Abstract
Derivatives of 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylic acid (chrysanthemic acid) are classic natural pyrethroids discovered in pyrethrum plants and show insecticidal activity. Chrysanthemic acid, with two asymmetric carbons, has four possible stereoisomers, and most natural pyrethroids have the (1R,3R)-trans configuration. Interestingly, chrysanthemic acid–related structures are also found in insect sex pheromones; carboxylic esters of (1R,3R)-trans-(2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropyl)methanol (chrysanthemyl alcohol) have been reported from two mealybug species. In the present study, another ester of chrysanthemyl alcohol was discovered from the striped mealybug, Ferrisia virgata (Cockerell), as its pheromone. By means of gas chromatography–mass spectrometry, nuclear magnetic resonance spectrometry, and high-performance liquid chromatography analyses using a chiral stationary phase column and authentic standards, the pheromone was identified as (1S,3R)-(−)-cis-chrysanthemyl tiglate. The (1S,3R)-enantiomer strongly attracted adult males in a greenhouse trapping bioassay, whereas the other enantiomers showed only weak activity. The cis configuration of the chrysanthemic acid–related structure appears to be relatively scarce in nature, and this is the first example reported from arthropods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Derivatives of 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylic acid (chrysanthemic acid) were first identified in pyrethrum plants, Chrysanthemum cinerariaefolium (now Tanacetum cinerariifolium) (Asteraceae), and are well known as naturally occurring pyrethroid insecticides (Khambay and Jewess 2010). Chrysanthemic acid has two asymmetric carbons with four possible configurations: (1R,3S)-cis, (1S,3R)-cis, (1R,3R)-trans, and (1S,3S)-trans. Most natural pyrethroids have the (1R,3R)-trans configuration, which generally shows more insecticidal activity than the corresponding cis-isomer (Khambay and Jewess 2010). Although cis-chrysanthemic acid derivatives are found in some plant essential oils (e.g., Wesołowska et al. 2015), they appear to be relatively scarce in nature.
Interestingly, chrysanthemic acid–related compounds—that is, carboxylic esters of (2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropyl)methanol (chrysanthemyl alcohol)—are produced as sex attractant pheromones by mealybugs. Females of the Madeira mealybug (Phenacoccus madeirensis) and citrophilus mealybug (Pseudococcus calceolariae) emit (1R,3R)-trans-chrysanthemyl (R)-2-methylbutanoate (Ho et al. 2009) and (1R,3R)-trans-chrysanthemyl (R)-2-acetoxy-3-methylbutanoate (El-Sayed et al. 2010; Unelius et al. 2011), respectively. In the present study, we identified another chrysanthemyl ester as the female-produced sex pheromone of the striped mealybug, Ferrisia virgata (Cockerell).
Ferrisia virgata is a cosmopolitan species and has been recorded from all biogeographic regions (Ben-Dov 1994; Kaydan and Gullan 2012), although most of the other members of the genus Ferrisia are New World species (Kaydan and Gullan 2012). This mealybug attacks various plants and has long been known as a destructive pest of crops or ornamental plants (e.g. Ghose and Paul 1972; Le Pellery 1968). Moreover, F. virgata has been reported to transmit several viral plant pathogens including cacao swollen shoot virus (Posnette and Strickland 1948) and citrus tristeza virus (Hughes and Lister 1953). The conventional management of mealybugs, including F. virgata, involves regular application of insecticides. However, the overuse of pesticides can cause resurgence and/or resistance of mealybug populations (Charles 2005; Morishita 2005). Monitoring the occurrence of pests informs decisions on when to spray insecticides in a more effective and efficient manner (Gut et al. 2004). Unlike other sampling methods that can be very time consuming and require technical expertise for pest identification, traps baited with species-specific pheromones are simple to use and are frequently employed in monitoring of mealybugs (e.g. Sawamura et al. 2015).
