Abstract
Insect enemies use several environmental cues for host or prey finding. In insects these cues are often chemical, deriving from the host plant or from the prey itself. The aphid alarm pheromone (E)-β-farnesene (EBF) that is emitted by aphids when attacked by a predator is believed to be such a cue, as it has been shown to be perceived by several aphid enemies. It is unclear, however, if EBF is used as an arrestant stimulus or a cue for short-range prey localization, i.e., attractant stimulus, on the plant. We observed the searching behavior of larvae of two aphid predators, lacewing (Chrysoperla carnea (Stephens), Neuroptera—Chrysopidae) and ladybird (Coccinella septempunctata L., Coleoptera—Coccinellidae), on a plant where an aphid was fixed, in the presence and absence of EBF, and under field and laboratory conditions. EBF had no effect on predator searching behavior, either when natural amounts of 50 ng EBF or unnaturally high amounts of 1,000 ng were used. EBF also did not induce longer predator patch residence times under laboratory (ladybird only: 600.8 ± 35.1 s) and field (ladybird: 644.9 ± 50.7 s, lacewing: 1,108.4 ± 49.5 s) conditions. Predators found the aphid on the plant within the allocated time in only 34.72 and 17.13 % of the cases in the laboratory and field, respectively, but the presence of EBF did not increase the foraging success. We conclude that aphid alarm pheromone is not used as an arrestant cue for these important aphid predators nor does it have a short-range attractant function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predators and parasitoids utilize various cues to localize their herbivore prey or host (Jervis 2005). These cues can be visual, auditory or mechanical, but in insects they are predominantly chemical (Carde and Bell 1995; Fellows et al. 2005). For insect enemies, potential chemical cues on which they can eavesdrop include prey pheromones and allelochemicals, i.e., compounds mediating interactions between the prey and other species, such as herbivore-induced plant volatiles (Agelopoulos et al. 1999; Fellows et al. 2005).
When searching for a host or prey (henceforth prey), insect enemies follow a hierarchical behavioral pattern that includes two steps before a prey is encountered (Vinson 1976): the first step is the prey habitat localization, i.e., long-range localization of the habitat where prey might be present. In case of insect herbivores that only feed on one plant species, a single plant individual may be the habitat. The second step is prey localization, i.e., short-range localization of the prey itself within the habitat, e.g., on the plant (Hatano et al. 2008b). In addition to the dichotomy of short-range and long-range localization, Fellows et al. (2005) described the dichotomy of attractant vs. arrestant stimuli. Attractant stimuli direct the searching forager to areas containing prey, independent of the range over which the forager is attracted. In contrast, the perception of arrestant stimuli results in a reduction in distance or area covered per unit of time, e.g., it may increase the residence time of a forager in the area where the stimulus is detected (Fellows et al. 2005). For example, Noldus et al. (1991) described arrestant effects of the sex pheromones of noctuid hosts for two parasitoid wasp species of the genus Trichogramma. In the experiment, natural emission amounts of the host’s sex pheromone induced higher patch residence times and prolonged locomotion of wasps compared to a set-up with clean air. As another example, aphid honeydew has been shown to act as both, an attractant for habitat localization (Saad and Bishop 1976) as well as a chemical arrestant stimulus for ladybird larvae that intensify their search in the prey patch in response to honeydew contact (Carter and Dixon 1984).
In many aphid species (Hemiptera: Aphididae), (E)-β-farnesene (EBF) is the only alarm pheromone component (Francis et al. 2005) and although it has the benefit of warning colony members of impending danger, EBF emission has been proposed to carry the cost of attracting aphid enemies (cf. Hatano et al. 2008b; Vandermoten et al. 2012). Several electroantennogram (EAG) studies and olfactometer assays demonstrate that a great variety of aphid enemies are capable of perceiving EBF or display a behavioral response to EBF presence (Hatano et al. 2008b). The evidence that EBF is used by predators or parasitoids as an attractant or arrestant stimulus is, however, weak, for a number of reasons. One problem is the low emission rate of EBF. While recent studies found that the EBF amounts released by individual aphids after an attack can range from less than 1 ng up to 50 ng (Schwartzberg et al. 2008; Joachim et al. 2013) with no further amplification by colony members (Hatano et al. 2008a; Verheggen et al. 2008), older studies on the attractant role of EBF used much higher amounts in their experiments, raising doubts about the biological significance. For example, Zhu et al. (1999) conducted EAG studies and showed that both the ladybird Coleomegilla maculata (Degeer) and the lacewing Chrysoperla carnea (Stephens) were able to perceive EBF, with increasing sensitivity in the range from 1 to 1,000 µg EBF, with the highest sensitivity at 1,000 µg. Thus, even the lowest concentration used was at least 20 times higher than the maximum amount released by aphids, a very unlikely scenario, since attacked aphids generally do not always emit maximum EBF amounts and the aphid colony would have dispersed after such frequent attacks (Minoretti and Weisser 2000; Joachim et al. 2013). In addition, EAG responses only give information of a predator’s potential to use EBF as a host-finding cue, rather than revealing if the predator actually employs EBF as a chemical signal in prey search. Results from EAG studies on EBF have also been shown to fail in correctly predicting the behavior of aphid enemies in the field (cf. Zhu et al. 1999).
