Abstract
Sexually mature male harlequin bugs produced a sex-specific compound, identified as one of the stereoisomers of the sesquiterpene epoxyalcohol 4-[3-(3,3-dimethyloxiran-2-yl)-1-methylpropyl]-1-methylcyclohex-2-en-1-ol (henceforth murgantiol), a compound with four chiral centers and 16 possible stereoisomers. Production of the compound was highest during the middle of the day. Individual virgin male bugs in separate containers produced the compound at a higher rate than virgin males in groups. The carbon skeleton was verified by synthesis of several mixtures which, in total, contained all possible isomers, one of which matched the insect-produced compound. The relative and absolute configurations of the insect-produced compound remain to be determined. In laboratory bioassays, insect-produced and synthetic murgantiol attracted harlequin bugs of both sexes, suggesting that murgantiol is a male-produced aggregation pheromone, analogous to those found in a number of other phytophagous bug species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The harlequin bug, Murgantia histrionica (Hahn), is an important pest of cabbage, broccoli, and other cole crops in the United States (McPherson 1982). Methods for monitoring this and other stink bug species, such as sampling the crop by using a sweep net or a beating tray, or by visual inspection of damage or presence of insects, are typically labor-intensive. Sampling at frequent intervals may be required to detect immigration of bug populations into crops, so that control measures can be applied before significant damage occurs. Consequently, attractive sex or aggregation pheromones used as baits in monitoring traps could prove of value in integrated pest management programs.
To date, pheromones have been identified for only a few stink bug species. For all phytophagous pentatomids for which sex or aggregation pheromones have been identified, the compounds were produced by males (reviewed in McBrien and Millar 1999; Ho and Millar 2001a, b; Millar 2005). In addition to using sex-specific pheromones, phytophagous stink bugs use species- and sex-specific substrate-borne vibrations for intraspecific communication over relatively short distances (Čokl and Doberlet 2003). The vibrational signals of M. histrionica were recently described (Čokl et al. 2004), with males producing five different vibrational songs and females producing only one song. Contrary to those found for most stink bugs investigated, the vibrational signals did not seem to play a role in orientation toward the source of the vibration (i.e., mate location). Rather, the vibrational signals appeared to function in close-range mate recognition and courtship, suggesting that signals other than vibrational signals may be important in bringing the two sexes together over greater distances.
Harlequin bugs produce or sequester a variety of chemicals. For example, the metathoracic scent gland secretion of adult M. histrionica consists of (2E,6E)-octadienedial and (2E,6E)-octadiene-1,8-diol diacetate (Aldrich et al. 1996). Additionally, when adults are squeezed, a sticky froth is expelled from the margins of the prothorax. This fluid contains aglycones of glucosinolates, sec-butyl isothiocyanate, methylthiopropyl nitrile, and methylthiobutyl nitrile, as well as two strong-smelling alkylmethoxypyrazines, 2-sec-butyl-3-methoxypyrazine and 2-isopropyl-3-methoxypyrazine, which have been hypothesized to serve as warning odors (Aldrich et al. 1996). These compounds are likely derived from glucosinolates sequestered from host plants, providing the vividly colored aposematic bugs with a formidable chemical defense against predation. The bugs can retain these compounds for an extended period of time (Aliabadi et al. 2002). However, none of these compounds has been reported to have a role as pheromones.
We describe herein the collection and analysis of volatile compounds released by live adult M. histrionica of both sexes. In addition to a number of compounds common to both sexes, one major sex-specific compound was found in the odors released by sexually mature adult males. This compound functions as an aggregation pheromone component for the harlequin bug.
Materials and Methods
Insect Colonies
Adults and nymphs of M. histrionica were collected from bladderpod, Isomeris arborea Nutt. (Caperaceae), from one site each in Riverside and San Diego, CA, USA. A colony was started in the laboratory in 2003 and augmented every year since with approximately 100 males, females, and nymphs from each location. Voucher specimens were submitted to the University of California, Riverside Entomology Research Museum (UCRC 145863–145882). Insects were reared at 26 ± 1°C, approximately 45% RH, with a photoperiod of 16:8 h (L:D) provided by fluorescent lights. Immature insects were held separately from adults in cylindrical plastic containers (20 × 15 cm), with two 4-cm holes on opposite sides of each container covered with brass screening. Adults were held in 30 × 17 × 18 cm glass-topped rearing cages. Insects were provided with Napa cabbage, cauliflower, and broccoli three times per week. Upon eclosion, adults were sexed and maintained for 14 d with florets of broccoli in 175 ml transparent plastic cups, or in groups in 3.8-l cardboard ice-cream cartons with ventilated lids, before use.
Collection of Insect-Produced Compounds from Live Bugs
A system for collecting volatile compounds from live organisms was set up as previously described (Millar and Sims 1998). Ten to fifteen virgin adult bugs and two large cauliflower florets were put into a two-part, 400 ml cylindrical glass aeration chamber, lined with screen upon which bugs could perch. Humidified, charcoal-filtered air (6–14 mesh activated charcoal granules) was drawn through the chamber by vacuum at 250 ml per min. Volatile chemicals were collected overnight on activated charcoal traps made from 4 mm ID glass tubes loaded with a 0.4-cm bed of 50–200 mesh activated charcoal (Cat. # 05–690A, Fisher Scientific, Pittsburgh, PA, USA), precleaned by heating at 200°C under a flow of clean N2 (~100 ml per minute). Aerations of groups of bugs were conducted continuously for 2–3 weeks at ~25°C and 50% RH with cohorts of male and female bugs of known age, changing the food and the collectors every other day. Aerations of individual bugs were conducted in the same way, but using smaller, 200-ml chambers. All aeration chambers were within the main colony rearing room. Additional cohorts of sexed virgin bugs, of the same age as those in the chamber, were maintained and used to replace any bugs in the chamber that died. As a control, two to three cauliflower florets were put in a 1-l aeration chamber and aerated for 20 h with an air flow rate of 1 l per minute. Trapped volatiles were stripped off the activated charcoal by elution with methylene chloride (500 μl; Optima grade, Cat. # D151–4, Fisher Scientific, Pittsburgh PA, USA), and the resulting extracts stored in glass vials with Teflon-lined screw caps at ~−20°C until needed.