The sex pheromone candidate that elicited a reproducible electroantennogram response from males, was isolated by several sequential chromatographic steps from crude extracts of airborne volatiles emitted by virgin females. Gas chromatography–mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) spectrometry were used to determine the chemical structure. All four stereoisomers of the pheromone candidate were synthesized from the purified stereoisomers of chrysanthemyl alcohol, and the absolute configuration was determined by high-performance liquid chromatography (HPLC) analyses using a chiral stationary phase column. Finally, attraction of males to each synthetic stereoisomer was tested in bioassays. The structure was determined to be (1S,3R)-(−)-chrysanthemyl tiglate with the relatively unusual cis configuration in the chrysanthemyl alcohol moiety.
Methods and Materials
Analytical Instruments
GC analyses were conducted on an Agilent 6890 N GC (Agilent Technology, Santa Clara, CA, USA) equipped with a split/splitless injector and a flame ionization detector. The injector and detector were each kept at 220 °C. A nonpolar DB-1 column (30 m × 0.25 mm internal diameter, 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA) was used at a constant flow rate (1.0 ml/min), with helium as the carrier gas. The column oven temperature was maintained at 60 °C for 1 min, raised to 220 °C at a rate of 10 °C/min, and held there for 8 min. GC-electroantennogram detection (GC-EAD) was performed on an HP5890 GC (Hewlett-Packard, Avondale, PA, USA) equipped with a biological amplifier for electrophysiological responses (AB-651 J; Nihon Kohden Co., Tokyo, Japan). A head was excised from a virgin male mealybug and was placed on the ground electrode. Then a few distal segments of an antenna were cut off, and the end of the antenna was connected to the capillary glass electrode of the EAD device with a drop of saline. GC-MS analyses were conducted with an SX-102A mass spectrometer (JEOL, Tokyo, Japan) with the temperatures of the interface and the ion source maintained at 210 and 220 °C, respectively. Preparative GC was carried out with a programmable injector for large-volume injection (OPTIC 3; ATAS GL International, Eindhoven, The Netherlands) maintained at 40 °C for 10 s and then raised to 200 °C at 5 °C/s and a preparative fraction collection system (Gerstel GmbH & Co. KG, Mulheim an der Ruhr, Germany) cooled by a dry ice–acetone bath, using a polar column (TC-FFAP; 0.53 mm internal diameter × 15 m length, 1 μm film thickness; GL Sciences Inc., Tokyo, Japan) maintained at 40 °C for 6 min and then raised to 210 °C at 8 °C/min.
Preparative HPLC for natural pheromone isolation was performed by using an HP1050 series HPLC (Hewlett-Packard) equipped with a silica gel column (Inertsil, 4.6 mm internal diameter × 250 mm length, 5 μm particle size; GL Science, Tokyo, Japan), operating at room temperature at a flow rate of 1.0 ml/min with a mobile phase of 3% of diethyl ether in hexane. HPLC for separation of geometric isomers of synthetic chemicals was performed by using an HP1050 HPLC with an AgNO3-impregnated Nucleosil 100-SA column (4.6 mm internal diameter × 250 mm length, 5 μm particle size; GL Science), operating at room temperature at a flow rate of 1.0 ml/min with a mobile phase of 10% diethyl ether in hexane. HPLC for chiral resolution was performed by using an HP1050 HPLC with a CHIRALCEL OD column (4.6 mm internal diameter × 250 mm length, 5 μm particle size; Daicel Co., Tokyo, Japan), operating at room temperature at a flow rate of 0.8 ml/min with a mobile phase of 0.15% of 2-propanol in hexane. The eluate was monitored with a UV detector (λ = 210 nm).
NMR spectra were obtained at 30 °C with a JNM-A600 spectrometer (JEOL; 1H: 600.05 MHz; 13C: 150.80 MHz). Micro-bottom NMR tubes (SP-501; 1.7 mm outside diameter; Shigemi Co., Tokyo, Japan) were used, with 35 μL C6D6 (min. 99.96%; Sigma-Aldrich, St. Louis, MO, USA) as the solvent.
Polarimetry was performed with a P-1020 polarimeter (JASCO, Easton, MD, USA). Enantiomeric excesses were calculated by comparing the chiral resolution HPLC peak areas.
