Abstract
When attacked by natural enemies some insect pests, including many aphid species, alert neighboring conspecifics with alarm pheromones. Cornicle secretions with pheromones benefit the attacked aphid but are costly to produce, while alarm pheromone benefits probably fall largely on alerted conspecifics. Given these variable benefits, the likelihood of a secretion may change depending on aphid density. Thus, we first hypothesized that the common alarm pheromone in aphids, E-ß-farnesene (EBF), was present in soybean aphid (Aphis glycines Matsumura) cornicle secretions and would elicit an alarm response in aphids exposed to it. Second, since aphids other than the secretor also benefit from cornicle secretions, we hypothesized that the likelihood of secretion would increase concurrently with the density of neighboring clonal conspecifics. Third, because alarm reaction behavior (e.g. feeding cessation) is probably costly, we hypothesized that alarm reaction behavior would decrease as conspecific density (i.e. alternative prey for an attacking natural enemy) increased. We found that soybean aphids 1) produce cornicle secretions using EBF as an alarm pheromone, 2) are less likely to release cornicle secretions when alone than in a small group (~10 individuals), but that the rate of secretion does not increase further with additional conspecific density, and 3) also exhibit alarm reaction behavior in response to cornicle secretions independent of aphid density. We show that soybean aphids can use their cornicle secretions to warn their neighbors of probable attack by natural enemies, but that both secretion and alarm reaction behavior does not change as density of nearby conspecifics rises above a few individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aphids and other arthropod pest species can occur in dense patches, making the number of neighboring conspecifics integral to understanding their interactions with natural enemies (Wrona and Dixon 1991). Aphid density is well known for its effect on natural enemy foraging and aggregation, such as causing the density of lady beetles to increase many times over where aphid colonies are large (Ives et al. 1993). While receiving relatively less attention, conspecific density may be just as important for modifying aphid defensive behaviors, including the use of alarm pheromones (Pickett and Griffiths 1980; Vandermoten et al. 2012). For example, since nearby conspecifics are alternative prey, increased aphid density could reduce the risk of attack and thus also reduce the propensity of aphids to respond to a warning signal (Wrona and Dixon 1991). There are also potential inclusive benefits to group defensive and/or alarm signaling behavior. Since aphids are often clonal species that live closely together, they might engage in behaviors benefitting their neighbors to increase their inclusive fitness (Wu et al. 2010). Despite forays into the effect of aphid density on alarm signaling behavior (Verheggen et al. 2009; Wu et al. 2010), there remains scant insight into how alarm reaction behavior occurs in aphid groups rather than in individual aphids.
Many aphid species exhibit behaviors used to limit enemy foraging or warn conspecifics of imminent danger (Vandermoten et al. 2012). When attacked these aphid species secrete droplets from cornicles on their posterior (Pickett et al. 1992; Mondor and Roitberg 2003). These “cornicle secretions” can physically encumber attackers and cause them to spend less time attacking prey (Wu et al. 2010), or may even kill the attacker (Butler and O’Neil 2006). Often included in the cornicle secretion is an alarm pheromone, most often E-β-farnesene (hereafter EBF; Francis et al. 2005 reviewed by Vandermoten et al. 2012) that commonly stimulates alarm reaction behaviors in nearby aphids. Pea aphids, perhaps the best studied example, kick, run, or may drop off the plant entirely when exposed to alarm pheromone (Roitberg and Myers 1978).
Alarm signaling and response behavior by aphids is contingent in part on aphid physiological state and local environment (Villagra et al. 2002; Beale et al. 2006). Cornicle secretions, composed of a large proportion of triglyceride fats (Strong 1967), are metabolically costly. Accordingly, there can be a reproductive cost to the aphid when released during certain developmental periods (Mondor and Roitberg 2003). Furthermore, as a signal of aphid presence, EBF may also attract aphid natural enemies (Du et al. 1998), making the signal even more counter-productive. Finally, alarm reaction behavior necessitates an interruption of aphid feeding and may indirectly induce aphid mortality (Nelson 2007). Because of these inherent tradeoffs in the costs and benefits of releasing a cornicle secretion and becoming alarmed, we hypothesized that these aphid behaviors would vary depending on the density of nearby conspecifics.
