Abstract
Preparation of a complete stereoisomeric library of 1,10-bisaboladien-3-ols and selected 10,11-epoxy-1-bisabolen-3-ols was pivotal for the identification of the aggregation pheromone of the brown marmorated stink bug, Halyomorpha halys. Herein, we describe syntheses of the remaining 10,11-epoxy-1-bisabolen-3-ols, and provide additional evidence on the assignment of relative and absolute configurations of these compounds by single-crystal X-ray crystallography of an intermediate, (3S,6R,7R,10S)-1-bisabolen-3,10,11-triol. To demonstrate the utility of this stereoisomeric library, we revisited the aggregation pheromone of the harlequin bug, Murgantia histrionica, and showed that the male-produced pheromone consists of two stereoisomers of 10,11-epoxy-1-bisabolen-3-ol. Employment of eight cis-10,11-epoxy-1-bisabolen-3-ol stereoisomeric standards, two enantioselective GC columns, and NMR spectroscopy enabled the identification of these compounds as (3S,6S,7R,10S)-10,11-epoxy-1-bisabolen-3-ol and (3S,6S,7R,10R)-10,11-epoxy-1-bisabolen-3-ol, which are produced by M. histrionica males in 1.4:1 ratio.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The bisabolane skeleton is a recurring structural motif in the semiochemistry of stink bugs (Hemiptera: Pentatomidae). Epoxides of bisabolene were identified as male-specific pheromones of Nezara viridula (Aldrich et al. 1987; Baker et al. 1987) and Chinavia hilaris (Aldrich et al. 1989; McBrien et al. 2001). Zingiberene, β-sesquiphellandrene and α-curcumene constitute part of the pheromone of Thyanta pallidovirens (McBrien et al. 2002), and β-sesquiphellandrene was identified as a pheromone component of Piezodorus hybneri (Leal et al. 1998). 1,10-Bisaboladien-3-ols were identified as part of the male-produced sex pheromone of the rice stalk stink bug, Tibraca limbativentris (Borges et al. 2006), and 10,11-epoxy-1-bisabolen-3-ol (“murgantiol”) has been reported as an aggregation pheromone of the harlequin bug, Murgantia histrionica (Zahn et al. 2008, 2012). Several sesquiterpenes were isolated from Zingiber officinale, among them a 1,10-bisaboladien-3-ol, called zingiberenol (Terhune et al. 1974). The stereo structures of the pheromones of both T. limbativentris and M. histrionica, as well as zingiberenol have not been determined. A sex pheromone of the rice stink bug, Oebalus poecilus, also has been recently identified as zingiberenol; more specifically, (3R,6R,7S)-1,10-bisaboladien-3-ol (de Oliveira et al. 2013). The absolute configuration of it has been assigned based on the correlation to natural zingiberene and similarities of 13C NMR spectra of a synthetic mixture containing the pheromone and (R,R)-quercivorol. Thus, until the recent synthesis of Khrimian et al. (2014), no single isomer of 1,10-bisaboladien-3-ol and/or 10,11-epoxy-1-bisabolen-3-ol had been synthesized to assist identifications of stereo structures of the above natural products. It is noteworthy that identification of the aggregation pheromones of O. poecilus, T. limbativentris and M. histrionica were supported by laboratory bioassays, but field trapping experiments with identified pheromones have not been reported. Murgantia histrionica is an important pest of cole crops in the U.S. (Wallingford et al. 2011), and development of an attractive pheromone as bait in monitoring traps or in management applications would be highly desirable.
Recently, we developed syntheses of all eight stereoisomers of 1,10-bisaboladien-3-ol and six stereoisomeric 10,11-epoxy-1-bisabolen-3-ols and established their relative and absolute configurations via single-crystal X-ray crystallography and chemical correlations (Khrimian et al. 2014). Utilizing enantioselective gas-chromatography, we identified two aggregation pheromone components of the brown marmorated stink bug, Halyomorpha halys, as (3S,6S,7R,10S)-10,11-epoxy-1-bisabolen-3-ol and (3R,6S,7R,10S)-10,11-epoxy-1-bisabolen-3-ol (Khrimian et al. 2014). In the current paper, we describe preparations of the remaining stereoisomers of 10,11-epoxy-1-bisabolen-3-ol, and the application of individual stereoisomers and enantioselective gas-chromatography to determine the stereochemistry of the pheromone of the harlequin bug.