Volatile Collection
Ferrisia virgata mealybugs were collected from fruits of Chrysophyllum cainito in Ishigaki City, Okinawa Prefecture, Japan (24.4°N, 124.2°E) on 16 July 2015. The insects were reared on squash (Cucurbita moschata) and maintained in a rearing room (16 h:8 h light:dark; 23 °C; 50% relative humidity). Males and females were manually separated immediately after the males had spun cocoons, and only females (~100 individuals) were transferred to a 1000-ml glass jar with a squash. Headspace volatiles containing sex pheromone were collected from the glass jar on an adsorbent trap (1.0 g of HayeSep Q; 60/80 mesh; Alltech, Deerfield, IL, USA) by pulling ambient air cleaned through activated charcoal (15 g) over the virgin females at a flow rate of 500 ml/min using a vacuum pump. Every 3 or 4 days the volatiles were extracted from the adsorbent with 15 ml of hexane; the extract was concentrated with a rotary evaporator at room temperature and the residue was kept at −20 °C. Collection of volatiles continued for 6 weeks, and six rounds of collection were performed to obtain approximately 25,200 female day-equivalents in total. The voucher specimens, partial DNA sequences of which were determined and deposited in the GenBank/DDBJ/EMBL database with accession numbers LC278435–278437, are stored at the National Agriculture and Food Research Organization (Tsukuba, Japan).
Pheromone Candidate Isolation
One compound in the extracts elicited an EAD response from antennae of males, and was selected as the pheromone candidate. The concentrated crude volatile extract was partially purified by open-column chromatography with 0.2 g of silica gel (Wakogel C-200; Wako Pure Chemicals, Osaka, Japan), successively eluting with 2 ml each of 0, 5, 15, and 50% diethyl ether in hexane. The pheromone candidate eluted in the 5% diethyl ether in hexane eluent fraction, which was concentrated and subjected to serial preparative HPLC and GC for further purification and isolation.
Authentic Chemicals
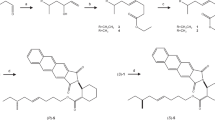
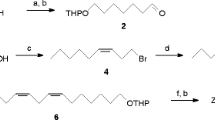
A commercially available ca. 1:2 mixture of geometric isomers of ethyl chrysanthemate (Tokyo Chemical Industry Co., Tokyo, Japan) was first separated into the (±)-cis- and (±)-trans-isomers by preparative HPLC using an AgNO3-impregnated Nucleosil column, injecting 10 μl aliquots of ethyl chrysanthemate in hexane (2 mg/ml). Pure ethyl (±)-cis- and (±)-trans-chrysanthemate, eluted at the retention times of 8.8 and 10.9 min, respectively. Then each (±)-cis- and (±)-trans-chrysanthemate was enantiomerically resolved by preparative HPLC using a CHIRALCEL OD column, injecting 40 μl aliquots of each isomer in hexane (2 mg/ml). Pure (1R,3S)-(+)-cis-, (1S,3R)-(−)-cis-, (1R,3R)-(+)-trans-, and (1S,3S)-(−)-trans-isomers eluted at retention times of 13.4, 6.2, 7.9, and 6.3 min, respectively, in the order described previously (Kim et al. 2003). Subsequently, each enantiomer of ethyl chrysanthemate was converted to chrysanthemyl alcohol; 1.5 mg of ethyl chrysanthemate was dissolved in dry THF and was added dropwise to a suspension of LiAlH4 (2 mg) in 1 ml of dry THF at room temperature under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 12 h. The reaction was quenched with saturated aqueous potassium sodium tartrate (3 ml) on ice, and the product was extracted with diethyl ether (4 × 2 ml). The combined extracts were washed with brine, dried over NaSO4, and concentrated under vacuum. The crude product including chrysanthemyl alcohol was then dissolved in 0.2 ml of dry CH2Cl2 with 1.0 mg of (E)-2-methyl-2-butenoic acid (tiglic acid; Tokyo Chemical Industry Co.), 0.24 mg of 4-dimethylaminopyridine (DMAP; Tokyo Chemical Industry Co.), and 1.3 mg of N,N′-diisopropylcarbodiimide (DIC; Wako Pure Chemicals). The mixture was stirred overnight at 35 °C, then poured into 2 ml of H2O, and extracted with hexane (3 × 2 ml). The extract was dried over Na2SO4, concentrated under vacuum, and purified by silica gel column chromatography eluting with 3% diethyl ether in hexane to give chrysanthemyl tiglate (0.94–1.01 mg; 52–58% yield over two steps). We also prepared a mixture of stereoisomers of chrysanthemyl tiglate (a “technical grade” pheromone; purity: 89%) by using a commercially available ca. 1:2 mixture of (±)-cis- and (±)-trans-chrysanthemyl alcohol (Sigma-Aldrich), tiglic acid, DMAP, and DIC, with the same procedure. Ethyl esters of tiglic acid and (Z)-2-methyl-2-butenoic acid (angelic acid; Tokyo Chemical Industry Co.) were prepared using ethanol and each acid in a similar manner.