Single-bug and group (5, 10, and 15) aerations were set up simultaneously to determine whether the male-specific compound was released at different rates by individuals or groups. For this, bugs were either held individually (N = 20) or in groups (N = 20 each group) for 2 weeks before use. Traps were changed every other day and eluted with 500 μl of CH2Cl2. The amounts of male-specific compound per bug hour were analyzed by one-way ANOVA and means were separated by Games–Howell tests.
To determine the diurnal cycle of release of the male-specific compound, five cohorts of five virgin, sexually mature, adult males (14–16 days old), and three florets of cauliflower were aerated in chambers as described above. After a cohort of bugs was confirmed to be producing the male-specific compound, the traps were changed every 2 h during the photophase. A single trap was used to collect volatiles produced between 23:00 and 07:00. An external standard of hexadecane in CH2Cl2 (1.6 µg/ml) was used to estimate the amount of male-specific compound present in each extract. The total amounts of the male-specific compound produced during each 24-h period were calculated along with the percentage of the total produced during each 2-h interval. For the 8-h period between 23:00 and 07:00, the average amount per 2 h was calculated. The percentage data from all cohorts were averaged, analyzed by ANOVA, and means were separated by Student–Newman–Keuls tests.
General Procedure for Y-tube Bioassays
Bioassays were conducted with a vertical glass Y-tube (5 cm ID; bottom arm = 18 cm long, top arms = 15 cm long, Y angle = 90°) with female ground glass joints, each fitted with male joints terminating in a hose nipple. The Y-tube was illuminated from above with a 1.2-m long light bank containing a Sylvania Octron 3500K F032/735 32 W fluorescent light and a GE F40PL/AQ Plant and Aquarium wide spectrum fluorescent light, providing light intensities of 350 lux at the bottom to 600 lux at the top of the Y-tube. Charcoal-purified air was pulled through the apparatus (2.5 liter per minute) by vacuum. Bugs were tested individually and used only once. Each bug was gently placed inside the bottom male joint of the Y-tube, which was then inserted into the female joint. M. histrionica are positively phototactic and negatively geotactic, and generally walked up toward the light. A positive response was classified as a bug crossing a line 12 cm up either arm from the junction. If a bug did not move within 10 min of the start of the bioassay, it was discarded. Each Y-tube and joint was used once, then washed, acetone rinsed, and oven-dried at 140°C. Bioassays were conducted in a temperature (22–25°C) and humidity (40–50% RH) controlled room, 4 to 8 h after the onset of photophase. Positions of treatments were alternated every second replicate. Choice data were analyzed with chi-square tests with one degree of freedom and α = 0.05.
The Y-tube olfactometer was also used to determine which sex of adult M. histrionica produced pheromone. The top joints of the Y-tube were connected to 200 ml glass aeration chambers that contained five sexually mature virgin bugs and two cauliflower florets, or two cauliflower florets alone. Chambers were positioned above the Y-tube, behind a cardboard screen so contents were not visible to the bugs in the Y-tube. Ten minutes after the chambers were set up, a test bug was introduced into the bottom of the Y-tube and its response recorded by an observer and on video tape (Panasonic high-density recording, time lapse video recorder, model AG-6740p). A fresh group of five unmated, sexually mature bugs was used as a pheromone source for each day of bioassay. Four separate experiments were conducted, testing the responses of females to odors from males, females to odors from females, males to odors from females, and males to odors from males. The responses of 40 bugs were tested in each experiment. Because females did not attract males, but males attracted females, only females were used as responders in the subsequent experiments, except where indicated otherwise.
The numbers of virgin female M. histrionica attracted to 5 bug-hour equivalents (1 bug-hour equivalent = the volatiles collected from 1 bug in 1 h) of crude extract in CH2Cl2 vs a CH2Cl2 blank were compared. Aliquots of extract or solvent were loaded on half-circular filter papers (1.5 cm in diameter) mounted on wires in the top of each arm of the Y-tube. Five bug-hours of extract contained ~30 ng of the major male-specific component, as determined by gas chromatography.
A pooled extract of male-produced odors was separated into eight fractions (see below), and the fraction containing the major male-specific compound (5 bug-hour equivalents) was bioassayed against a CH2Cl2 control. Equal aliquots of the remaining seven fractions were combined and tested against a CH2Cl2 control. Having determined that only the fraction containing the major male-specific compound was attractive to females, this fraction was then tested against an equivalent amount of crude extract.
Mixtures containing synthetic enantiomers (see below) of the major male-specific compound were tested using 14-day-old sexually mature adults of both sexes. The mixture derived from (S)-citronellal was tested vs a solvent control, and both synthetic mixtures were tested vs the crude extract. Finally, equal doses of the two synthetic mixtures were tested against each other. The mixtures (240 ng of each mixture of four stereoisomers, ~60 ng/isomer), in CH2Cl2, and solvent controls, were loaded onto filter paper discs as described above.
Gas Chromatography (GC) and Mass Spectrometry (MS) Analyses of Extracts
Extracts were concentrated under a gentle stream of nitrogen, and analyzed by splitless gas chromatography with a Hewlett–Packard (H–P) 5890 GC fitted with a DB-5 column (30 m × 0.32 mm ID × 0.25 μm film, J&W Scientific, Folsom, CA, USA), using a temperature program of 40°C for 1 min, then 15°C min−1 to 250°C. The injector and detector temperatures were 250°C and 280°C, respectively, and helium was used as carrier gas. Extracts were also analyzed by GC-MS, using an HP 5890 GC, fitted with a DB5-MS column (20 m × 0.2 mm ID × 0.33 μm film), interfaced to an HP 5970B mass selective detector (electron impact ionization, 70 eV). The same temperature program described above was used, with injector and transfer line temperatures of 250 and 280°C, respectively. Injections (1 µl) were made in splitless mode. Previously known compounds were tentatively identified from their mass spectra, and from matches with the NBS-NIH mass spectral database. Identifications were confirmed by matching retention times and mass spectra with those of authentic standards (see below).