Methods and Materials
General Methods
Routine GC analyses were performed on an Agilent Technologies 6890N instrument equipped with a flame ionization detector and a DB-5 capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness). Hydrogen was used as carrier gas at 1 ml/min. Column temperature was maintained at 50 °C for 3 min, and then raised to 270 °C at 10 °C/min. Enantioselective GC analyses were performed on a Hydrodex β-6TBDM capillary column (25 m × 0.25 mm ID; Macherey-Nagel GmbH & Co. KG, Düren, Germany), column #1, and an Astec Chiraldex G-TA column (30 m × 0.25 mm i.d. × 0.12 μm film; Sigma-Aldrich/Supelco, Bellefonte, PA, USA), column #2. Electron impact ionization (EI) mass spectra were obtained at 70 eV with an Agilent Technologies 5973 mass selective detector interfaced with 6890N GC system equipped with either an HP-5MS (30 m × 0.25 mm i.d. × 0.25 μm film) column, or one of the chiral columns described above. The HP-5MS column temperature was maintained at 50 °C for 5 min, and then raised to 270 °C at 10 °C/min. Helium was used as a carrier gas at 1 ml/min.
TLC analyses were conducted on Whatman AL SIL G/UV plates using 20 % ethanol solution of phosphomolybdic acid, and/or UV for visualization of spots. Flash chromatography was carried out with 230–400 mesh silica gel (Fisher Scientific, Fair Lawn, NJ, USA).
NMR spectra of compounds 17, 18, and 20 were collected on a Bruker Avance 500 spectrometer running Topspin 1.4 pl8 using a 5 mm BBO probe. Spectra were recorded in CD2Cl2 at 500 MHz for 1H and 125 MHz for 13C NMR. Chemical shifts are reported as parts per million from tetramethylsilane based on the lock solvent. COSY, 13C-DEPT 135, HMBC, and HSQC spectra also were recorded to assign protons and carbons in the synthetic molecules. 1H NMR spectra of other compounds were obtained at 600 MHz and 13C spectra at 151 MHz on a Bruker AVIII-600 MHz spectrometer. Chemical shifts are reported in δ units and referenced to the residual CD2Cl2 solvent signal.
Optical rotations were obtained using a Perkin-Elmer 241 polarimeter with a 1.0 ml cell. GC-HRMS analyses were performed by time-of-flight in EI, or ESI modes on a Waters GCT Premier instrument equipped with a DB5-MS column.
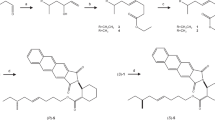
All reagents and solvents were purchased from Aldrich Chemical Co., unless otherwise specified. All eight stereoisomeric 1,10-bisaboladien-3-ols (Scheme 1, 1–8), seven intermediate triols, 9, 10, 13, 14, 15, 16, 21, and six 10,11-epoxy-1-bisabolen-3-ols, 25, 26, 29, 30, 31, 37, have been described previously (Khrimian et al. 2014). Syntheses of these and remaining stereoisomeric triols and epoxybisabolenols are presented below and in Scheme 1.