Chemical data on cis-chrysanthemyl tiglate are as follows: GC-MS (EI 70 eV)—Rt 13.66 min (on DB-1), m/z 55 (38%), 67 (11%), 83 (95%), 93 (40%), 105 (18%), 121 (100%), 136 (7%), 154 (26%), 167 (3%), 236 (8%, M+); 1H–NMR δH—0.98 [3H, s, (CH 3)(CH3)C<], 0.99 [3H, s, (CH3)(CH 3)C<], 1.15 [1H, ddd, J = 7.6, 7.6, 8.3 Hz, OCH2CH<], 1.37 [1H, m, 1.35–1.39 ppm, C = CHCH<], 1.38 [3H, d, J = 7.2 Hz, (CH 3)CH=], 1.60 [3H, s, (CH 3)(CH3)C=], 1.64 [3H, s, (CH3)(CH 3)C=], 1.83 [3H, s, CH 3C(CO)=], 4.28 [1H, dd, J = 7.6, 11.7 Hz, >CHCHHO], 4.35 [1H, dd, J = 7.6, 11.7 Hz, >CHCHHO], 5.05 [1H, d, J = 6.9 Hz, >CHCH = C(CH3)(CH3)], 6.96 [1H, q, J = 7.2 Hz, CH3CH = C(CH3)(CO)]; 13C–NMR δC—12.2, 13.9, 15.7, 18.6, 20.6, 25.6, 26.9, 27.4, 28.7, 62.8, 119.4, 129.4, 135.4, 136.4, 167.8; (1R,3S)-cis-chrysanthemyl tiglate—HPLC Rt 8.7 min (on CHIRALCEL OD), [α]25.0 D = +48.9 (observed, >99.9% ee, c 0.089, hexane); (1S,3R)-cis-chrysanthemyl tiglate—HPLC Rt 9.3 min (on CHIRALCEL OD), [α]25.0 D = −44.1 (observed, 98.6% ee, c 0.087, hexane).
Chemical data on trans-chrysanthemyl tiglate are as follows: GC-MS (EI 70 eV)—Rt 13.54 min (on DB-1), m/z 55 (37%), 67 (12%), 83 (96%), 93 (46%), 105 (19%), 121 (100%), 136 (9%), 154 (26%), 167 (3%), 236 (10%, M+);1H–NMR δH—0.95 [1H, ddd, J = 5.5, 6.9, 8.3 Hz, OCH2CH<], 0.99 [3H, s, (CH 3)(CH3)C<], 1.04 [3H, s, (CH3)(CH 3)C<], 1.17 [1H, dd, J = 5.5, 6.9 Hz, C = CHCH<], 1.37 [3H, d, J = 7.6 Hz, (CH 3)CH=], 1.61 [3H, s, (CH 3)(CH3)C=], 1.64 [3H, s, (CH3)(CH 3)C=], 1.82 [3H, s, CH 3C(CO)=], 4.10 [1H, dd, J = 6.9, 11.7 Hz, >CHCHHO], 4.42 [1H, dd, J = 8.3, 11.7 Hz, >CHCHHO], 4.93 [1H, d, J = 6.9 Hz, >CHCH = C(CH3)(CH3)], 6.96 [1H, q, J = 7.6 Hz, CH3CH = C(CH3)(CO)]; 13C–NMR δC—12.2, 13.9, 18.3, 21.5, 22.4, 22.6, 25.6, 29.4, 31.7, 65.5, 123.7, 129.4, 133.3, 136.4, 167.7; (1R,3R)-trans-chrysanthemyl tiglate—HPLC Rt 10.9 min (on CHIRALCEL OD), [α]25.0 D = +23.0 (observed, >99.9% ee, c 0.102, hexane); (1S,3S)-trans-chrysanthemyl tiglate—HPLC Rt 10.5 min (on CHIRALCEL OD), [α]25.0 D = −22.6 (observed, >99.9% ee, c 0.168, hexane).