An aliquot of the purified male specific compound was also analyzed by chemical ionization mass spectrometry (NH3 reagent gas) by direct insertion on a VG-7070B instrument.
Fractionation of Extracts of Volatiles Collected from Sexually Mature Male Bugs
Thirty aeration extracts of sexually mature male bugs, prepared from May 2003 to August 2005, were combined and concentrated to ~1 ml by fractional distillation of the solvent through an 8-cm long Vigreux column. The concentrated extract was transferred to a vial and the solvent removed under a gentle stream of nitrogen. The sample was reconstituted to ~250 μl with pentane, and then loaded onto a 2.8-ml solid phase extraction (SPE) cartridge (J.T. Baker silica gel SPE, stock #7086–01, precleaned by flushing with 3 × 1 ml diethyl ether, then 3 × 1 ml pentane). The extract was rinsed onto the SPE bed with two to three drops of pentane, and eluted with 2 × 1 ml pentane, 2 × 1 ml 10% ether in pentane, and 4 × 1 ml ether, collecting each elution as a separate fraction.
Aliquots (100 µl) of crude extract were subjected to a series of microchemical tests to ascertain characteristics of the male-specific compound.
-
a)
To test for the presence of a conjugated diene, an aliquot was treated with two drops of a solution of the dienophile, 4-methyl-1,2,4-triazolin-3,5-dione (MTAD; Young et al. 1990) in CH2Cl2 (2 mg/ml), producing a pale pink solution which was analyzed immediately by GC-MS.
-
b)
To test for an ester function, an aliquot was concentrated just to dryness, and then treated with three drops of 1 M NaOH in ethanol to water (95:5). After standing at room temperature for 4 h, 200 µl of water were added and the mixture extracted with 500 µl pentane. The pentane extract was dried over Na2SO4, concentrated under a stream of nitrogen, and analyzed by GC-MS.
-
c)
To test for an alcohol function, an aliquot was treated with one drop each of pyridine and acetyl chloride. The resulting mixture was stirred for 2 h, treated with 200 µl of saturated aqueous NaHCO3, and extracted with 0.5 ml pentane. The pentane extract was concentrated and analyzed as above.
-
d)
To test for reducible functional groups, an aliquot in diethyl ether was treated with ~1 mg of LiAlH4, with stirring at room temperature for 2 h. The mixture was quenched with 1 M HCl and extracted with 0.5 ml pentane. The pentane extract was dried over Na2SO4, concentrated, and analyzed.
-
e)
To test for unsaturation, an aliquot of extract and ~1 mg of 5% Pd on carbon were placed in a 1.5-ml screwcap vial with a Teflon-lined septum. The septum was punctured with a syringe needle attached to a balloon filled with hydrogen, the lid was loosened for 10 s to flush the vial with hydrogen and resealed, and the mixture stirred for 2 h. The reactant mixture was filtered through a small plug of Celite and analyzed.
NMR Analysis of the Male-Specific Compound
Forty-two extracts were combined, concentrated, and fractionated as described above, and the fraction containing the male-specific was compound concentrated under a gentle stream of nitrogen, taking the solution just to dryness. Approximately 200 µl of CD2Cl2 were added, and the solution was concentrated just to dryness again to remove traces of non-deuterated solvent. The residue (estimated at >100 µg) was then taken up in CD2Cl2 and transferred to a 3-mm NMR tube. 13C, 1H, and 1H-1H COSY spectra were obtained with both a Varian INOVA-400 and a Brucker 600 MHz NMR spectrometer; the latter instrument was also used to obtain NOESY, 13C-DEPT 135, HMBC, and HSQC spectra.
Chemicals
Cineole, limonene, myrcene, dodecane, and dimethyldisulfide were purchased from Aldrich Chemical Co. (Milwaukee, WI, USA). Dimethyltrisulfide was obtained from Alfrebro (Monroe, OH, USA). The bisabolene epoxide pheromones of Acrosternum hilare were available from previous studies (McBrien et al. 2001). The sex-specific compound produced by male harlequin bugs was synthesized as described below.
For the syntheses, unless stated otherwise, all reactions were carried out under argon. Tetrahydrofuran (THF) used in the synthesis was distilled from sodium/benzophenone ketyl under argon. 1H- and 13C-NMR spectra were taken as CDCl3 or CD2Cl2 solutions, recorded on a Varian INOVA-400 (400 and 100 MHz, respectively) spectrometer. Chemical shifts are expressed in parts per million (ppm) (δ-scale) relative to CDCl3 (7.26 and 77.23 ppm) and CD2Cl2 (5.32 and 54.00 ppm) signals for 1H and 13C NMR, respectively. Routine GC analyses were performed with an HP 5890 series II GC, equipped with a DB-5 column (30 m × 0.25 μm ID, 0.25 μm film thickness, J&W Scientific, programmed from 100°C for 1 min, then at 15°C min−1 to 250°C) or a DB-WAX column (30 m × 0.25 μm ID, 0.25 μm film thickness, J&W Scientific), programmed from 40°C for 1 min, then at 5°C min−1 to 250°C).
