic aphid prey location by Episyrphus balteatus larvae (Diptera : Syrphidae). Eur J Entomol 102:431–436
Guerrieri E, Poppy GM, Powell W, Tremblay E, Pennacchio F (1999) Induction and systemic release of herbivore-induced plant volatiles mediating in-flight orientation of Aphidius ervi. J Chem Ecol 25:1247–1261
Hatano E, Kunert G, Bartram S, Boland W, Gershenzon J, Weisser WW (2008a) Do aphid colonies amplify their emission of alarm pheromone? J Chem Ecol 34:1149–1152
Hatano E, Kunert G, Weisser WW (2008b) Chemical cues mediating interaction in a food chain: aphid-location by natural enemies. Eur J Entomol 105:797–806
Heuskin S, Lorge S, Godin B, Leroy P, Frere I, Verheggen FJ, Haubruge E, Wathelet JP, Mestdagh M, Hance T, Lognay G (2012) Optimisation of a semiochemical slow-release alginate formulation attractive towards Aphidius ervi Haliday parasitoids. Pest Manag Sci 68:127–136
Joachim C, Hatano E, David A, Kunert M, Linse C, Weisser WW (2013) Modulation of aphid alarm pheromone emission of pea aphid prey by predators. J Chem Ecol 39:773–782
Kislow CJ, Edwards LJ (1972) Repellent odor in aphids. Nature 235:108–109
Kost C (2008) Chemical communication. In: Jorgensen SE, Fath B (eds) Encyclopedia of ecology. Elsevier, Oxford, pp 557–575
Kourtchev I, Bejan I, Sodeau JR, Wenger JC (2009) Gas-phase reaction of (E)-β-farnesene with ozone: rate coefficient and carbonyl products. Atmos Environ 43:3182–3190
Kunert G, Otto S, Röse USR, Gershenzon J, Weisser WW (2005) Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecol Lett 8:596–603
Meyhofer R (2001) Intraguild predation by aphidophagous predators on parasitised aphids: the use of multiple video cameras. Entomol Exp Appl 100:77–87
Micha SG, Wyss U (1996) Aphid alarm pheromone (E)-β-farnesene: a host finding kairomone for the aphid primary parasitoid Aphidius uzbekistanicus (Hymenoptera: Aphidiinae). Chemoecology 7:132–139
Minoretti N, Weisser WW (2000) The impact of individual ladybirds (Coccinella septempunctata, Coleoptera: Coccinellidae) cell aphid colonies. Eur J Entomol 97:475–479
Ninkovic V, Al Abassi S, Pettersson J (2001) The influence of aphid-induced plant volatiles on ladybird beetle searching behavior. Biol Control 21:191–195
Nordlund D, Lewis WJ (1976) Terminology of chemical releasing stimuli in intraspecific and interspecific interactions. J Chem Ecol 2:211–220
Powell W, Pennacchio F, Poppy GM, Tremblay E (1998) Strategies involved in the location of hosts by the parasitoid Aphidius ervi Haliday (Hymenoptera : Braconidae : Aphidiinae). Biol Control 11:104–112
Roelofs WL (1978) Threshold hypothesis for pheromone perception. J Chem Ecol 4(6):685–699
Schwartzberg EG, Kunert G, Stephan C, Biedermann A, Rose USR, Gershenzon J, Boland W, Weisser WW (2008) Real-time analysis of alarm pheromone emission by the pea aphid (Acyrthosiphon pisum) under predation. J Chem Ecol 34:76–81
Schwartzberg EG, Boroczky K, Tumlinson JH (2011) Pea aphids, Acyrthosiphon pisum, suppress induced plant volatiles in broad bean, Vicia faba. J Chem Ecol 37:1055–1062
Verheggen FJ, Fagel Q, Heuskin S, Lognay G, Francis F, Haubruge E (2007) Electrophysiological and behavioral responses of the multicolored asian lady beetle, Harmonia axyridis Pallas, to sesquiterpene semiochemicals. J Chem Ecol 33:2148–2155
Verheggen FJ, Arnaud L, Bartram S, Gohy M, Haubruge E (2008a) Aphid and plant volatiles induce oviposition in an aphidophagous hoverfly. J Chem Ecol 34:301–307
Verheggen FJ, Mescher MC, Haubruge E, Moraes CM, Schwartzberg EG (2008b) Emission of alarm pheromone in aphids: a non-contagious phenomenon. J Chem Ecol 34:1146–1148
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Vinson BB (1976) Host selection by insect prasitoids. Annu Rev Entomol 21:109–133
Wohlers P (1981) Effects of the alarm pheromone (E)-β-farnesene on dispersal behavior of the pea aphid Acyrthosiphon pisum. Entomol Exp Appl 29:117–124
Zhu JW, Cosse AA, Obrycki JJ, Boo KS, Baker TC (1999) Olfactory reactions of the twelve-spotted lady beetle, Coleomegilla maculata and the green lacewing, Chrysoperla carnea to semiochemicals released from their prey and host plant: Electroantennogram and behavioral responses. J Chem Ecol 25:1163–1177
Zhu J, Obrycki JJ, Ochieng SA, Baker TC, Pickett JA, Smiley D (2005) Attraction of two lacewing species to volatiles produced by host plants and aphid prey. Naturwissenschaften 92:277–281
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG), grant WE-3081/2-3. The authors thank Jennifer Falk from the Chair of Chemistry of Biogenic Resources, Technische Universität München, for advice and help preparing the slow releasers and Dr. Manfred Türke for introducing the video system.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 369 kb)
Rights and permissions
About this article
Cite this article
Joachim, C., Weisser, W.W. Does the Aphid Alarm Pheromone (E)-β-farnesene Act as a Kairomone under Field Conditions?. J Chem Ecol 41, 267–275 (2015). https://doi.org/10.1007/s10886-015-0555-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0555-0