Ethanolysis and Esterification
The pheromone candidate (100 ng) was dissolved in 1 ml of 0.5 M KOH in ethanol. The reaction mixture was held overnight at room temperature, poured into 1 ml of H2O, and extracted with hexane (3 × 1 ml). The combined extracts were concentrated to approximately 20 μl at room temperature, and an aliquot (2 μl) was used for GC-MS analysis. The residue was dissolved in CH2Cl2 and was re-esterified with tiglic acid by using the method described above.
Bioassay
The attractiveness of each stereoisomer of the synthetic chrysanthemyl tiglate was tested in a greenhouse (3.0 × 4.8 × 2.8 m) at the National Agriculture and Food Research Organization (Tsukuba), where mealybugs naturally occurred on potted potato plants. Males were captured with white delta-traps with a sticky board (12 × 22 cm), baited with 0.1 mg of the synthetic pheromone impregnated into a red rubber septum (8 mm outside diameter × 19 mm height; Wheaton, Millville, NJ, USA). A trap baited with a blank (solvent only) septum was prepared as a control. Traps were placed 0.6 m above the ground at approximately 1.5-m intervals. From 5 to 11 April 2016, seven replicates of the assay were performed, randomly changing the trap locations at each count. Data were log-transformed and analyzed by analysis of variance followed by Tukey-Kramer HSD tests for multiple comparisons. The attractiveness of the (1S,3R)-cis-isomer was further compared to that of a ca. 1:2 mixture of (±)-cis- and (±)-trans-isomers (technical grade pheromone) in a choice bioassay in a glass dish (9 cm diameter, 2 cm height); adult males (34 individuals in total) were released into the dish, and two pieces of filter paper (Toyo Roshi Co. Ltd., Tokyo, Japan), each of which was impregnated with 1 μg of the pure (1S,3R)-cis-isomer or the technical grade pheromone, were placed at opposite sides of the dish. After 5 min, the number of males on each sample was used as an index of attractiveness.
Results
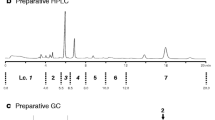
One pheromone candidate (X) that elicited a reproducible response from the antennae of male mealybugs eluted from a silica gel column in the 5% diethyl ether in hexane fraction, the fraction in which esters typically elute (Fig. 1a). Because no other EAD-active compounds were found, we focused on X as the pheromone candidate, and it was isolated by sequential preparative HPLC and GC. In the GC-MS analyses, X had a Kovats index of 1576 on a DB-1 column. High-resolution electron-impact MS data (m/z 236.17892) showed that the molecular formula was C15H24O2 (calcd. 236.17763), with four double bonds or rings. Characteristic mass fragments were observed at m/z 154 [M+ − 82 (C5H6O)], 136 [M+ − 100 (C4H7COOH)], 121 [136–15 (CH3)], and 93 [136–43 ((CH3)2CH)] (Fig. 1b). After basic ethanolysis, an alcohol with a molecular ion at m/z 154 (C10H17OH) (Fig. 1b) and the transesterification product of an acid with a molecular ion at m/z 128 (Fig. 1b) were generated. The GC retention time (7.96 min) and the mass spectrum of the alcohol were identical to those of authentic cis-chrysanthemyl alcohol. The GC retention time (4.63 min) and the mass spectrum of the ethyl-esterified acid were identical to those of authentic ethyl tiglate and were distinct from the GC retention time (4.11 min) and the mass spectrum of authentic ethyl angelate (the geometric isomer). Re-esterification of the ethanolysis product with tiglic acid restored the retention time and the mass spectrum pattern of the original X. These results suggested that X was cis-chrysanthemyl tiglate. Chrysanthemyl alcohol or related alcohols were not detected in the original crude volatile extracts.