Preparation of (3S)-α-Methylene-3,7-dimethyl-6-octen-1-al (2)
Piperidine (5.39 g, 63.3 mmol) was added dropwise over ~5 min to a three-necked, round-bottomed flask, containing (S)-citronellal 1 (4.69 g, 30.4 mmol; Aldrich Chem. Co., Cat. #37375-3, 96%), attached to a condenser. The temperature was increased to 44°C and the mixture became cloudy, and then cleared again. Formaldehyde (37% aqueous solution, 7.8 ml) was added dropwise, keeping the temperature under 40°C, followed by glacial acetic acid (0.78 ml) dropwise. The mixture was stirred overnight at room temperature and then heated to 80°C for 1.5 h. The mixture was cooled to room temperature, diluted with hexanes (80 ml), then washed sequentially with HCl (2 M in water, 2 × 30 ml), saturated aqueous NaHCO3 (20 ml), and brine (20 ml). The organic phase was separated and dried with anhydrous Na2SO4 and concentrated under vacuum. The crude product was Kugelrohr distilled (64–67°C, 1.6 mm Hg), affording 4.01 g of aldehyde 2 (79.3% yield). 1H NMR (400 MHz, CDCl3): δ 1.07 (d, 3H, J = 7.2 Hz), 1.33–1.44 (m, 1H), 1.48–1.56 (m, 1H), 1.57 (s, 3H), 1.67 (s, 3H), 1.86–2.00 (m, 2H), 2.70 (sext, 1H, J = 7.0 Hz), 5.08 (br t, 1H, J = 7.0 Hz), 5.99 (s, 1H), 6.23 (s, 1H), 9.53 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 17.85, 19.75, 25.89, 25.96, 31.18, 35.79, 124.34, 131.88, 133.25, 155.71, 194.89. MS (m/z, rel. abundance): 166 (M+, 8), 151 (8), 137 (2), 135 (3), 133 (4), 124 (4), 123 (8), 121 (1), 110 (8), 109 (41), 107 (3), 106 (2), 105 (4), 96 (3), 95 (18), 93 (9), 91 (5), 84 (10), 83 (15), 81 (18), 69 (28), 67 (26), 56 (12), 55 (51), 53 (16), 43 (16), 41 (100).
Preparation of 4-[(1S)-1,5-Dimethylhex-4-enyl]cyclohex-2-en-1-one (3)
Sodium (177 mg, 7.7 mmol) was added to a 200-ml, three-necked, round-bottomed flask containing methanol (23 ml) and attached to a condenser. After the sodium had dissolved, a solution of methyl acetoacetate (2.74 g, 23.6 mmol) in methanol (35 ml) was slowly added (~20–25 min) and the mixture was stirred 30 min at room temperature. A solution of aldehyde 2 (3.92 g, 23.6 mmol) in methanol was added dropwise over 40 min. The resulting mixture was stirred 2 h at room temperature and then refluxed for 2 h. After cooling, the methanol was removed under vacuum, the residue diluted in hexanes (100 ml), washed with saturated aqueous NH4Cl, water and brine, dried over anhydrous Na2SO4, and concentrated under vacuum. The crude product was purified by vacuum flash chromatography on silica gel, eluting with hexane/ethyl acetate (97:3). The resulting product was purified further by Kugelrohr distillation (112–117°C, 0.35 mm Hg), affording 2.19 g of cyclohexenone 3 (45.1% yield) as a ~1:1 mixture (estimated from 1H and 13C NMR spectra) of inseparable diastereomers. 1H NMR (CDCl3): δ 0.90 (d, 1.5H, J = 6.8 Hz), 0.94 (d, 1.5H, J = 7.2 Hz), 1.20–1.32 (m, 1H), 1.38–1.48 (m, 1H), 1.62 (s, 3H), 1.70 (s, 3H), 1.90–2.15 (m, 4H), 2.30–2.56 (m, 4H), 5.06–5.14 (m, 1), 6.02 (dt, 1H, J = 3.2 and 10.2 Hz), 6.82–6.90 (m, 1H). 13C NMR (CDCl3): d 16.23, 16.75, 17.91, 24.21, 25.92, 26.07, 34.14, 34.34, 36.29, 36.35, 37.74, 37.93, 41.30, 41.85, 124.34, 124.37, 129.91, 130.17, 132.03, 132.04, 154.54, 155.64, 200.34. Several of these peaks corresponded to composite signals from carbons in both diastereomers. MS (m/z, rel. abundance) 206 (M+, 9), 191 (4), 177 (1), 163 (5), 149 (4), 136 (6), 135 (5), 124 (5), 123 (36), 122 (30), 121 (16), 109 (13), 107 (10), 96 (18), 95 (13), 94 (16), 91 (4), 79 (16), 77 (6), 69 (61), 67 (25), 55 (31), 53 (15), 43 (10), 41 (100).
Preparation of 4-[(1S)-1,5-Dimethylhex-4-enyl)-1-methylcyclohex-2-en-1-ol (4)
A solution of cyclohexenone 3 (2.18 g, 10.6 mmol) in diethyl ether (20 ml) was cooled to −78°C, and methyl lithium (26.4 ml of a 1.2-M solution in hexanes, 31.7 mmol) added dropwise. The resulting mixture was stirred 4 h, warmed to room temperature, and stirred overnight. The reaction was quenched with dilute aqueous NH4Cl and extracted with ethyl acetate (4 × 20 ml). The combined organic phase was washed with brine, dried over anhydrous Na2SO4, and concentrated by rotary evaporation. The residue was purified by flash chromatography on silica gel, eluting with 5% ethyl acetate in hexane, yielding alcohols 4 in two fractions (less polar fraction, higher Rf, 0.681 g; more polar fraction, lower Rf, 1.272 g; 83.1% yield). Each fraction consisted of mixtures of two diastereomers that were inseparable by flash chromatography; each mixture eluted as a single peak on a DB-5 GC column. Less polar diastereomers: 1H NMR (CDCl3): δ 0.84 (d, 1.5H, J = 7.2 Hz), 0.89 (d, 1.5H, J = 6.8 Hz), 1.28 (s, 3H), 1.61 (s, 3H), 1.69 (s, 3H), 1.14–1.60 (m, 7H), 1.80–2.08 (m, 4H), 5.07–5.14 (m, 1H), 5.59–5.70 (m, 2H). MS (m/z, rel. abundance): 222 (M+, 3), 207 (10), 204 (M+-18, 7), 189 (5), 179 (2), 161 (8), 151 (4), 148 (6), 147 (4), 138 (12), 137 (17), 133 (7), 123 (15), 121 (12), 119 (39), 109 (22), 108 (4), 107 (10), 105 (9), 95 (23), 94 (22), 93 (24), 91 (12), 82 (12), 81 (13), 79 (19), 77 (14), 69 (91), 67 (25), 55 (37), 53 (15), 43 (80), 41 (100). More polar diastereomers: 1H NMR: (CDCl3): δ 0.82 (d, 1.5H, J = 6.4 Hz), 0.85 (d, 1.5H, J = 7.2 Hz), 1.10–1.22 (m, 1H), 1.27 (s, 3H), 1.32–1.55 (m, 4H), 1.60 (s, 3H), 1.68 (s, 3H), 1.60–1.75 (m, 2H), 1.83–2.14 (m, 4H), 5.06–5.13 (m, 1H), 5.48–5.65 (m, 2H). MS (m/z, rel. abundance): 222 (M+, 2), 207 (11), 204 (M+-18, 10), 189 (3), 179 (2), 161 (8), 151 (4), 148 (3), 147 (3), 138 (14), 137 (20), 133 (6), 123 (20), 121 (14), 119 (38), 109 (19), 108 (4), 107 (10), 105 (9), 95 (23), 94 (11), 93 (23), 91 (12), 82 (13), 81 (13), 79 (14), 77 (14), 69 (86), 67 (24), 55 (37), 53 (15), 43 (83), 41 (100).