In contrast to EAG studies, olfactometer assays reveal behavioral responses to a chemical signal, but also here the amounts of EBF used are often well above natural emission rates. For example, Micha and Wyss (1996) found that naive females of the parasitoid Aphidius uzbekistanicus Luzhetski showed attractant behavior to EBF in an olfactometer, but only at concentrations of 5.7 µg and not for lower concentrations. Further, Francis et al. (2004) found an attractant role of EBF only for amounts above 2 µg for larvae and adults of the ladybird Adalia bipunctata L.–concentrations more than 40 times higher than the maximum emitted amounts measured in aphids attacked by a predator. It is unclear whether reactions found for very high doses have any implications for aphid enemy behavior under natural conditions.
Another problem is that studies so far have rarely distinguished between the potential attractant and potential arrestant role of EBF. While EAG studies cannot distinguish between these two possibilities, olfactometers and wind tunnels (Du et al. 1998; Zhu et al. 1999; Al Abassi et al. 2000; Verheggen et al. 2007) focus on the role as an attractant stimulus. It is thus unknown if EBF can act as an arrestant stimulus, thus triggering an aphid enemy to stay and search longer or more intensively within a habitat.
In this paper, we investigate the influence of EBF on the foraging behavior of two different aphid predators on a plant. Here, in contrast to former studies on the kairomone effect of EBF, we will ascertain if EBF concentrations that naturally occur in aphid–predator interactions evoke a behavioral change in aphid predators. Further, we addressed the following questions under both laboratory and field conditions since laboratory studies do not always reflect natural conditions: (1) does EBF act as an arrestant stimulus for aphid predators? (2) Is short-range prey localization in aphid predators faster in the presence of EBF, i.e., does it serve as an attractant stimulus?
Materials and methods
Experimental plants and animals
Identical rearing conditions were established for aphids and predators (20 °C, 75 % humidity, photoperiod: L16:D8). Experiments were conducted with red clones of the pea aphid Acyrthosiphon pisum Harris (Hemiptera—Aphididae), originally collected in Bayreuth, Germany. Aphids were reared on two-week-old broad bean plants, Vicia faba L. (The Sutton; Nickerson-Zwaan, UK). To avoid aphid escape, plants were covered with air-permeable cellophane bags (Unipack GmbH, Germany). A split-brood design was employed according to Kunert et al. (2005). By distributing individuals from one line equally among treatments, any variation due to rearing conditions is equally distributed over all treatments. To do so, we established 22 lines for the laboratory experiment (experiment 1) and 36 lines for the field experiment (experiment 2) by placing 22 and 36 adult foundress aphids (F 0 generation), randomly collected from a single population consisting of the same clone, on 22 or 36 bean plants, respectively, where they were allowed to reproduce for 24 h. After 8–9 days, the offspring (F 1 generation) reached the adult stage. For each line, one F 1 individual was selected and transferred to a new plant where it was allowed to reproduce for 24 h. The resulting offspring (F 2 generation) were eventually used for the experiments. A split-brood design was achieved for each experiment by choosing for all lines 1 F 2 individual for each EBF treatment. So, one aphid line was used once for each treatment.
Predators were chosen to show differences in foraging strategies. Lacewing larvae, Chrysoperla carnea (Stephens) (Neuroptera—Chrysopidae), are piercing-sucking predators, slowly consuming their prey and ladybird larvae, Coccinella septempunctata L.(Coleoptera—Coccinellidae), are chewing predators, quickly consuming their prey. Predators were obtained from a commercial supplier (Katz Biotech AG, Germany). Lacewings were reared on bean plants infested with pea aphids until they reached the 3rd instar, at which point they were used in the experiments. Hatching larvae of the seven-spot ladybird were reared individually in Petri dishes with sufficient pea aphid supply until they reached the 4th instar, at which point they were used in the experiments. All predators were starved 1.5 h (laboratory experiment) and 24 h (field experiment) before each experiment, respectively.
General experimental setup
For the behavioral assay “The Observer XT” Version 10.5 (Noldus Information Technology, Netherlands) was run on a laptop PC. Predator behavior was classified as follows: Search—walking or foraging on the plant, Stop—resting or cleaning on the plant without moving, Encounter—the predator comes in physical contact with the aphid prey, and Walk Off—the predator leaves the plant.