a Gas chromatography–flame ionization detection (FID) and gas chromatography–electrophysiological antenna detection (EAD) chromatograms of the headspace volatile extracts of virgin females of the striped mealybug (Ferrisia virgata). The compound X, which elicited a clear male EAD signal, was selected as the pheromone candidate. b Mass spectra of X and its ethanolysis products
Approximately 7 μg of the isolated natural pheromone dissolved in 35 μL of C6D6 was used to obtain 1H–NMR spectra (Fig. 2) and 1H–1H correlation spectra. 1H–NMR analysis showed two olefinic protons, six sets of methyl protons, one set of methylene protons, and two methine protons. The chemical shifts of four of the methyl groups (singlets at 1.60, 1.64, and 1.83 ppm and a doublet at 1.38 ppm) indicated that they might be allylic. Two were expected to form an isopropylidene group, and the other two, including a doublet signal coupled with one olefinic proton, were expected to be included in the tigloyl structure. Moreover, the characteristic olefinic proton signal at 6.96 ppm indicated a tigloyl structure (6.60 ppm in prediction), rather than an angeloyl structure (6.03 ppm in prediction) with the opposite geometric configuration. The presence of the doublet methyl signal ruled out the other possible structure of the acid moiety (senecioyl group). The other olefinic proton (doublet at 5.05 ppm) was coupled with an allylic methine proton signal (1.37 ppm), which was further coupled with the other methine proton signal (1.15 ppm), which in turn was coupled to the methylene protons with chemical shifts of 4.28–4.35 ppm, indicating that the methylene was attached to the ester oxygen. Because no other protons except for two methyl signals (singlets at 0.98 and 0.99 ppm) were observed, the two methines were considered to be on a cyclopropyl ring with the third carbon of the ring having two geminal methyl groups. Accordingly, the pheromone candidate (X) was confirmed to be (2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropyl)methyl (E)-2-methyl-2-butenoate (chrysanthemyl tiglate).
We then prepared the four pure isomers, (1R,3S)-cis-, (1S,3R)-cis-, (1R,3R)-trans-, and (1S,3S)-trans-chrysanthemyl tiglate. The cis and trans configurations were confirmed by the 1H–NMR coupling constant between the two vicinal protons on the cyclopropane ring; the vicinal H–H coupling constant was expected to be relatively large in the cis configuration (J = 8.3 Hz) compared to that of a trans configuration (J = 5.5 Hz), because of their respective dihedral angles, according to the Karplus equation (Hesse et al. 2008). The 1H–NMR spectrum, the mass spectrum, and the GC retention time of the natural pheromone candidate (X) were identical to those of synthetic cis-chrysanthemyl tiglate. Chiral stationary phase HPLC analyses indicated the absolute configuration of X to be (1S,3R)-cis; both X and the (1S,3R)-(−)-cis-isomer eluted at 9.3 min, whereas the (1R,3S)-(+)-cis-isomer eluted at 8.7 min. Subsequently, the attractiveness of the four synthetic isomers of chrysanthemyl tiglate was tested in a greenhouse bioassay. The (1S,3R)-cis-isomer, with the natural configuration, was most attractive to males (Fig. 3). The other isomers were less attractive, but still more attractive than the control (Wilcoxon test, approximated χ 2 = 8.95–11.3, df = 1, P < 0.05). In a follow-up bioassay comparing the pure (1S,3R)-cis-isomer versus technical grade pheromone comprised of a ca. 1:2 mixture of the (±)-cis- and (±)-trans-isomers, 20 males were attracted to the former while 14 males were attracted to the latter, indicating no significant difference between their attractiveness (χ 2 = 1.05, df = 1, P = 0.303).
Responses of Ferrisia virgata males to stereoisomers of synthetic chrysanthemyl tiglate. Purities of each stereoisomer are >99.9% in geometric isomerism. Enantiomeric excesses of (1R,3S)-cis, (1S,3R)-cis, (1R,3R)-trans, and (1S,3S)-trans are >99.9%, 98.6%, >99.9%, and >99.9%, respectively. Blank: solvent (hexane) only. Significant differences are indicated with lowercase letters (ANOVA followed by Tukey-Kramer HSD test, N = 7, P < 0.05)
Discussion
We identified the female-produced sex pheromone of the striped mealybug (F. virgata) to be (1S,3R)-(−)-cis-chrysanthemyl tiglate. Chrysanthemyl esters now have been identified as sex pheromones of three mealybug species, Ph. madeirensis, Ps. calceolariae, and F. virgata, although the former two pheromones have the (1R,3R)-trans-chrysanthemyl structure. These three mealybugs all produce only one enantiomer of their own chrysanthemyl pheromones, with no contamination from the other isomers, indicating that each species biosynthesizes its pheromone de novo with strict stereoselectivity.