Preparation of 4-[3S-(3,3-Dimethyloxiran-2-yl)-1-methylpropyl]-1-methylcyclohex-2-en-1-ol ((S)-murgantiol—5)
m-Chloroperbenzoic acid (ca. 70%, 0.333 g, ~1.35 mmol) was added in small portions over 30 min to a well-stirred suspension of the less polar, higher Rf, fraction of alcohols 4 (0.300 g, 1.35 mmol) in an aqueous solution of NaHCO3 (0.5 M, 10 ml) in an ice-bath (Fringuelli et al. 1992). The mixture was stirred 3 h at 0°C, then brine (50 ml) added, and the mixture was extracted with hexanes (4 × 20 ml). The combined organic phases were dried with anhydrous Na2SO4 and concentrated by rotary evaporation. The residue was purified by vacuum flash chromatography (hexanes/ethyl acetate 5:1) to yield 0.240 g of epoxyalcohols 5 (74.5%) as an inseparable mixture of diastereomers. 1H NMR (400 MHz, CD2Cl2): δ 0.86 (d, 1.5H, J = 6.8 Hz), 0.90 (d, 1.5H, J = 6.0 Hz), 1.24 (S, 6H), 1.27 (S, 3H), 1.35–1.70 (m, 9H), 1.76–1.87 (m, 1H), 1.99–2.10 (M, 1H), 2.63–2.70 (M, 1H), 5.58–5.70 (m, 2H). The 13C spectrum was complex because of the mixture of stereoisomers, but contained peaks that matched those of insect-produced murgantiol. MS (m/z, rel. abundance) 205 (2), 165 (9), 147 (6), 138 (10), 135 (4), 134 (13), 132 (10), 123 (10), 122 (4), 121 (12), 119 (11), 109 (15), 107 (9), 106 (6), 105 (9), 95 (15), 94 (19), 93 (17), 91 (11), 81 (15), 79 (18), 77 (11), 71 (27), 69 (12), 67 (15), 59 (20), 55 (21), 43 (100), 41 (47).
Preparation of 4-[3R-(3,3-Dimethyloxiran-2-yl)-1-methylpropyl]-1-methylcyclohex-2-en-1-ol ((R)-murgantiol—5)
An analogous mixture of diastereomers containing the other enantiomer of murgantiol was prepared in identical fashion, and in similar yield, using (R)-citronellal (Aldrich Chem. Co, technical grade, 90%) as the starting material.
Results
Y-tube Bioassays with Odors from Live Insects, or Insect Extracts
Sexually mature females were significantly attracted to odors from live males in Y-tube bioassays (Fig. 1). Males were also significantly attracted to odors from other males. In contrast, odors from live females were not attractive to either sex (Fig. 1).
Results of vertical Y-tube bioassays that tested attraction of sexually mature virgin Murgantia histrionica to odors from live, sexually mature virgin conspecifics on a cauliflower floret vs odors from a cauliflower floret control (N = 24). Data were analyzed with χ 2 tests. **P < 0.001; ns not significant, P > 0.05
Crude extracts of male-produced odors were significantly more attractive to female M. histrionica than a solvent control (Fig. 2). Fractionation of the extract and bioassay of the fractions showed that the fraction containing the male-specific component (eluted from the silica gel cartridge with the first aliquot of 100% ether) was attractive to females (Fig. 2), whereas the recombination of the remaining seven fractions appeared to be repellent (Fig. 2). In a follow-up bioassay, the active fraction was as attractive as the crude extract (N = 24, χ 2 = 0.22, P > 0.05), suggesting that the fraction containing the male-specific compound, and possibly the compound itself, may be responsible for all the attractiveness of the extract.
Production of the Male-Specific Compound by Individual Males or Males in Groups
Males that were aerated individually, or in groups of only five, produced more murgantiol per bug than males aerated in groups of ten or more (Fig. 3). Individual bugs and bugs aerated in groups of five produced the male-specific compound at similar rates (Games–Howell test, P = 0.41; Games and Howell 1976).
Diurnal Cycle of Pheromone Production
The production of the male-specific compound followed a defined diurnal cycle (Fig. 4), with production increasing from midmorning and peaking in the early afternoon.