One particularly important species, the soybean aphid (Aphis glycines Matsumura, Aphididae), is a common agricultural pest and invasive in the Upper Midwest, USA (Ragsdale et al. 2004). Like many aphid species, soybean aphid produces cornicle secretions and may also engage in alarm pheromone production and response (Butler and O’Neil 2006), and while much about soybean aphid ecology has already been studied (Ragsdale et al. 2011), the mere presence and identity of compound(s) used to signal danger to closely-related conspecifics has not yet been positively identified. Because of recent interest in aphid alarm pheromones and the nature of soybean aphids as an economically important pest species, we wanted to examine the impact of conspecific density on potential soybean aphid cornicle secretion and alarm reaction behavior. We hypothesized that 1) soybean aphids and their cornicle secretions contain EBF, 2) the likelihood of a soybean aphid producing a cornicle secretion increases concurrently with conspecific density, and 3) soybean aphid alarm reaction behavior decreases as conspecific density increases.
Materials and Methods
We evaluated our hypotheses by using a chemical analysis to test for the presence of EBF in soybean aphid cornicle secretions, and using two experimental designs: A) comparing the cornicle secretion behavior of a single aphid to an aphid in a small group of conspecifics and B) comparing the likelihood of cornicle secretion and alarm reaction behavior at aphid densities ranging from 5 to 130+ aphids per leaf. Unlike larger and more active aphid species (e.g. pea aphids) soybean aphid alarm reaction behavior is relatively subdued and consists mostly of feeding sessation and slow dispersal instead of vigorous kicking or dropping behavior seen in other species.
Soybeans and Soybean Aphids
Soybean plants used in our experiments were an aphid-susceptible soybean variety (RG607RR, NDSU Research Foundation, Fargo, ND) grown individually in plastic pots (10.2 × 10.2 cm, Tessman Seed Co, St. Paul, MN). Pots were filled with a commercial sphagnum peat moss-based horticultural mix that also included horticultural perlite, dolomitic limestone, added nutrients, and a wetting agent (Sunshine Mix LC1, Sun Gro Horticulture, Vancouver, BC) and plants with and without aphids grew under standard lab conditions (16:8 light:dark cycle under florescent lighting at room temperature 20–22 C).
Colony soybean aphids were wild collected from soybean fields in Prosper, North Dakota during the summer of 2012. Colonies were provided with fresh soybean plants (grown as above) with 1–2 trifoliate leaves every 1–2 weeks, and new field collected aphids from the same location were added to the colony during the summer of 2013. Very few alate aphids (<1 %) were present in the colonies and none were used in experiments, and aphids were never observed to produce a cornicle secretion during handling prior to or during the setup of experiments. Aphids were transferred using small brushes at close visual range (~10 cm) or under low magnification.
Identification of Alarm Pheromone
We used gas chromatography (GC) to determine if EBF was present in both the aphids and their cornicle secretions. To detect EBF in the bodies of soybean aphids, ten late stage juvenile or adult instar individuals were randomly removed from a lab colony and placed into a small aliquot (~0.5 mL) of hexane. Because soybean aphids are small, we used ten individuals to increase the likelihood that the GC detection limit for EBF would be reached and also focused on these two largest size classes of soybean aphids that prior research in other species suggests produce the greatest amounts of EBF (Mondor et al. 2000). Similarly, to detect EBF in the cornicle secretions of soybean aphids we mechanically stimulated (see below) a cornicle secretion from ten soybean aphid individuals, collected the secretion on small (~0.5 cm2) piece of clean filter paper, then placed all ten pieces into a small amount (~0.1 mL) of hexane. Cornicle secretions and aphid extracts were analyzed by gas chromatography/mass spectrometry (GC/MS) on a Hewlett-Packard 5890/5972 (Palo Alto, California, USA). The GC was fitted with a 30 m × 0.25 mm i.d. ZB-Wax column (Phenomenex, Torrance, California, USA) and a splitless injector. Helium was the carrier gas at a constant flow of 1 ml min−1. The GC column oven was programmed from 80 to 180 °C at 15 °C min−1 (initial delay of 1 min), and then to 220 °C at 3 °C min−1. We compared the GC retention time and mess spectra of the soybean aphid/cornicle solutions to a synthetic EBF standard (Bedoukian Research, Inc., Danbury, Connecticut, USA).