Each predator was observed until it encountered the aphid, until it left the plant, or until the maximum time was reached (15 min, hence 900 s, for experiment 1; 25 min, hence 1,500 s, for experiment 2), whatever came first. Longer observation times were chosen for in experiment 2 to account for abiotic factors, such as wind, and hence the second application of alarm pheromone (see below).
The following target, i.e., response, variables were used for analysis: (1) experimental outcome, the frequency of replicates in which the predator either found the prey, left the plant before the end of the experiment (900/1,500 s) or stayed on the plant for the whole time of the experiment—hence three possible outcomes; (2) foraging success, the frequency of replicates in which the predator found the aphid on the plant; (3) patch residence time, the duration of an replicate in seconds before the observation ended. In cases where the predator found the aphid prey, we refer to the patch residence time also as time until prey encounter. In case the predator left the plant without encountering the aphid and before the experimental time had ended we refer to the patch residence time also as time until the predator left the plant. If the predator stayed on the plant for the full 15/25 min without finding the aphid or leaving the plant a patch residence time of 900/1,500 s was noted as patch residence time. (4) Predator search time, the duration in seconds a predator was in motion, hence walking or searching for potential prey, not resting during a replicate; (5) Predator rest time, the duration in seconds a predator was motionless on the plant, not walking during a replicate.
Experiment 1—predator search behavior in the laboratory
Experiment 1 tested the influence of different EBF concentrations (EBF treatment), as evaporated from a filter paper, on the foraging behavior of ladybird predators under laboratory conditions. One aphid was glued by its hind legs close to the leaf edge of the 2nd leaf pair (as seen from the base) of three- to four-week-old Vicia faba plants (three to five leaf pairs) and 1.5 cm away from the leaf stem using glue (UHU Alleskleber 45015 (all purpose adhesive), UHU GmbH & Co. KG, Germany) 1.5 h before a predator was introduced to the plant. Although pea aphids are known to feed on the underside of the leaf, close to the leaf petiole at (c.f. Keiser et al. 2013), due to an enhanced visibility aphids were fixed to the upper side of the leaf, but close the petiole in the experiments. While the glue mainly consists of methyl acetate, and also ethanol and acetone, the delay time before the start of the experiment allowed for evaporation of the solvents and hence solidification of the adhesive to an inert, physiologically indifferent and neutral drop. After they have been glued to the plant, at the start of each replicate, the aphids were all alive, looked healthy (no signs that the glue had an influence on the physiological appearance) and predominantly continued feeding (i.e., the aphid's stylet pierced the plant tissue).
Each plant with an aphid was subjected to one of three levels of the EBF treatment: (1) control (hexane), (2) low EBF amount (50 ng), and (3) high EBF amount (1,000 ng). Treatment three was established to check for possible overdose effects. Two plants, each within their flowerpot and with a fixed aphid, were placed in a no-choice experiment, on a lab table at a distance of ~50 cm to each other to prevent any confounding effects from EBF application or aphid presence in the neighboring treatment. A piece of filter paper (0.5 × 1 cm), attached to a wooden stick, was placed close (~1–2 cm) to the aphid without touching the plant. Alarm pheromone solutions were created in hexane (Carl Roth Germany, 99.8 %) at an EBF concentration of 12.5 and 250 µg mL−1 (Bedoukian Research Inc., USA). For each treatment, 4.0 µL of the respective solution, hence 50 and 1,000 ng EBF, respectively, was applied with a syringe to the filter paper at the start of each replicate. So, EBF was released by the filter paper during the experiment, as demonstrated by Joachim and Weisser (2013). After EBF application, one predator was carefully set free from a Petri dish (5 cm in diameter) at the base of the plant stem. The behavioral monitoring started at the same time as the predator release.
Two replicates of the same treatment were observed simultaneously, to increase the sample size. Each treatment was replicated at least 16 times, resulting in N = 54 total replicates.
Experiment 2—predator search behavior in the field
Experiment 2 was set up similar to experiment 1, with some modifications. In this experiment we used two different predator species, lacewings and ladybirds (predator treatment). Because the experiment was carried out under field conditions, some parameters were changed to account for the particular abiotic factors, including wind. One aphid was glued by its hind legs using glue to the base of a leaf of the uppermost leaf-pair of two-week-old Vicia faba plants (three leaf pairs), as mentioned above. After they have been glued to the plant, at the start of each replicate, the aphids were all alive, looked healthy (no signs that the glue had an influence on the physiological appearance) and predominantly continued feeding (i.e., the aphid's stylet pierced the plant tissue).