Syntheses of 10,11-epoxy-1-bisabolene-3-ols (25–40) from 1,10-bisaboladien-3-ols (1–8) via 1-bisabolen-3,10,11-triols (9–24). Compounds marked with asterisks were described in Khrimian et al. 2014
Preparation of 1-Bisabolen-3,10,11-Triols Via Asymmetric Dihydroxylation of 1,10-Bisaboladien-3-ols
Solutions of alcohols (2, 5, 6, 7, and 8, 1 mmol) in tert-butanol (4.7 ml) were added to a mixture of AD-mix-α (1.38 g), and methanesulfonamide (91 mg) in water (4.7 ml) at 0 °C. Mixtures were stirred at 0–2 °C for 24 h, then treated with sodium sulfite (1.47 g), and the temperature was allowed to rise to 20–25 °C within 0.5 h. The mixtures were extracted with methylene chloride (4 × 30 ml), the combined organic extracts were washed with 2 N KOH, brine, and dried with Na2SO4. After evaporation of the solvent, residues were chromatographed on SiO2 with ethyl acetate to yield 10S triols 12 (90 % yield; M.p. 95–97 °C, ethyl acetate/heptane, 1:2), 18 (90 %), 20 (76 %), 22 (82 %), and 24 (80 %; M.p. 97–99 °C , ethyl acetate/hexane, 1:2), respectively. Analogously, alcohols 2, 5, 6, and 8 were dihydroxylated with AD-mix-β to yield 10R triols 11 (78 %; M.p. 96–98 °C, ethyl acetate), 17 (96 %), 19 (86 %; M.p. 123–124 °C, tert-butyl methyl ether), and 23 (87 %; M.p. 94–95 °C, tert-butyl methyl ether), respectively. GC-MS (e.g., 17, m/z, %): 238 (1), 223 (3), 220 (2), 205 (6), 180 (18), 162 (19), 147 (20), 134 (58), 132 (47), 121 (83), 105 (42), 94 (100), 93 (66), 79 (47), 71 (54), 59 (97), 43 (89), 41 (27). Two other cis-1-bisabolen-3,10,11-triols, 18 and 22, have mass spectra similar to that of 17, which were consistent with those published (Khrimian et al. 2014). GC/MS (12, m/z, %): 238 (2), 223 (3), 220 (4), 205 (5), 180 (11), 159 (15), 145 (21), 134 (70), 132 (84), 121 (87), 105 (42), 94 (42), 93 (91), 79 (35), 71 (57), 59 (100), 43 (84), 41 (27). Other trans-1-bisabolen-3,10,11-triols 11, 19, 23, 20, and 24 display fragmentations similar to 12 that were consistent with those published (Khrimian et al. 2014). Specific rotations and 1H and 13C NMR data of synthesized triols were presented in Table 1. All triols displayed correct molecular weights in HRESIMS analyses corresponding to 279.1936 calculated for C15H28O3Na.
Preparation of 10,11-Epoxy-1-bisabolen-3-ols
Methanesulfonyl chloride (77 μl, 1.14 mmol) was added to a stirred solution of a triol (11, 12, 16–20, 22–24, 1.0 mmol) in dry pyridine (1.5 ml) at 0–5 °C. The mixture was allowed to warm to room temperature and stirred for 1 h. Then, it was poured into ice-water (4 ml) and extracted with CH2Cl2 (3 × 10 ml). Combined organic extracts were washed with ice-water, dried with Na2SO4, and concentrated to yield a crude mesylate. This was taken into methanol (5 ml), cooled to 0 °C, and treated with a solution of KOH (112 mg, 2 mmol) in MeOH (1.3 ml), which resulted in an instantaneous precipitation of inorganic salts. The reaction mixture was warmed to room temperature, stirred for 0.5 h, and concentrated to remove most of MeOH. The residue was combined with NH4Cl solution (pH 7–8), and extracted with ether (3 × 10 ml). Combined organic extracts were washed with ice-water and brine, dried with Na2SO4, and concentrated. Flash chromatography (hexane/ethyl acetate, 3:2) yielded epoxybisabolenols 27 (56 % yield, 95 % dr, column #1), 28 (36 %, 98 % dr, column #1), 32 (61 %, 98 % dr, column #1), 33 (61 %, 94 % dr, column #1), 34 (39 %, 92 % dr, column #1), 35 (71 %, 91 % dr, column #2), 36 (56 %, 87 % dr, column #2), 38 (50 %, 98 % dr, column #1), 39 (20 %, 92 % dr, column #2), 40 (36 %, 78 % dr, column #2). (Scheme 1, Table 