We also conducted a behavioral response assay to confirm if EBF is an active semiochemical in the soybean aphid’s cornicle secretion. We placed approximately ten 3rd or 4th instar soybean aphids on a piece of fresh soybean leaf inside a 50 mm petri dish, allowing the aphids to acclimate for at least 30 min, a time long enough for aphids to begin probing the leaf but not so long as to degrade leaf tissue (Diaz-Montano et al. 2007). We then exposed the aphids to two treatments, 1) a n-hexane control treatment (n = 12), or 2) a 10 nanogram EBF (Bedoukian Research, Inc., Danbury, Connecticut, USA) per 1 μL n-hexane EBF treatment (n = 13). To deliver the stimulus in each trial we put a small (circular, made with a regular office hole punch, ~9 mm2) piece of filter paper inside the small Petri dishes already containing the leaf with aphids, and then delivered 5 μL of each individual solution using a micropipette. We recorded each trial using an iPhone® 5 (Apple Corp., Cupertino, CA) affixed to the eyepiece of a dissecting microscope in a Magnifi ™ case (Arcturus Labs, Palo Alto, California, USA) for 60 s, scoring signs of altered (above background levels of activity) and/or agitated behavior (e.g. “waggling” of the body, removal of stylet, ambulation) as “alarm” behavior. Unlike larger and more active aphid species (e.g. pea aphids) soybean aphid alarm behavior is relatively subdued and consists mostly of feeding sessation and slow dispersal instead of vigorous kicking or dropping behavior seen in other species. To differentiate from incidental activity, aphids were scored as alarmed only if they reacted for longer than a full second.
Individual Vs. Group Experiment
To test if the likelihood of soybean aphid emitting a cornicle secretion was dependent on the presence of conspecifics, we mechanically stimulated aphids when they were alone compared to when they were in a group, while monitoring the response of the focal aphid and nearby conspecifics. Late stage juvenile or adult soybean aphids were collected from the colony and transferred with a fine camel hair paintbrush to a detached leaf of a V1 (first set of trifoliate leaves expanded) stage soybean plant in a 5 cm petri dish and were left undisturbed for 10–15 min. Petri dishes contained either a single aphid (n = 60) or a group of 8–10 conspecifics (n = 53), the latter of which were never farther than 2.5 cm from the focal aphid. Using a size 00 stainless steel insect pin (Ento Sphinx, Pardubice, Czech Republic) the focal aphid was jabbed in the thorax for 1–2 s from directly above (modified from Butler & O’Neil, 2006). Following the attack, the presence of any secretion from the focal aphid’s cornicles was assessed from 60 s of video recorded using the iPhone 5/Magnifi ™ case as in the EBF trials. This experiment was performed in March–April of 2013.
Group Density Experiment
To test if soybean aphid cornicle secretion and alarm reaction behavior varies with aphid density we manipulated aphid numbers, stimulated them to produce a secretion, and monitored nearby conspecifics. Late stage juvenile or adult soybean aphids from the lab colony were placed together on a single unifoliate leaf of a V1 or V2 (first or second set of trifoliate leaves expanded) soybean plant at approximate densities of 5, 10, 15, 20, and 25 individuals (n = 8 each). A 50 mm clip cage was placed over the leaf, allowing the contained aphids to move about the upper and lower surface. This experiment was performed twice, once on 22 January 2014 and again on 5 February 2014.
After six days the clip cages were removed and the leaf was clipped from the plant. During this time aphid numbers had naturally increased to <5–130+ per leaf. Because the effective range of alarm pheromones from aphid cornicle secretions is limited (Nault et al. 1973), we focused our observations of secretion and alarm reaction behavior on aphids within a small (~15 mm diameter) area and maintained 15X magnification on the leaf in this area. We counted the total number of “large” (putative 3rd-4th instar and adults) and “small” (putative 1st-2nd instar) aphids within view, haphazardly selecting a single late (3rd-4th) instar juvenile “focal” soybean aphid in the center of the field of view to be stimulated. We observed only these nearby aphids since their numbers were highly correlated with the total number of aphids on the leaf, and the effective range of EBF is rather limited (1–3 cm; Nault et al. 1973). Using the 00 stainless steel insect pins the focal aphid was jabbed in the thorax from directly above. Following this attack we recorded the presence of any secretion from one or both of the focal aphid’s cornicles, then recorded signs of altered or alarm reaction behavior in the other surrounding aphids for 60 s.
Data Analysis
We used a Pearson’s Chi-Squared test to compare the cornicle secretion response of soybean aphids alone and in a small group. Aphids were classified as either “single” (n = 60) or “grouped” (n = 53) and we compared the observed frequency of response to the frequency of response expected if treatment was not important.