Each plant with an aphid was subjected to one of three levels of the EBF treatment: (1) control (methanol), (2) low EBF amount (2 × 50 ng EBF), and (3) high EBF amount (2 × 500 ng). Treatment three was again established to check for possible overdose effects. Further, in contrast to experiment 1, EBF application was done twice to counteract EBF drift and fast EBF evaporation due to a slight but permanent breeze. Thus, in experiment 2, there were three EBF treatments × two predator treatments, i.e., six treatment combinations in a no-choice experiment. Methanol was used in contrast to experiment 1 since preliminary experiments showed that hexane might influence the behavior of additional aphid enemies, such as ants. As experiment 2 was conducted in the field with the possibility of attracting additional predators, a different solvent was chosen to exclude potential hexane effects on other predators.
Six plants, each within their flowerpot and with a fixed aphid, were placed in an experimental field site in Freising, Germany (fallow grassland, geographic coordinates: latitude, longitude: 48.405191, 11.6910). Plants were placed on the ground at a distance of ~40 cm to each other in an open semicircle.
Thus, to be able to observe them all at the same time, the plants were arranged slightly closer to each other as in experiment 1. Preliminary behavioral observations revealed that this distance seems to be still adequate to prevent any confounding effects from EBF application or aphid presence in the neighboring treatment since EBF is only applied in small amounts, in the presence of wind, and is believed to degrade fast, thus having an impact only at a local scale (Kourtchev et al. 2009). Further, when predators left the plant, observations showed that their search was not instantly directed to plants with (higher) EBF concentration. A piece of filter paper (0.5 × 1 cm), attached to a wooden stick, was placed near to the aphid in the distance of 1–2 cm without touching the plant. Alarm pheromone solutions were created in methanol (Carl Roth Germany, 99.8 %) at an EBF concentration of 100 and 1,000 µg mL−1, respectively. For each treatment 0.5 µL of the respective solution was applied with a syringe to the filter paper at the start of each replicate and again after 10 min. So, EBF was released by the filter paper during the experiment, as demonstrated by Joachim and Weisser (2013). Subsequently the predators, each kept individually in a Petri dish (5 cm in diameter) were carefully set free at the base of the plant stems. The behavioral monitoring started at the same time as the predator release. All six treatment combinations were observed simultaneously and the block was replicated 36 times, resulting in N = 216 total replicates.
Statistical analysis
Data were analyzed using the R software 3.0.1 (www.r-project.org). All data are presented as mean ± standard error (SE).
The count data for frequencies of occurrences, i.e., the variables of experimental outcome and foraging success, were analyzed following Crawley (2007): after fitting saturated generalized linear models (GLM) with a Poisson distribution to account for count data, the interaction of interest was removed and the updated model was compared with the saturated model using analysis of variance (ANOVA) with a Chi-squared test. The interaction of interest was either the influence of the treatment or the influence of the predator species on the experimental outcome or foraging success. For example, in experiment 2, the interaction between predator species, EBF concentration and foraging success was tested first by removing it from the saturated model, i.e., it was tested if foraging success of the predator depended on the interaction between predator and EBF concentration. In the same way we proceeded to remove further terms where necessary.
Patch residence time, time until prey encounter, time until the predator left the plant, the predator search time, and predator rest time were compared using GLMs with model simplification, i.e., stepwise backward selection of independent variables to obtain the minimal adequate model. Due to the time-bound nature of the data, a Gamma distribution with an ‘inverse’ link function was used.
Results
Experiment 1—predator search behavior in the laboratory
When only predator foraging success was considered (i.e., aphid found/not found), there was no effect of the EBF treatment on the frequencies an aphid was found on the plant or not (GLM: χ 2 = 2.48, df = 2, P = 0.290).
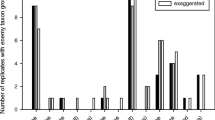
When all experimental outcomes were considered, i.e., the frequencies of whether a predator found the prey, left the plant before the end of the replicate time or searched unsuccessfully for the whole 900 s of the experiment, there was also no effect of the EBF treatment on the outcome (GLM: χ 2 = 3.12, df = 4, P = 0.539, Fig. 1a).