2). GC-MS (34, m/z, %): 220 (3), 205 (4), 187 (4), 165 (15), 147 (16), 134 (43), 132 (38), 123 (23), 121 (34), 119 (42), 109 (32), 105 (29), 93 (69), 91 (50), 79 (38), 71 (50), 55 (29), 43 (100), 41 (42). Other cis-10,11-epoxy-1-bisabolen-3-ols (33, 38) had mass-spectra similar to 34 mass-spectra corresponding to those reported (Khrimian et al. 2014; Zahn et al. 2008). GC-MS (28, m/z, %): 220 (7), 205 (7), 187 (5), 165 (26), 147 (21), 145 (20), 134 (57), 132 (81), 123 (28), 121 (50), 119 (83), 109 (43), 105 (42), 93 (96), 91 (73), 79 (34), 71 (49), 55 (34), 43 (100), 41 (46). Other trans-10,11-epoxy-1-bisabolen-3-ols (27, 32, 35, 36, 39, and 40) had mass spectra similar to that of 28. Optical rotations and NMR data of 10,11-epoxy-1-bisabolen-3-ols are presented in Table 2. All synthesized 10,11-epoxy-1-bisabolen-3-ols displayed correct molecular weights in HRESIMS analyses corresponding to 261.1830 calculated for C15H26O2Na.
X-ray Structure Determination of Triol 12
Triol 12 was crystallized from ethyl acetate/heptane, 1:2, then re-crystallized as follows. Triol 12 (2.8 mg) was placed in an NMR tube and dissolved in dichloromethane (150 μl). Then, toluene (100 μl) was added. Lath-like crystals slowly precipitated and were analyzed for X-ray structure determination. All reflection intensities were measured at 110(2) K using a SuperNova diffractometer (equipped with Atlas detector) with Cu Kα radiation (mirror optics, λ = 1.5418 Å) under the program CrysAlisPro (Version 1.171.36.24 Agilent Technologies 2012). The program CrysAlisPro (Version 1.171.36.24 Agilent Technologies 2012) was used to refine the cell dimensions. Data reduction was done using the program CrysAlisPro (Version 1.171.36.24 Agilent Technologies 2012). The structure was solved with the program SHELXS-97 (Sheldrick 2008), and was refined on F 2 with SHELXL-97 (Sheldrick 2008). Analytical numeric absorption corrections based on a multifaceted crystal model were applied using CrysAlisPro (Version 1.171.36.24 Agilent Technologies 2012). The temperature of the data collection was controlled using the system Cryojet (manufactured by Oxford Instruments). The H atoms (unless otherwise specified) were placed at calculated positions using the instructions AFIX 13, AFIX 23, AFIX 43, or AFIX 137 with isotropic displacement parameters having values 1.2 or 1.5 times Ueq of the attached C atoms. The H atoms attached to O1, O2, and O3 were found from difference Fourier maps. Their atomic coordinates (the O−H distances were restrained to be 0.84(3) Å using the DFIX instruction), and isotropic temperature factors were refined freely. The structure is ordered. The absolute configuration has been established by anomalous dispersion effects in diffraction measurements on the crystal. The Flack and Hooft parameters (Flack 1983; Hooft et al. 2008) refine to 0.01(14) and −0.05(5), respectively. The model has chirality at C3 S, C6 R, C7 R, and C10 S (Fig. 1). Triol 12, Fw = 256.37, thin colorless lath, 0.56 × 0.10 × 0.04 mm3, monoclinic, P21 (no. 4), a = 9.4105(3), b = 6.49971(18), c = 12.2958(3) Å, β = 99.261(2)°, V = 742.28(4) Å3, Z = 2, D x = 1.147 g cm−3, μ = 0.614 mm−1, abs. corr. range: 0.807–0.977. Seven thousand eight hundred thirty reflections were measured up to a resolution of (sin θ/λ)max = 0.62 Å−1. Two thousand nine hundred reflections were unique (R int = 0.0206), of which 2844 were observed [I >2σ(I)]. One hundred eighty parameters were refined using 4 restraints. R1/wR2 [I >2σ(I)]: 0.0306/0.0833. R1/wR2 [all refl.]: 0.0312/0.0842. S = 1.055. Residual electron density found between −0.16 and 0.21 e Å−3.