Diurnal rhythm of release of the male-specific compound by Murgantia histrionica males (N = 5 cohorts). Two-way ANOVA for cohort effect, F = 3.08, df = 4, 39, P = .03; for time interval effect F = 107.1, df = 7, 39, P < 0.001. Bars surmounted by the same letter are not significantly different (Student–Newman–Keuls test, P < 0.05)
Analysis of Extracts of Odors from Adult Bugs
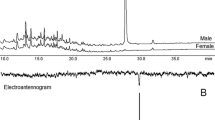
Typical gas chromatograms of extracts of odors from groups of 10–15 sexually mature, virgin adult bugs of both sexes are shown in Fig. 5. Several compounds appeared in extracts from both females and males, including dodecane, dimethyldisulfide, dimethyltrisulfide, S-methylmethanethiosulfonate, myrcene, limonene, and cineole. With the exception of S-methylmethanethiosulfonate, for which a standard was not available, the identifications of these compounds were verified by comparison of their retention times and mass spectra with those of authentic standards. Myrcene, limonene, and cineole were also found in cauliflower odor controls, whereas the sulfur-containing compounds were only seen in the insect odor extracts.
Comparison of extracts collected from sexually mature virgin males and females (>14 day old) by gas chromatography (Fig. 5) revealed the presence of two male-specific compounds. The first was identified by retention time and mass spectral matches as tridecane, a common component of stink bug odors (e.g., Ho and Millar 2001a, b). The second compound (Kovats index, KI, 1743 on DB-5 column; Kovats 1965) was not found in aerations of sexually immature males, nor was it a component of the frothy defensive emissions (2-sec-butyl-3-methoxypyrazine and 2-isopropyl-3-methoxy-pyrazine; (Aldrich et al. 1996)) or one of the two components reported from the metathoracic glands of male bugs ((2E,6E)-octadienedial and (2E,6E)-octadien-1,8-diol diacetate; Aldrich et al. 1996). The spectrum also gave no meaningful matches with any literature spectra in the NBS-NIH mass spectral database.
Electron impact ionization GC-MS analysis (70 eV) of the compound gave an apparent molecular ion at m/z = 220, with an ion at m/z = 202 probably due to loss of water (Fig. 6), for a possible molecular formula of C15H24O. The base peak at m/z 93 suggested a methylcyclohexadiene fragment. The spectrum bore some similarity to the mass spectra of sesquiterpenoids, and the overall complexity of the spectrum suggested that the compound was not aromatic. Based upon comparison of the retention indices of known compounds with the KI for the Murgantia compound, it seemed unlikely that the molecular weight was actually 220 amu. For example, the bisabolene epoxide pheromone components (m/z = 220) of Nezara viridula and Acrosternum hilare had retention indices on a DB-5 GC column of 1609 (trans isomer) and 1616 (cis isomer) vs the Murgantia compound with KI 1743. Thus, we suspected that the loss of 18 amu from m/z = 220 to give an ion at m/z = 202 in the 70 eV EI mass spectrum of the Murgantia compound was not the first loss of water but the second, and that the actual molecular ion at m/z 238, corresponding to a molecular formula of C15H26O2, was too unstable to be seen under EI-MS conditions. This molecular formula requires three rings or sites of unsaturation. A molecular ion at m/z 238 also was not observed with chemical ionization mass spectrometry (NH3 reagent gas) using a direct insertion probe.
Microchemical tests provided further information. The compound did not react with the dienophile 4-methyl-1,2,4-triazoline-3,5-dione, ruling out the presence of a conjugated diene. The compound was not changed by treatment with NaOH in aqueous alcohol (ruling out an ester), by acetyl chloride and pyridine in ether (ruling out primary and secondary alcohols), or by treatment with LiAlH4 at room temperature (ruling out aldehydes, ketones, or esters). This suggested that the two postulated oxygen functionalities were probably present as ethers or tertiary alcohols. The presence of at least one polar functional group was suggested by fractionation of the crude extracts on silica gel: the compound appeared in the first fraction eluted with 100% diethyl ether.
Reduction of the compound by catalytic hydrogenation gave a mixture of compounds with apparent molecular weights of 224 or 226 by EI GC-MS, corresponding to loss of water from the actual molecular ions, indicative of two or three double bonds or other functionalities susceptible to catalytic hydrogenation. Most of these derivatives had a base peak or large fragment at m/z 95 or 97, suggestive of a methylcyclohexane substructure, such as that found in bisabolane-type sesquiterpenoids. Furthermore, males of several stinkbug species are known to produce pheromones with bisabolene skeletons, such as the bisabolene epoxides from Nezara viridula (Aldrich et al. 1987; Baker et al. 1987) and Acrosternum hilare (McBrien et al. 2001), zingiberene and sesquiphellandrene from Thyanta spp. (McBrien et al. 2002), and zingiberenol from Tibraca limbativentris (Borges et al. 2006). In total, the evidence supported a bisabolene-type sesquiterpenoid structure, with two oxygens present as ethers or tertiary alcohols.
With this fragmentary information about the possible structure of the unknown, crude aeration extracts from multiple cohorts of virgin males were combined to obtain sufficient material (>100 µg) for NMR analysis, and fractionated by liquid chromatography. The fraction enriched in the male-specific compound was concentrated, and taken up in CD2Cl2 rather than the usual CDCl3 solvent to avoid possible decomposition of the sample by the traces of DCl commonly found in CDCl3.
The 13C spectrum showed the expected fifteen carbons (Table 1). The three carbons with chemical shifts between δ 58.5 and 67.6 ppm were in the correct position for carbons with an attached oxygen, indicating that one of the two oxygens was bonded to two carbons as a straight-chain or cyclic ether. The two carbons at 134.2 and 134.3 ppm were in the correct range for alkene carbons, indicative of a single C–C double bond and, by default, indicating that there were two rings in the structure. The ten other carbons ranging from 16.1 to 41.2 ppm were within the expected range for aliphatic carbons with no directly attached heteroatoms (Table 1).