Behavior of ladybird predators as a function of the presence of aphid alarm pheromone in experiment 1 (laboratory experiment) in which a ladybird larva was placed on plants where an aphid was fixed. EBF treatments: low = 50 ng, high EBF = 1,000 ng, control = 0 ng, N = 54. a Outcome of the experiment. Full bars—successful search (encounter), i.e., the predator found the aphid on the plant. Gray bars—the predator left the plant before the experimental run time of 900 s had elapsed without having encountered the aphid (leave). Open bars: the predator stayed on the plant for the entire experimental period of 900 s but did not encounter the aphid (stay). b Patch residence time of replicates in which the ladybird predator encountered the aphid (time until prey encounter, N = 16), (c) patch residence time of the predator for replicates in which the ladybird predator left the plant before the maximum time (900 s) had elapsed, without finding the aphid (time until the predator left the plant, N = 15). Box plots show the median value (solid line), the 25 and 75th percentile; the error bars below and above the box indicate the 10 and 90th percentile, respectively. Black dots indicate outliers. ns not significant
Patch residence time of ladybirds was 612.6 ± 41.6 s and not affected by the EBF treatment (GLM: F 2,51 = 0.45, P = 0.642). In trials where the predator found the prey, the time until prey encounter was 415.9 ± 65.7 s and also not affected by the presence of EBF (GLM: F 2,13 = 0.09, P = 0.913, Fig. 1b). For replicates where the predator did not encounter the prey item and left the plant before 15 min had elapsed, the time until the predator left the plant was 335.1 ± 49.0 s, also not affected by the EBF treatment (F 2,12 = 1.68, P = 0.240, Fig. 1c).
Ladybird search time was 297.6 ± 26.9 s and not affected by the EBF treatment (GLM: F 2,51 = 0.92, P = 0.405). Predator rest time was 314.3 ± 37.3 s and also not influenced by the EBF treatment (GLM: F 2,51 = 0.03, P = 0.973).
Experiment 2—predator search behavior in the field
Overall, the outcome of the field experiment was similar to the laboratory experiment.
Under field conditions, the aphid prey was also rarely found by the predator. In only 37 out of 216 replicates (17.13 %), the prey was encountered by the predator, half as often as under laboratory conditions. This foraging success, i.e., whether an aphid was found on the plant or not, did not depend on the interaction between predator species and EBF treatment (GLM: χ 2 = 1.58, df = 2, P = 0.453). The main effects, predator treatment (GLM: χ 2 = 2.67, df = 3, P = 0.102) and EBF treatment (GLM: χ 2 = 0.45, df = 4, P = 0.797), were also not significant.
When all experimental outcomes were considered, i.e., the frequencies of whether a predator found the prey, left the plant before the 1,500 s had elapsed, or stayed on the plant for the entire time, there was a difference between the predator species (GLM: χ 2 = 42.71, df = 6, P < 0.001, Fig. 2): lacewings stayed on the plant more often for the entire time period while ladybirds left the plant more often before the experimental time elapsed. This was independent of the EBF treatment (main effect EBF: GLM: χ 2 = 2.39, df = 8, P = 0.665, interaction predator treatment × EBF treatment GLM: χ 2 = 1.29, df = 4, P = 0.864).
Behavior of ladybird and lacewing predators as a function of the presence of aphid alarm pheromone in experiment 2 (field experiment) in which a predator larva was placed on plants where an aphid was fixed. EBF treatments: low = 2 × 50 ng = 100 ng, high = 2 × 500 ng = 1,000 ng, control = 2 × 0 ng. N = 36 (per predator and treatment). Full bars—successful search (encounter), i.e., the predator found the aphid on the plant. Gray bars—the predator left the plant before the experimental run time of 900 s had elapsed without having encountered the aphid (leave). Open bars: the predator stayed on the plant for the entire experimental period of 1,500 s but did not encounter the aphid (stay)
Patch residence times of ladybirds and lacewings were 644.9 ± 50.7 s and 1,108.4 ± 49.5 s, respectively, and differed between the predator species (GLM: F 1,212 = 34.88, P > 0.001), but were not affected by the EBF treatment (GLM: F 2,213 = 0.13, P = 0.882). The interaction between EBF and predator treatment was also not significant (GLM: F 2,210 = 0.19, P = 0.828).
In replicates where the predator found the aphid, the time until prey encounter did not differ between predator species (GLM: F 1,33 = 1.54, P = 0.224). There was, however, a slight effect of the EBF treatment on the time until prey encounter, but in the opposite direction as expected (GLM: F 2,34 = 4.30, P = 0.023, Fig. 3a): aphids were found after 549.6 ± 91.5 s in the control treatment, 615.7 ± 152.3 s at low EBF concentrations, and 986.5 ± 113.9 s at high EBF concentrations, i.e., the predators searched longer until the aphid was found when more EBF was present in the headspace of the plant. The interaction between predator treatment and EBF treatment was also significant (GLM: F 2,31 = 4.30, P = 0.023): lacewings encountered aphids after longer time periods than ladybirds in the presence of EBF, but not in the control.
The time until a predator left the plant (replicates where the predator did not encounter the aphid and left the plant before 25 min had elapsed, Fig. 3b) did not differ between ladybirds (438.2 ± 41.8 s) and lacewings 579.9 ± 79.6 s (GLM: F 1,100 = 2.57, P = 0.112). There was also no effect of EBF treatment (GLM: F 2,101 = 2.76, P = 0.068) and the interaction was also not significant (GLM: F 2,98 = 0.11, P = 0.894).