Insect Rearing and Semiochemical Collection
Harlequin bug adults and nymphs were collected by hand from their host plants, primarily collards, kale, forage radish, and rape (cv. Dwarf Essex) on gardens and small farms within 80 km of Beltsville, Maryland, and reared under conditions of 25° ±1 °C, 50 ± 10 % RH, and 16:8 h L:D photoperiod in a walk-in growth chamber. Nymphs were reared on collard plants (Brassica oleracea L. (acephala group)) (cv. Champion or Vates) grown in 3.8-l pots. Newly-molted adults were separated by observing sexual differences in the terminal abdominal segments, and males were retained separately in small (375 ml) ventilated containers on commercial organically-grown broccoli florets until at least 7-d-old as adults, after which volatile collections were initiated.
Five adult harlequin bug males then were placed in a glass jar (500 ml) aeration system with a moistened cotton ball at the bottom and commercial organic cauliflower florets as food. The sample was aerated with 100 ml/min activated carbon filtered air-flow for 72 h. Volatiles released from M. histrionica males on the cauliflower florets were trapped onto 50 mg activated charcoal (50/80 mesh; Sigma-Aldrich, USA) in glass tubing between two plugs of glass wool. Trapped volatiles were removed with 1 ml dichloromethane into a 2-ml glass vial. The aeration extract was kept in freezer (−20 °C) before GC/MS analyses. The control aeration extract was obtained by conducting aeration without insects. Five replicates of the aeration extract were collected.
Results and Discussion
Syntheses
Recently, we developed syntheses of all eight stereoisomers of 1,10-bisaboladien-3-ols (Scheme 1, 1–8), which were used to prepare selected 10,11-epoxy-1-bisabolen-3-ols via intermediate triols (Scheme 1, compounds marked with asterisks) (Khrimian et al. 2014). Herein, we describe the remaining stereoisomers of 10,11-epoxy-1-bisabolen-3-ol and 1-bisabolen-3,10,11-triol, which completes full stereoisomeric libraries of three classes of 1-bisabolen-3-ols. In addition to triol 16 described earlier (Khrimian et al. 2014), five more triols turned out to be crystalline. X-ray structure determination of triol 12 (Fig. 1) clearly demonstrates trans arrangement of the OH group at C-3 and alkyl group at C-6 of the cyclohexene ring, thus providing an additional confirmation of our earlier assignments of relative and absolute configurations of these stereogenic centers. Also, the 10S configuration of triol 12 provided further proof of the stereochemistry of the Sharpless asymmetric dihydroxylation, whereby AD-mix α delivered (S)- and AD-mix β (R)-products (Sharpless et al. 1992). Triols were converted to the corresponding mesylates of the secondary hydroxy groups, and the mesylates were cyclized to epoxides with inversion of configuration by treatment with KOH in MeOH (Frater and Müller 1989; Moore et al. 1999). All stereoisomers of 10,11-epoxy-1-bisabolen-3-ols and 1-bisabolen-3,10,11-triols were characterized by NMR spectroscopy. Thus, 13C NMR is the method of choice to distinguish between cis (3R*,6R*) and trans (3R*,6S*) stereoisomers (Khrimian et al. 2014), with trans isomers displaying greater difference in chemical shifts of C-1 and C-2 (3.2–5.2 ppm) than cis-isomers (0.1–1.7 ppm) regardless of configurations at C-7 and C-10. Additionally, in trans isomers, chemical shifts of C-3 (69.4–69.9 ppm) are higher and those of C-15 (28.2–28.7 ppm) lower than corresponding signals of cis isomers (67.1–67.5 and 29.6–30.0 ppm, respectively), consistent with those previously reported (Blair and Tuck 2009; Khrimian et al. 2014).