For both the alarm pheromone and density response trials, we used logistic regression to determine if the probability of aphid alarm reaction behavior and cornicle secretion production differed among treatments, treating a positive event (“alarm” or “secrete”) as a success, and its alternative (“no alarm”, “no secrete”) as a failure. For the alarm pheromone trials we compared the likelihood of alarm reaction behavior in our two treatments. In the density trials, the number of nearby aphids (i.e. within view at 15X magnification) was used as a continuous proportion of “successes” (aphid alarmed) versus “failures” (non-alarmed aphid) that were assessed independently for each replicate, and a categorical factor of “date” (22 January or 5 February) was used as a factor. In both analyses we used the function “glm” within the “stats” package, in the case of the alarm reaction behavior analysis adding a further categorical factor of “secrete” (yes/no) and using a quasibinomial error distribution due to overdispersion of variance.
All statistical analyses were performed using R v2.13.2 (R Development Core Team 2013).
Results
Identification of Alarm Pheromone
We detected E-β-farnesene (EBF) in the bodies and cornicle secretions of soybean aphids. Furthermore, soybean aphids displayed alarm reaction behavior 30 % of the time when exposed to synthetic EBF, while only showing some kind of alarm reaction behavior 15 % of the time when exposed to the hexane control (t 1,23 = 2.44, P = 0.023).
Individual Vs. Group Experiment
Soybean aphids are less likely to secrete when alone than when in a group (χ2 = 7.38, df = 1, P = 0.007; Fig. 1). From a total of 60 isolated soybean aphids only 10 (17 %) produced a cornicle secretion. However, when in a small group the proportion of aphids producing a secretion increased to over 40 % (22 of 53).
Proportion of soybean aphids producing a cornicle secretion when they are alone as compared to when they are in groups of 5–10 individuals. “Single” aphids experienced a simulated natural enemy “attack” when alone while “group” aphids were surrounded by a small cluster of conspecifics (8–10 individuals) at the time of “attack”
Group Density Experiment
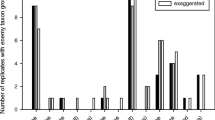
Soybean aphid density had no effect on either the probability of cornicle secretion release after stimulation (t 1,77 = 0.59, P = 0.56; Fig. 2), or on the probability of neighboring conspecifics to exhibit alarm reaction behavior (t 1,76 = 1.55, P = 0.13; Fig. 3). At all aphid densities the proportion of focal aphids releasing a cornicle secretion after being jabbed was consistently ~50 % regardless of aphid density. However, following the release of a cornicle secretion by a nearby aphid, alarm reaction behavior was almost always observed (t 1,76 = 6.45, P < 0.0001; Fig. 3), with approximately 20 + % of all nearby aphids demonstrating some alarm reaction behavior regardless of aphid density. When the focal aphid did not produce a cornicle secretion, alarm reaction behavior occurred <5 % of the time (Fig. 3).
Relationship between soybean aphid density and the probability of an “attacked” aphid to produce a cornicle secretion. The bars are a histogram illustrating the frequency of secretion as a function of aphid density with “no” the bottom bars and “yes” the top bars: frequency scale on right y-axis. The dashed line is the aerage probability of releasing a cornicle secretion as a function of aphid density (NS): probability scale on left y-axis
Probability of soybean aphids exhibiting alarm reaction behavior following the release of a cornicle secretion by a neighboring conspecific. Filled circles are instances in which no cornicle secretion was released by the focal aphid, while open circles are cases in which there was a cornicle secretion release. The long dashed line is the model fit to the “no secretion” data, while the short dashed line is the model fit to the “yes secretion” data
Discussion
We found that soybean aphid cornicle secretions do contain EBF, and that while they are more likely to secrete from their cornicles at densities of 10 neighboring aphids than if alone, the frequency of cornicle secretions and alarm reaction behavior is not related to conspecific density above that level. Our first experiments demonstrated that like many other aphid species like the green peach aphid and the pea aphid (Vandermoten et al. 2012), soybean aphids emit EBF as part of their alarm pheromone. While EBF may not be the only chemical used as part of the alarm pheromone, we found that soybean aphids do respond to pure synthetic EBF. This confirms previous suggestions about soybean aphid cornicle secretions containing alarm pheromone (Butler and O’Neil 2006).
Additional experiments investigated if behaviors related to cornicle secretion depend on how many conspecific aphids are nearby. While we found that soybean aphids are more likely to produce cornicle secretions when in a group than when alone, we did not find any difference in the likelihood that soybean aphids produce or respond to cornicle secretions when in the company of 5–50+ conspecifics. Despite aphid density having little effect, we found a consistent and congruent response by individual soybean aphids to the release of a nearby cornicle secretion, just as we had with synthetic EBF; in both cases the likelihood of observing alarm reaction behavior was ~20–30 %.