Predator search time was 343.5 ± 28.5 s and 303.9 ± 19.0 s for ladybirds and lacewings, respectively, and did not differ between predator species (GLM: F 1,214 = 1.42, P = 0.235) or between EBF treatments (GLM: F 2,212 = 0.61, P = 0.543). The interaction of EBF and predator treatments was also not significant (GLM: F 2,210 = 0.82, P = 0.442). Predator rest time was much shorter for ladybirds (313.4 ± 42.8 s) than for lacewings (838.4 ± 44.6 s, GLM: F 1,212 = 43.11, P < 0.001). There was no influence of the EBF treatment on predator rest time (GLM: F 2,213 = 0.09, P = 0.915) and the interaction between EBF and predator treatment was also not significant (GLM: F 2,210 = 0.495, P = 0.611).
Behavior of ladybird and lacewing predators as a function of the presence of aphid alarm pheromone in experiment 2 (field experiment) in which a predator larva was placed on plants where an aphid was fixed. EBF treatments: low = 2 × 50 ng = 100 ng, high = 2 × 500 ng = 1,000 ng, control = 2 × 0 ng. a Patch residence time of replicates in which the ladybird and lacewing predator encountered the aphid (time until prey encounter, N = 37), (b) patch residence time of the predator for replicates in which the ladybird and lacewing predator left the plant before the maximum time (1,500 s) had elapsed, without finding the aphid (time until the predator left the plant, N = 104). Box plots show the median value (solid line), the 25 and 75th percentile; the error bars below and above the box indicate the 10 and 90th percentile, respectively. Black dots indicate outliers. ns not significant
Discussion
Our study suggests that lacewings and ladybirds do not use the aphid alarm pheromone (E)-β-farnesene (EBF) as an environmental foraging cue to increase their foraging efficiency on the plant; in particular, it does not serve as either an arrestant or an attractant stimulus (Fellows et al. 2005; Purandare and Tenhumberg 2012). In both our laboratory and our field experiment, none of the different aspects of predator foraging behavior, in particular patch residence time and the chance of finding the aphid, was affected by the presence of EBF, either when predators were exposed to natural levels of EBF emission, or when they were exposed to exaggerated high amounts of EBF in the headspace.
In the process of prey localization by predators, EBF could play two distinct roles after the predator has arrived on a plant that may host aphid prey: first, it may disclose the presence of aphids to the predator. Consequently predators should search more intensively on the plant, given that the chances of encountering prey are higher than on a plant where no EBF is perceived. EBF would thus act as an arrestant stimulus and one would expect that patch residence times and ultimately also the fraction of replicates where the fixed aphid is found are higher than in the non-EBF treatments, which was not the case. This is consistent with findings of Nakamuta (1991) who showed for ladybirds that EBF presence had no influence on area-concentrated search behavior, which is typically displayed by coccinellids after having consumed an aphid prey.
The second potential role of EBF for a foraging predator on a plant could be that of a short-range attractant, i.e., it could serve as a guiding signal for the predators. Consistent with a large number of studies that have shown that lacewing and ladybird larvae do not show directed search towards aphids on a plant (e.g., Bänsch 1964; Clark and Messina 1998; Minoretti and Weisser 2000), our study also found no evidence that the presence of EBF changed the efficiency of ladybird or lacewing search. As EBF in our study was placed close to or behind the aphid (from the position of the predator), both the probability of finding the aphid and also the time needed to find it should have been shorter in the presence of EBF, which was not the case.
There are at least two reasons why it may not be adaptive for aphid predators to use EBF as a stimulus for within-plant foraging behavior. First, the presence of EBF in the headspace of an aphid colony indicates a predation event which implies that the aphid colony is disturbed, i.e., that aphids may be walking, may have dropped off the plant or are otherwise scattered over the plant (e.g., Kislow and Edwards 1972; Wohlers 1981; Minoretti and Weisser 2000). Thus, particularly when the aphid colony was initially small, there may in fact be no aphids available for consumption. Second, the presence of EBF in the headspace indicates the presence of a competitor or even intraguild predator on the plant. Thus, foraging success may in fact be very low on the plant and there is an additional mortality risk for the foraging predator. In addition to these disadvantages, there may also be physiological constraints: EBF is only emitted in very low quantities and it is not amplified by the colony (Verheggen et al. 2008; Hatano et al. 2008a; Joachim et al. 2013), yet EBF degenerates when in contact to air (Kourtchev et al. 2009). However, very little is known about how EBF disperses within the plant and how far it can be detected, not only by the predator but also by conspecifics. More information is needed on the movement of EBF molecules when emitted by an attacked aphid, and the rate of decay with distance. For EBF to be a short-range attractant, a gradient needs to build up.