Stereoisomeric Composition of Pheromone of Harlequin Bug
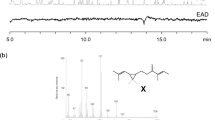
Aeration of male M. histrionica fed on cauliflower with subsequent analysis of volatiles by GC-MS revealed two major compounds (Fig. 2). The first was identified as tridecane, also found in earlier work (Zahn et al. 2008), and the second one as the expected aggregation pheromone, murgantiol (Zahn et al. 2008). The mass-spectrum and GC-retention time on HP-5MS column of murgantiol (compound A) matched those of cis-10,11-epoxy-1-bisabolen-3-ols. The male aeration extract was examined further on two enantioselective GC columns against all eight synthetic stereoisomers of cis-10,11-epoxy-1-bisabolen-3-ol. The murgantiol, eluted as a single peak on HP-5MS, split into two peaks on Chiraldex G-TA column (Fig. 3, III), signifying that the aggregation pheromone of M. histrionica consists of more than one stereoisomer. Earlier, we showed that Chiraldex G-TA column separated all four cis-10,11-epoxy-1-bisabolen-3-ols with 7R configurations (Khrimian et al. 2014). We used that feature in the current study (Fig. 3, I) and determined that (3S,6S,7R,10S)-epoxide 25 co-eluted with the main murgantiol peak (A) and (3S,6S,7R,10R)-epoxide 26 with the minor murgantiol peak (B) (Fig. 3, II). On a Hydrodex-β-6TBDM, murgantiol again produced two peaks, but the order of elution was reversed (Fig. 4. I). All four cis-10,11-epoxy-1-bisabolen-3-ols with 7S configurations were baseline separated (Fig. 4, II) on this column (Khrimian et al. 2014). Co-injection of this mixture with M. histrionica male aeration extract showed that the stereoisomer 37 eluted closely to peak A but did match it, and that (3S,6S,7S,10R)-epoxide 34 co-eluted with peak B. Thus, analyses of M. histrionica male aeration extract on two enantioselective columns revealed that the major male-specific component A matched only one out of eight cis-10,11-epoxy-1-bisabolen-3-ols, and hence, was unequivocally identified as (3S,6S,7R,10S)-epoxyalcohol 25. The minor component B in the male aeration could be either epoxide 26, or epoxide 34, or a mixture thereof. The last two compounds did not separate on Chiraldex G-TA either; therefore, we used NMR spectroscopy to finalize the stereochemical identification of component B.
Segments of GC-MS total ion chromatograms on Chiraldex G-TA column: (I) mixture of four cis-(7R)-10,11-epoxy-1-bisabolen-3-ols; peaks were assigned using individual stereoisomers; (II) co-injection of Murgantia histrionica male aeration and the mixture of cis-(7R)-10,11-epoxy-1-bisabolen-3-ols; (III) M. histrionica male aeration
Segments of GC-MS total ion chromatograms on Hydrodex-β-6TBDM column: (I) Murgantia histrionica male aeration; (II) cis-(7S)-10,11-epoxy-1-bisabolen-3-ols; compounds were assigned using individual stereoisomers; (III) co-injection of M. histrionica male aeration and cis-(7S)-10,11-epoxy-1-bisabolen-3-ols