We expected that elicitation of cornicle secretion could depend on conspecific density because of the variable costs (Nelson 2007) and benefits associated with this behavior (Butler and O’Neil 2006; Wu et al. 2010). Conspecific density is important to alarm reaction behavior in other aphid species. For example, pea aphids raised individually produce less EBF than those raised in groups (Verheggen et al. 2009), and green peach aphids in groups are more sensitive to EBF than single aphids (Montgomery and Nault 1978). Similar to our results, Wu et al. (2010) found that another aphid species, the English grain aphid, Sitobion avenae, was more likely to smear parasitoids with cornicle secretions in moderate sized groups (6–12 aphids) than in a pair of aphids. However, they did not examine responses at aphid density exceeding 12 individuals, and it was at this point in our study that the effect of aphid density on cornicle secretions seemed to plateau. These results suggest that the alarm reaction behaviors of soybean and other aphid species using EBF as an alarm pheromone like the green peach and pea aphids may be sensitive to conspecific density only at a relatively low range of densities. Possible explanations for this pattern include the inability of aphids to determine conspecific density beyond a limited distance, or the irrelevance of aphids outside the limited range of EBF (Nault et al. 1973).
Besides conspecific density, numerous other factors outside the scope of our study can also influence aphid cornicle secretion and alarm response behavior. Cornicle secretion and EBF production in pea aphids occurs most frequently in the late juvenile stages of development when conspecific numbers are usually high (Mondor et al. 2000). Host plant quality moderates aphid defense behaviors, with increasing physiological stress leading to lower rates of alarm reaction behavior (Villagra et al. 2002). At high concentrations of carbon dioxide aphid alarm pheromone responses are muted (Awmack et al. 1997), while transgenic plants engineered to release EBF stimulate aphid activity (Beale et al. 2006). Even the species identity of the natural enemy may influence the amount of EBF released following an attack (Joachim and Weisser 2013). Further complicating matters, a recent study shows that chronic EBF exposure causes aphid alarm reaction behaviors to be lost or regained in as few as three generations, suggesting that aphid gene expression rather than genetic mutation is responsible for inter-generational plasticity of aphid defense behaviors (de Vos et al. 2010). While expressing itself as a rather simple set of behaviors, alarm signaling in aphids is much more complex set within a specific ecological, environmental, and physiological context. It is possible that conspecific density, with weak effects in the context that were examined in our study, might interact with one or more of these other factors (e.g. plant quality or carbon dioxide levels) and be more important in other ecological circumstances.
Many aphids are important agricultural pests that can use cornicle secretion defensive behavior to combat natural enemy attacks and warn nearby conspecifics of danger. Because of their ubiquity and ability to inflict significant crop damage, predicting or defeating aphid defenses may prove valuable. Soybean aphids, a common soybean pest in Asia and North America, uses a common aphid alarm pheromone (EBF) to warn its conspecific neighbors of potential attack, but this behavior is mostly unaffected by local aphid density. Together with the relatively low proportion of secreting aphids under any condition, this insight suggests that the cost of cornicle secretion in soybean aphids frequently outweighs the benefits.