The only significant effect of EBF presence was when unnaturally high amounts (1,000 ng) of EBF were offered in the field experiment. Interestingly, this led to longer but not shorter times until the aphid was found, compared to the control, in replicates where the foraging was successful. It is, however, unclear why this effect is observed. It has, however, been observed that unnaturally high concentrations of a semiochemical can lead to unusual behaviors in the receiver, which are not displayed, when natural amounts of that semiochemical are present, as e.g., in males of different moth species after perceiving different ratios of their sex pheromone (e.g., Roelofs 1978).
In contrast to alarm pheromones, which trigger prey dispersal, other chemical signals, such as products of prey feeding or prey body odor, may better disclose the presence of prey on a plant, and are potentially more suitable as arrestant or short-range attractant cues. For example, honeydew that is released by aphids when feeding is such a cue and is indeed known to acts as a contact kairomone in ladybirds (Carter and Dixon 1984; Buitenhuis et al. 2004; Ide et al. 2007). Ladybird larvae show a difference in foraging behavior only after they have been in close (physical) contact with honeydew. A number of other aphid enemies also induce intensive area-restricted foraging behavior or increase their rate of oviposition upon direct contact with honeydew or, for syrphid flies (Diptera—Syrphidae), after perceiving honeydew volatiles (Budenberg 1990; Budenberg and Powell 1992).
To summarize, our study provides no evidence for a role of aphid alarm pheromone in the foraging behavior of predators on the host plant. While we present evidence that (E)-β-farnesene is not utilized as an arrestant or short-range attractant cue for two important aphid predators, a more detailed knowledge of its role as a kairomone is desirable for a better understanding of the costs associated with chemical alarm signaling.
References
Agelopoulos N, Birkett MA, Hick AJ, Hooper AM, Pickett JA, Pow EM, Smart LE, Smiley DWM, Wadhams LJ, Woodcock CM (1999) Exploiting semiochemicals in insect control. Pestic Sci 55(3):225–235
Al Abassi S, Birkett MA, Pettersson J, Pickett JA, Wadhams LJ, Woodcock CM (2000) Response of the seven-spot ladybird to an aphid alarm pheromone and an alarm pheromone inhibitor is mediated by paired olfactory cells. J Chem Ecol 26(7):1765–1771
Bänsch R (1964) Vergleichende Untersuchungen zur Biologie und zum Beutefangverhalten aphidivorer Coccinelliden, Chrysopiden und Syrphiden. Zool Jahrb, Abt Syst Ökol Geogr Tiere 91:271–340
Budenberg WJ (1990) Honeydew as a contact kairomone for aphid parasitoids. Entomol Exp Appl 55(2):139–147
Budenberg WJ, Powell W (1992) The role of honeydew as an ovipositional stimulant for two species of syrphids. Entomol Exp Appl 64(1):57–61
Buitenhuis R, Mcneil JN, Boivin G, Brodeur J (2004) The role of honeydew in host searching of aphid hyperparasitoids. J Chem Ecol 30(2):273–285
Carde RT, Bell WJ (1995) Chemical ecology of insects 2. Chapman and Hall, New York
Carter MC, Dixon AFG (1984) Honeydew: an arrestant stimulus for coccinellids. Ecol Entomol 9(4):383–387
Clark TL, Messina FJ (1998) Foraging behavior of lacewing larvae (Neuroptera : Chrysopidae) on plants with divergent architectures. J Insect Behav 11(3):303–317
Crawley MJ (2007) The R book. Wiley, Chichester, pp 553–556
Du YJ, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM (1998) Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol 24(8):1355–1368
Fellows MDE, Van Alphen JJM, Jervis MA (2005) Foraging behaviour. In: Jervis MA (ed) Insects as natural enemies: a practical perspective. Springer, Dordrecht, pp 1–71
Francis F, Lognay G, Haubruge E (2004) Olfactory responses to aphid and host plant volatile releases: (E)-β-farnesene an effective kairomone for the predator Adalia bipunctata. J Chem Ecol 30(4):741–755
Francis F, Vandermoten S, Verheggen F, Lognay G, Haubruge E (2005) Is the (E)-β-farnesene only volatile terpenoid in aphids? J Appl Entomol 129(1):6–11
Hatano E, Kunert G, Bartram S, Boland W, Gershenzon J, Weisser WW (2008a) Do aphid colonies amplify their emission of alarm pheromone? J Chem Ecol 34(9):1149–1152
Hatano E, Kunert G, Weisser WW (2008b) Chemical cues mediating interaction in a food chain: aphid-location by natural enemies. Eur J Entomol 105:797–806