We compared 1H and 13C NMR spectra of synthetic (3S,6S,7R,10S)-epoxide 25, (3S,6S,7R,10R)-epoxide 26 and (3R,6R,7R,10S)-epoxide 29 with those of natural murgantiol (Zahn et al. 2008). Compound 29 (which is an enantiomer of 34 and hence has identical NMR spectra) was chosen instead of 34 because the spectra of 25, 26, and 29 were obtained on the same instrument under identical conditions (Khrimian et al. 2014). Resonances from H-14 of epoxyalcohols 25 and 26 in 1H NMR spectra occur in the same field, 0.89 ppm, due to identical configurations of C-3, C-6, and C-7. On the other hand, the signal of H-14 in 29 appeared at 0.93 ppm signifying that if 29 (or 34) is mixed with either 25, or 26, there should be two distinctly separated doublets from H-14 methyl groups in 1H NMR spectra. Indeed, a small amount of 29 is easily detectable in synthetic 25 (Khrimian et al. 2014), but only one doublet from H-14 methyl group was present in the 1H NMR of the M. histrionica male volatiles (Zahn et al. 2008; also see Acknowledgement). This confirms the presence of epoxide 25, which was earlier proven to be the main constituent A, as well as epoxide 26 as the minor component B in the male volatiles. The absence of a second (downfield) doublet from H-14 rules out the (3S,6S,7S,10R)-epoxide 34 as a possible male-specific compound in M. histrionica. Finally, field bioassays with different stereoisomers confirmed that (3S,6S,7R,10S)-epoxide 25 and (3S,6S,7R,10R)-epoxide 26 were both attractive to M. histrionica, but (3S,6S,7S,10R)-epoxide 34 was not (Weber et al. 2014).
In summary, we completed the synthetic library of stereoisomers of 10,11-epoxy-1-bisabolen-3-ol, which was proven essential in determining the stereoisomeric composition of the harlequin bug aggregation pheromone. Field studies have established the attractiveness of both epoxyalcohols 25 and 26, and especially their mixtures, to harlequin bug males, females, and nymphs, in the field (Weber et al. 2014). Murgantia histrionica and Halyomorpha halys share the same compound, (3S,6S,7R,10S)-10,11-epoxy-1-bisabolen-3-ol (25), as the main aggregation pheromone component, whereas the minor pheromone components are different in these bugs. Further studies will investigate the sensitivity of life stages of each species to ratios of the three identified aggregation pheromone components as well as other attractants, in order to target each or both bugs for monitoring and management.
References
Aldrich JR, Oliver JE, Lusby WR, Kochansky JP, Lockwood JA (1987) Pheromone strains of the cosmopolitan pest Nezara viridula (Heteroptera: Pentatomidae). J Exp Zool 244:171–175
Aldrich JR, Lusby WR, Marron BE, Nicolaou KC, Hoffmann MP, Wilson LT (1989) Pheromone blends of green stink bugs and possible parasitoid selection. Naturwissenschaften 76:173–175
Baker R, Borges M, Cooke NG, Herbert RH (1987) Identification and synthesis of (Z)-(1′S,3′R,4′)-(−)-2-(3′,4′-epoxy-4′-methylcyclohexyl)-6-methylhepta-2,5-diene, the sex pheromone of the southern green stink bug, Nezara viridula (L.). J Chem Soc Chem Commun 414–416
Blair M, Tuck KL (2009) A new diastereoselctive entry to the (1S,4R)- and (1S,4S)-isomers of 4-isopropyl-1-methyl-2-cyclohexen-1-ol, aggregation pheromones of the ambrosia beetle Platypus quercivorus. Tetrahedron Asymmetry 20:2149–2153
Borges M, Birkett M, Aldrich JR, Oliver JE, Chiba M, Murata Y, Laumann RA, Barrigossi JA, Pickett JA, Moraes MCB (2006) Sex attractant pheromone from the rice stalk stink bug, Tibraca limbativentris Stal. J Chem Ecol 32:2749–2764
de Oliveira MWM, Borges M, Andrade CKZ, Lauman RA, Barrigossi JAF, Blassioli-Moraes MC (2013) Zingiberenol, (1S,4R,1′S)-4-(1′,5′-dimethylhex-4′-enyl)-1-methylcyclohex-2-en-1-ol, identified as the sex pheromone produced by males of the rice stink bug Oebalus poecilus (Heteroptera: Pentatomidae). J Agric Food Chem 61:7777–7785