References
Awmack C, Woodcock C, Harrington R (1997) Climate change may increase vulnerability of aphids to natural enemies. Ecol Entomol 22:366–368. doi:10.1046/j.1365-2311.1997.00069.x
Beale MH, Birkett MA, Bruce TJA, et al. (2006) Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc Natl Acad Sci 103:10509–10513. doi:10.1073/pnas.0603998103
Butler CD, O’Neil RJ (2006) Defensive response of soybean aphid (Hemiptera: Aphididae) to predation by insidious flower bug (Hemiptera: Anthocoridae). Ann Entomol Soc Am 99:317–320. doi:10.1603/0013-8746(2006)099[0317:DROSAH]2.0.CO;2
de Vos M, Cheng WY, Summers HE, et al. (2010) Alarm pheromone habituation in Myzus persicae has fitness consequences and causes extensive gene expression changes. Proc Natl Acad Sci 107:14673–14678. doi:10.1073/pnas.1001539107
Development Core Team R (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Diaz-Montano J, Reese JC, Louis J, et al. (2007) Feeding behavior by the soybean aphid (Hemiptera: Aphididae) on resistant and susceptible soybean genotypes. J Econ Entomol 100:984–989. doi:10.1093/jee/100.3.984
Du Y, Poppy GM, Powell W, et al. (1998) Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol 24:1355–1368. doi:10.1023/A:1021278816970
Francis F, Vandermoten S, Verheggen FJ, et al. (2005) Is the (E)-b-farnesene only volatile terpenoid in aphids? J Appl Entomol 129:6–11
Ives AR, Kareiva P, Perry R (1993) Response of a predator to variation in prey density at three hierarchical scales lady beetles feeding on aphids. Ecology 74:1929–1938
Joachim C, Weisser WW (2013) Real-time monitoring of (E)-β-farnesene emission in colonies of the pea aphid, Acyrthosiphon pisum, under lacewing and ladybird predation. J Chem Ecol 39:1254–1262. doi:10.1007/s10886-013-0348-2
Mondor EB, Roitberg BD (2003) Age-dependent fitness costs of alarm signaling in aphids. Can J Zool 81:757–762. doi:10.1139/z03-053
Mondor EB, Baird DS, Slessor KN, Roitberg BD (2000) Ontogeny of alarm pheromone secretion in pea aphid, Acyrthosiphon pisum. J Chem Ecol 26:2875–2882. doi:10.1023/A:1026402229440
Montgomery ME, Nault LR (1978) Effects of age and wing polymorphism on the sensitivity of Myzus persicae to alarm pheromone. Ann Entomol Soc Am 71:788–790
Nault LR, Edwards LJ, Styer WE (1973) Aphid alarm pheromones: secretion and reception. Environ Entomol 2:101–105
Nelson EH (2007) Predator avoidance behavior in the pea aphid: costs, frequency, and population consequences. Oecologia 151:22–32. doi:10.1007/s00442-006-0573-2
Pickett JA, Griffiths DC (1980) Composition of aphid alarm pheromones. J Chem Ecol 6:349–360. doi:10.1007/BF01402913
Pickett JA, Wadhams LJ, Woodcock CM, Hardie J (1992) The chemical ecology of aphids. Annu Rev Entomol 37:67–90. doi:10.1146/annurev.en.37.010192.000435
Ragsdale DW, Voegtlin DJ, O’Neil RJ (2004) Soybean aphid biology in North America. Ann Entomol Soc Am 97:204–208. doi:10.1603/0013-8746(2004)097[0204:SABINA]2.0.CO;2
Ragsdale DW, Landis DA, Brodeur J, et al. (2011) Ecology and Management of the Soybean Aphid in North America. Annu Rev Entomol 56:375–399. doi:10.1146/annurev-ento-120709-144755
Roitberg BD, Myers JH (1978) Adaptation of alarm pheromone responses of the pea aphid Acyrthosiphon pisum (Harris). Can J Zool 56:103–108. doi:10.1139/z78-014
Strong FE (1967) Observations on aphid cornicle secretions. Ann Entomol Soc Am 60:668–673
Vandermoten S, Mescher MC, Francis F, et al. (2012) Aphid alarm pheromone: an overview of current knowledge on biosynthesis and functions. Insect Biochem Mol Biol 42:155–163. doi:10.1016/j.ibmb.2011.11.008
Verheggen FJ, Haubruge E, Moraes CMD, Mescher MC (2009) Social enviroment influences aphid production of alarm pheromone. Behav Ecol 20:283–288. doi:10.1093/beheco/arp009
Villagra CA, CC Ramı́rez, HM N (2002) Antipredator responses of aphids to parasitoids change as a function of aphid physiological state. Anim Behav 64:677–683. doi:10.1006/anbe.2002.4015
Wrona FJ, Dixon RWJ (1991) Group size and predation risk: a field analysis of encounter and dilution effects. Am Nat 137:186–201
Wu G-M, Boivin G, Brodeur J, et al. (2010) Altruistic defence behaviours in aphids. BMC Evol Biol 10:19. doi:10.1186/1471-2148-10-19
Acknowledgments
The authors thank members of the Harmon and Prischmann-Voldseth Labs at NDSU for valuable insights and material/logistic support. J. Kopco and A. Valls aided in the EBF trials. Funding for this research was provided in part by the North Dakota Soybean Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eichele, J.L., Dreyer, J., Heinz, R. et al. Soybean Aphid Response to their Alarm Pheromone E-ß-Farnesene (EBF). J Insect Behav 29, 385–394 (2016). https://doi.org/10.1007/s10905-016-9567-z
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-016-9567-z