A DEPT experiment, in combination with HSQC data (see below) confirmed that there were four methyl carbons (δ 16.1, 19.0, 25.2, and 30.1), four methylene carbons (δ 20.7, 27.7, 31.3 and 37.8), five methine carbons (δ 37.1, 41.2, 64.9, 134.2, and 134.3), and two quaternary carbons (δ 58.5 and 67.6) (Table 1). The fact that both alkene carbons were methines showed that the single alkene present was 1,2-disubstituted. Combining the information from the 13C and DEPT experiments suggested the presence of a tertiary alcohol (67.6 ppm) and a trisubstituted epoxide (δ 64.9 and 58.5), accounting for all three carbons bonded to oxygens and one ring. It was noteworthy that treatment with LiAlH4 during the microchemical tests did not open the epoxide ring, presumably due to the mild conditions used.
This also confirmed the presence of the two postulated oxygens and, with the evidence for 15 carbons, the likely molecular weight of 238 amu and molecular formula of C15H26O2. Thus, the single remaining site of unsaturation had to be a second ring which, from the mass spectral and NMR data, was probably a methyl-substituted cyclohexene with one oxygen attached as a tertiary alcohol.
The 1H spectrum corroborated much of the above information (Table 1). There were three, three-proton singlets at 1.23, 1.23, and 1.27 ppm, from three methyl groups attached to quaternary carbons. The fourth methyl group appeared as a doublet at 0.85 ppm, indicative of a methyl group attached to a tertiary carbon. There was a one-proton triplet at 2.66 ppm (J = 6.6 Hz), consistent with a proton attached to an epoxide carbon, and a possible allylic hydrogen as a complex multiplet centered around 2.04 ppm. The alkene configuration was Z, based on the coupling constant (10.2 Hz) between the two broadened single-proton doublets at 5.65 and 5.61 ppm. The remaining protons were accounted for by overlapped multiplets between about 1.6 and 1.25 ppm.
The 1H-1H COSY spectrum indicated that the two alkene hydrogens (5.61 and 5.65 ppm) on C2 and C3, respectively, were coupled to each another, as expected. The possible allylic proton (2.04 ppm) on C1 was coupled to one of the two protons on C6 (~1.46 ppm). The single epoxide proton (2.66 ppm) on C10 was correlated with the two protons on C9 in the overlapped multiplets between 1.47–1.60 ppm, and one of the two protons on C5 (1.80 ppm) was coupled with multiple protons in the overlapped 1.47–1.60 ppm range.
Further information on connectivity through two and three bonds was obtained from the HMBC experiment (Table 1). The HMBC experiment showed correlations between the quaternary C4 (67.6 ppm) and the alkene proton H3 (5.65 ppm), the methyl protons at 1.23 ppm (H15), and one of the protons (1.80 ppm) of the C5 methylene carbon at 37.8 ppm, indicative of a tertiary allylic alcohol flanked by a CH2 group. The H5 proton at 1.80 ppm showed a further correlation to the C6 methylene carbon at 20.7 ppm. To finish establishing the connectivity within the cyclohexene ring, the remaining olefin proton at 5.61 ppm on C2 was correlated with the ring junction carbon C1 at 41.2 ppm and with the methylene carbon at C6.
The epoxide carbons at 64.9 and 58.5 ppm showed interactions with protons of two methyl groups (singlets) at 1.23 (H13) and 1.26 (H12) ppm, with the two methyl groups being directly bonded to the C11 quaternary carbon (58.5 ppm), confirming the trisubstituted epoxide fragment in the side chain. The epoxide proton H10 at 2.66 ppm was correlated with the C9 methylene carbon at 27.7 ppm. The methyl protons at 0.85 ppm (H14) showed correlations with C1 (41.2 ppm), C7 (37.1 ppm), and C8 (31.3 ppm), establishing the rest of the connectivity in the side chain, and between the side chain and the ring junction.
The NOESY spectrum (summarized in Table 1) provided further corroboration of several parts of the structure, with correlations between the epoxide proton and the adjacent methylene protons and the C12 methyl, between one of the protons (1.80 ppm) on C5 and the C15 methyl group, between the alkene proton H2 at 5.61 ppm and the bridgehead proton on C1 (2.04 ppm), and between the bridgehead proton H1 and the C14 methyl.
In total, the evidence unequivocally supported the bisabolane-type skeleton shown in Fig. 7, with one double bond in the cyclohexene ring, a tertiary alcohol at position 4 of the ring, and a trisubstituted epoxide in the side chain. This structure has four chiral centers, for a total of 16 possible isomers. The gross structure was confirmed by synthesis as described below but, to date, we have not been able to assign unequivocally the relative and absolute configurations of the insect-produced compound, henceforth referred to as murgantiol.
Synthesis of Murgantiol (Scheme 1)
Because it was not possible to determine the relative stereochemistry of the insect-produced murgantiol, a short synthetic route was developed that would produce all possible stereoisomers of the compound, one of which should then match the natural compound (Scheme 1). Citronellal, available in both enantiomeric forms, was chosen as the starting material, so that one chiral center in the molecule would be known. Two parallel syntheses were carried out, using (R)- and (S)-citronellal as starting materials, respectively, to ensure that all 16 possible stereoisomers would be represented. Thus, citronellal 1 was converted into α,ß-unsaturated aldehyde 2 by α-methylenation with formaldehyde (79.3% yield) (Chavan et al. 1997). Treatment with methyl acetoacetate and sodium methoxide then produced the 4-(1,5-dimethylhex-4-en-1-yl)cyclohex-2-en-1-one 3, presumably by conjugate addition followed by an aldol condensation, as a mixture of diastereomers (∼1:1, determined by NMR) at the ring junction (Chavan et al. 1997). The mixture was not resolved on capillary GC (DB-5). Addition of methyl lithium to enone 3 afforded a mixture (38:62 – less/more polar isomer) of diastereomers of alcohol 4 in 83.1% yield (Hagiwara et al. 2002). The diastereoisomeric mixture was separated by flash chromatography into two fractions, and both were epoxidized individually with buffered m-chloroperbenzoic acid. Each diastereomeric mixture thus yielded a further mixture of diastereoisomeric epoxyalcohols 5. NMR and mass spectra, and GC retention times on two columns (DB-5 and DB-Wax) of the epoxidation products 5 were compared with those of the insect-produced compound. One of the stereoisomers in the product mixture 5, from epoxidation of the less polar, higher Rf diastereomer of 4, matched the retention times of the insect-produced compound on DB-5 and DB-Wax GC columns. Comparisons of the mass, 1H, and 13C NMR spectra of the synthetic mixture from the less polar alcohol 4 with the natural pheromone gave good agreement, confirming the gross structure of the insect-produced compound as one of the stereoisomers of 4-[3-(3,3-dimethyloxiran-2-yl)-1-methylpropyl]-1-methylcyclohex-2-en-1-ol (5). The relative and absolute stereochemistry of the insect-produced compound remain to be determined.