Flack HD (1983) On enantiomorph-polarity estimation. Acta Cryst A39:876–881
Frater G, Müller U (1989) Synthesis of (+)-(4S,8R)-8-epi- and (−)-(4R,8S)-4-epi-β-bisabolol. Helv Chim Acta 72:653–658
Hooft RWW, Straver LH, Spek AL (2008) Determination of absolute structure using Bayesian statistics on Bijvoet differences. J Appl Crystallogr 41:96–103
Khrimian A, Zhang A, Weber DC, Ho H-Y, Aldrich JR, Vermillion KE, Siegler MA, Shirali S, Guzman F, Leskey TC (2014) Discovery of the aggregation pheromone of the brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1-bisabolen-3-ols. J Nat Prod 77:1708–1717
Leal WS, Kuwahara S, Shi XA, Higuchi H, Marino CEB, Ono M, Meinwald J (1998) Male-released sex pheromone of the stink bug Piezodorus hybneri. J Chem Ecol 24:1817–1829
McBrien HL, Millar JG, Gottlieb L, Chen X, Rice RE (2001) Male-produced sex attractant pheromone of the green stink bug Acrosternum hilare (Say). J Chem Ecol 27:1821–1839
McBrien HL, Millar JG, Rice RE, McElfresh JS, Cullen E, Zalom FG (2002) Sex attractant pheromone of the red-shouldered stink bug Thyanta pallidovirens: a pheromone blend with multiple redundant components. J Chem Ecol 28:1797–1817
Moore CJ, Possner S, Hayes P, Paddon-Jones GC, Kitching W (1999) An asymmetric dihydroxylation route to (3R,5E)-2,6-dimethyl-2,3-epoxyocta-5,7-diene: the major volatile component from male fruit-spotting bugs. J Org Chem 64:9742–9744
Sharpless KB, Amberg W, Bennani YL, Crispino GA, Hartung J, Jeong K-S, Kwong H-L, Morikawa K, Wang Z-M, Xu D, Zhang X-L (1992) The osmium-catalyzed asymmetric dihydroxylation: a new ligand class and a process improvement. J Org Chem 57:2768–2771
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A64:112–122
Terhune SJ, Hogg JW, Bromstein AC, Lawrence BM (1974) Four new sesquiterpene analogs of common monoterpenes. Can J Chem 53:3287–3293
Wallingford AK, Kuhar TP, Schultz PB, Freeman JH (2011) Harlequin bug biology and pest management in Brassicaceous crops. J Integr Pest Mgmt 2:H1–H4
Weber DC, Cabrera Walsh G, DiMeglio AS, Athanas MM, Leskey TC, Khrimian A (2014) Attractiveness of harlequin bug, Murgantia histrionica (Hemiptera: Pentatomidae), aggregation pheromone: field response to isomers, ratios and dose. J Chem Ecol, in press
Zahn DK, Moreira JA, Millar JG (2008) Identification, synthesis, and bioassay of a male-specific aggregation pheromone from the harlequin bug, Murgantia histrionica. J Chem Ecol 34:238–251
Zahn DK, Moreira JA, Millar JG (2012) Erratum to: identification, synthesis, and bioassay of a male-specific aggregation pheromone from the harlequin bug, Murgantia histrionica. J Chem Ecol 38:126
Acknowledgments
We thank Dr. Jocelyn G. Millar, University of California, Riverside, for sharing 1H NMR spectrum of the murgantiol described in Zahn et al. 2008. We express our gratitude to Michael M. Athanas, Anthony DiMeglio, Matthew Klein, and Meiling Z. Webb, for collecting, rearing, and volatile collection of the insects.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 101 kb)
Rights and permissions
About this article
Cite this article
Khrimian, A., Shirali, S., Vermillion, K.E. et al. Determination of the Stereochemistry of the Aggregation Pheromone of Harlequin Bug, Murgantia histrionica . J Chem Ecol 40, 1260–1268 (2014). https://doi.org/10.1007/s10886-014-0521-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-014-0521-2