In addition to using different chrysanthemol isomers in the alcohol moieties of their pheromones, Ph. madeirensis, Ps. calceolariae, and F. virgata also use different acid moieties to form the pheromone esters: (R)-2-methylbutanoate (Ho et al. 2009), (R)-2-acetoxy-3-methylbutanoate (El-Sayed et al. 2010; Unelius et al. 2011), and tiglate (present study), respectively. In general, such differences are expected to be essential for discrimination among sympatric species when they occur simultaneously on the same host plants. However, these three mealybugs from different genera and different geographic regions have no long history of coevolution, and so their related but unique pheromone structures have evolved independently of any interference among them. But it is possible and even likely that they have faced competition for their pheromone channels for other, as yet unknown species in their native regions.
On the basis of morphological and molecular data, mealybugs of the genus Ferrisia were recently revised to include 18 species, and F. virgata, the most widespread pest in the genus, is distinguished from many Nearctic and Neotropical populations previously recognized as F. virgata (Kaydan and Gullan 2012). Some of these species are very closely related, making identifications difficult. For example, F. dasylirii exhibits morphological and molecular variation among some populations and is difficult to distinguish from F. virgata (Kaydan and Gullan 2012). Further pheromone studies of other members of the genus would be valuable to support taxonomic and systematic studies of this group, which includes important agricultural pests, because mealybug pheromone structures appear to be species-specific and are clearly different even among some closely related congeners (Tabata et al. 2017; Zou and Millar 2015).
Some mealybugs have recently expanded their distributions, apparently in association with increasing movement of plants by humans and/or climate change (Gullan et al. 2003; Jara et al. 2013). Because F. virgata is a highly polyphagous species, which potentially damages a broad range of plants (Ben-Dov 1994), it is important to detect incursions into new areas of the world. Traps baited with pheromones would be useful monitoring tools for mealybug pests (Tabata and Ichiki 2015, 2016); pheromone traps can capture these cryptic and morphologically indistinguishable insects effectively, with minimal cost and effort. Furthermore, a mixture of stereoisomers of chrysanthemyl tiglate, easily produced from commercially available chrysanthemyl alcohol and tiglic acid, can be used for lures for F. virgata, because its attractiveness appeared to be equivalent to that of the pure (1S,3R)-cis-isomer with the natural configuration.
References
Ben-Dov Y (1994) A systematic catalogue of the mealybugs of the world. Intercept, Andover, p 686
Charles JG (2005) Mealybug insecticide resistance management strategy. In: Martin NA, Beresford RM, Harrington KC (eds) Pesticide resistance: prevention and management strategies. New Zealand Plant Protection Society, Hastings, pp 126–132
El-Sayed AM, Unelius CR, Twidle A, Mitchell V, Manning L-A, Cole L, Suckling DM, Flores MF, Zaviezo T, Bergmann J (2010) Chrysanthemyl 2-acetoxy-3-methylbutanoate: the sex pheromone of the citrophilous mealybug, Pseudococcus calceolariae. Tetrahedron Lett 51:1075–1078
Ghose SK, Paul PK (1972) Observation on the biology of the mealybug, Ferrisia virgata (Cockerell) (Pseudococcidae: Hemiptera). Proc Zool Soc (Calcutta) 25:39–48
Gullan PJ, Downie DA, Steffan SA (2003) A new pest species of the mealybug genus Ferrisia Fullaway (Hemiptera: Pseudococcidae) from the United States. Ann Entomol Soc Am 96:723–737