Ide T, Suzuki N, Katayama N (2007) The use of honeydew in foraging for aphids by larvae of the ladybird beetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae). Ecol Entomol 32(5):455–460
Jervis MA (2005) Insects as natural enemies: a practical perspective. Springer, Dordrecht
Joachim C, Weisser WW (2013) Real-time monitoring of (E)-β-farnesene emission in colonies off the pea aphid Acyrthosiphon pisum, under lacewing and ladybird predation. J Chem Ecol 39(10):1254–1262
Joachim C, Hatano E, David A, Kunert M, Linse C, Weisser WW (2013) Modulation of aphid alarm pheromone emission of pea aphid prey by predators. J Chem Ecol 39(6):773–782
Keiser CN, Sheeks LE, Mondor EB (2013) The effect of microhabitat feeding site selection on aphid foraging and predation risk. Arthropod-Plant Interact. 7(6):633–641
Kislow CJ, Edwards LJ (1972) Repellent odor in aphids. Nature 235(5333):108–109
Kourtchev I, Bejan I, Sodeau JR, Wenger JC (2009) Gas-phase reaction of (E)-β-farnesene with ozone: rate coefficient and carbonyl products. Atmos Environ 43(20):3182–3190
Kunert G, Otto S, Röse USR, Gershenzon J, Weisser WW (2005) Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecol Lett 8(6):596–603
Micha SG, Wyss U (1996) Aphid alarm pheromone (E)-β-farnesene: a host finding kairomone for the aphid primary parasitoid Aphidius uzbekistanicus (Hymenoptera: Aphidiinae). Chemoecology 7:132–139
Minoretti N, Weisser WW (2000) The impact of individual ladybirds (Coccinella septempunctata, Coleoptera: coccinellidae) cell aphid colonies. Eur J Entomol 97(4):475–479
Nakamuta K (1991) Aphid alarm pheromone component, (E)-β-farnesene, and local search by a predatory lady beetle, Coccinella septempunctata bruckii Mulsant (Coleoptera, Coccinellidae). Appl Entomol Zool 26(1):1–7
Noldus LPJJ, Lenteren JCV, Lewis WJ (1991) How Trichogramma parasitoids use moth sex pheromones as kairomones: orientation behaviour in a wind tunnel. Physiol Entomol 16(3):313–327
Purandare SR, Tenhumberg B (2012) Influence of aphid honeydew on the foraging behaviour of Hippodamia convergens larvae. Ecol Entomol 37(3):184–192
Roelofs WL (1978) Threshold hypothesis for pheromone perception. J Chem Ecol 4(6):685–699
Saad AAB, Bishop GW (1976) Attraction of insects to potato plants through use of artificial honeydews and aphid juice. Entomophaga 21(1):49–57
Schwartzberg EG, Kunert G, Stephan C, Biedermann A, Rose USR, Gershenzon J, Boland W, Weisser WW (2008) Real-time analysis of alarm pheromone emission by the pea aphid (Acyrthosiphon pisum) under predation. J Chem Ecol 34(1):76–81
Vandermoten S, Mescher MC, Francis F, Haubruge E, Verheggen FJ (2012) Aphid alarm pheromone: an overview of current knowledge on biosynthesis and functions. Insect Biochem Molec Biol 42(3):155–163
Verheggen FJ, Fagel Q, Heuskin S, Lognay G, Francis F, Haubruge E (2007) Electrophysiological and behavioral responses of the multicolored asian lady beetle, Harmonia axyridis Pallas, to sesquiterpene semiochemicals. J Chem Ecol 33(11):2148–2155
Verheggen FJ, Mescher MC, Haubruge E, Moraes CM, Schwartzberg EG (2008) Emission of alarm pheromone in aphids: a non-contagious phenomenon. J Chem Ecol 34(9):1146–1148
Vinson BB (1976) Host selection by insect prasitoids. Annu Rev Entomol 21:109–133
Wohlers P (1981) Effects of the alarm pheromone (E)-β-farnesene on dispersal behavior of the pea aphid Acyrthosiphon pisum. Entomol Exp Appl 29(1):117–124
Zhu JW, Cosse AA, Obrycki JJ, Boo KS, Baker TC (1999) Olfactory reactions of the twelve-spotted lady beetle, Coleomegilla maculata and the green lacewing, Chrysoperla carnea to semiochemicals released from their prey and host plant: electroantennogram and behavioral responses. J Chem Ecol 25(5):1163–1177
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG), grant WE-3081/2-3. The authors thank Katz Biotech AG for providing aphid predators free of charge.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael Heethoff.
Rights and permissions
About this article
Cite this article
Joachim, C., Vosteen, I. & Weisser, W.W. The aphid alarm pheromone (E)-β-farnesene does not act as a cue for predators searching on a plant. Chemoecology 25, 105–113 (2015). https://doi.org/10.1007/s00049-014-0176-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-014-0176-z