Gut LJ, Stelinski LL, Thomson DR, Miller JR (2004) Behaviour-modifying chemicals: prospects and constraints in IPM. In: Koul O, Dhaliwal GS, Cuperus GW (eds) Integrated pest management: potential, constraints and challenges. CABI Publishing, Oxford, pp 73–122
Hesse M, Meizer H, Zeeh B (2008) Spectroscopic methods in organic chemistry. Georg Thieme Verlag KG, Stuttgart, pp 74–241
Ho H-Y, Su Y-T, Ko C-H, Tsai M-Y (2009) Identification and synthesis of the sex pheromone of the Madeira mealybug, Phenacoccus madeirensis green. J Chem Ecol 35:724–732
Hughes WA, Lister CA (1953) Lime die-back in the gold coast, a virus disease of the lime, Citrus aurantifolia (Christmann) Swingle. J Hort Sci 28:131–140
Jara V, Meza FJ, Zaviezo T, Chorbadjian R (2013) Climate change impacts on invasive potential of pink hibiscus mealybug, Maconellicoccus hirsutus (green), in Chile. Clim Chang 117:305–317
Kaydan MB, Gullan PJ (2012) A taxonomic revision of the mealybug genus Ferrisia Fullaway (Hemiptera: Pseudococcidae), with descriptions of eight new species and a new genus. Zootaxa 3543:1–65
Khambay BPS, Jewess PJ (2010) Pyrethroids. In: Gilbert LI, Gill SS (eds) Insect control. Academic Press, London, pp 1–29
Kim B-H, Lee SU, Kim KT, Lee J-Y, Choi NH, Han Y-K, Ok JH (2003) Enantiomeric discrimination of pyrethroic acid esters on polysaccharide derived chiral stationary phases. Chirality 15:276–283
Le Pellery RH (1968) Pest of coffee. Longmans, London, p 590
Morishita M (2005) Resurgence of Japanese mealybug, Planococcus kraunhiae (Kuwana), in persimmon induced by a synthetic pyrethroid cypermethrin. Annu Rep Kansai Plant Protect Soc 47:125–126
Posnette AF, Strickland AH (1948) Virus diseases of cacao in west Africa. Ann Appl Biol 35:53–63
Sawamura N, Narai Y, Teshiba M, Tsutsumi T, Mochizuki M, Toda S, Suzuki T, Ichihashi H, Tabata J, Sasaki R (2015) Forecasting the occurrence of young Japanese mealybug larva Planococcus kraunhiae (Kuwana) (Hemiptera: Pseudococcidae) using sex pheromone traps and total effective temperature for persimmon. Jpn J Appl Entomol 59:183–189
Tabata J, Ichiki RT (2015) A new lavandulol-related monoterpene in the sex pheromone of the grey pineapple mealybug, Dysmicoccus neobrevipes. J Chem Ecol 41:194–201
Tabata J, Ichiki RT (2016) Sex pheromone of the cotton mealybug, Phenacoccus solenopsis, with an unusual cyclobutane structure. J Chem Ecol 42:1193–1200
Tabata J, Ichiki RT, Moromizato C, Mori K (2017) Sex pheromone of a coccoid insect with sexual and asexual lineages: fate of an ancestrally essential sexual signal in parthenogenetic females. J R Soc Interface 14:20170027
Unelius CR, El-Sayed AM, Twidle A, Bunn B, Zaviezo T, Flores MF, Bell V, Bergmann J (2011) The absolute configuration of the sex pheromone of the citrophilous mealybug, Pseudococcus calceolariae. J Chem Ecol 37:166–172
Wesołowska A, Grzeszczuk M, Kulpa D (2015) GC-MS analysis of the essential oil from flowers of Chrysanthemum coronarium L. propagated conventionally and derived from in vitro cultures. Acta Chromatogr 27:525–539
Zou Y, Millar JG (2015) Chemistry of the pheromones of scale and mealybug insects. Nat Prod Rep 32:1067–1113
Acknowledgments
NMR spectrometry analyses were carried out with the support of the Advanced Analysis Center at the National Agriculture and Food Research Organization. We acknowledge a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (no. 16 K08103) to JT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabata, J., Ichiki, R.T. (1S,3R)-cis-Chrysanthemyl Tiglate: Sex Pheromone of the Striped Mealybug, Ferrisia virgata . J Chem Ecol 43, 745–752 (2017). https://doi.org/10.1007/s10886-017-0879-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0879-z