Bioassays of Synthetic Murgantiol
The mixtures of murgantiol diastereomers prepared from (R)- and (S)-citronellal were both attractive to female bugs in Y-tube bioassays. Thus, 21 of the 24 females tested were attracted to murgantiol derived from (S)-citronellal vs three females attracted to the crude extract control (χ 2 test, P < 0.001), whereas 18 of 24 females tested were attracted to murgantiol derived from (R)-citronellal vs the control (χ 2 test, P < 0.014). When the mixtures of murgantiol diastereomers derived from (R)- and (S)-citronellal were tested against one another, 17 of 24 females tested were attracted to the mixture derived from (S)-citronellal (χ 2 test, P < 0.04). Similarly, when these mixtures were tested against each other with sexually mature virgin males as the responding animals, 19 of 24 males tested were attracted to the mixture derived from (S)-citronellal (χ 2 test, P < 0.004).
Discussion
Both female and male bugs were attracted to odors from live males in Y-tube bioassays, indicating that mature males produce an aggregation pheromone, as previously observed for other phytophagous stink bug species including Nezara viridula (Harris and Todd 1980), several Euschistus spp. (Aldrich et al. 1991), Biprorulus bibax (James et al. 1994), and Plautia stali (Moriya and Shiga 1984; Sugie et al. 1996). In contrast, two other pentatomid species [Thyanta pallidovirens (Millar 1997) and Acrosternum hilare (McBrien et al. 2001)] have been reported to produce compounds attractive to females only.
Single males released significantly more murgantiol than males aerated in groups of ten or more, suggesting that males in groups may detect each other’s presence influencing their release of pheromone. An analogous situation was recently reported for the predatory stink bug Eocanthecona furcellata, with single individuals producing orders of magnitude more pheromone than groups of bugs (Ho et al. 2005). Although the full biological significance of these results has not been determined, for practical purposes, they suggest that collection of pheromone from individual bugs or small groups of bugs will be most efficient for analysis or identification of the pheromone.
The quantity of pheromone released peaked between 13:00–15:00, mirroring the peak of reproductive activity of the bugs (Zahn et al. 2007). Murgantiol was detected throughout most of the 24 h-sampling period, but the pattern of release suggested that the compound was released primarily during the photophase. The small amounts of murgantiol detected during the scotophase may have been due to the slow release of murgantiol absorbed in the insects’ cuticular lipids or on the glass walls of the aeration chamber. Insects of both sexes were dissected to look for possible pheromone glands, but no obvious macroscopic male-specific glands that may be the site of pheromone production were found. Other phytophagous pentatomid species produce pheromones from sex-specific unicellular glands on the ventral surface of the abdomen (Pavis and Malosse 1986; Lucchi 1994).
The diurnal peak in pheromone production also corresponded with the general activity cycle of the insect. M. histrionica are aposematic, with effective chemical defenses, which protect them from predation during daylight hours (Aliabadi et al. 2002). Unlike other stink bug species that are heavily parasitized by Diptera and Hymenoptera which use the bugs’ pheromone for location, no parasitoids of nymphal and adult M. histrionica are known, although there are several egg parasitoids (Chittenden 1920; Mitchell and Mau 1971; Ludwig and Kok 1998; Amarasekare 2000; Millar et al. 2001). For other phytophagous pentatomids, the majority of reproductive activity occurs in the late afternoon or around dusk, which allows them to evade predation or parasitization, and to avoid unfavorable temperature conditions. In particular, the risk of desiccation may limit the activity period of bug species such as Thyanta spp., Nezara viridula, and Chlorochroa spp., that typically feed on seeds rich in oils and proteins but with little water content (Hall and Teetes 1982; Panizzi et al. 1995; Wang and Millar 1997; Zalom et al. 1997; Ho and Millar 2001a, b; Nuessly et al. 2004; Numata 2004). In contrast, M. histrionica typically feeds on a wide range of plant tissues that are high in water, and thus the risk of desiccation through being active during the hottest part of the day is probably low.
Murgantiol contains four chiral centers at carbons 1, 4, 7, and 10 (Fig. 7), for a total of 16 possible stereoisomers (eight diastereomeric pairs of enantiomers). Although we have been able to match the insect-produced compound to a component in a synthetic blend of stereoisomers, we have not yet determined which isomer this is. The difficulty in trying to determine exactly which stereoisomer the bugs produce was exacerbated by our synthesis of murgantiol not being stereoselective. We separated alcohol mixture 4 into two fractions, each containing two stereoisomers, with the alcohol in either the axial or the equatorial orientation. However, after epoxidation of each of these fractions, we were unable to separate the resulting mixtures, which may contain as many as four stereoisomers, varying in the configurations of C1 at the ring junction and C10 in the side chain. Similar problems have been reported in the separation of diastereomers of less functionalized, but related, sesquiterpenes such as zingiberene and its ring junction epimer (Breeden and Coates 1994). Furthermore, because the epoxide in murgantiol is several carbons removed from the other chiral centers in the molecule, diastereomers differing only in the configuration of the chiral epoxide carbon might not be expected to separate easily by chromatographic methods. It is also noteworthy that epoxidation under buffered conditions at 0°C (Fringuelli et al. 1992) was highly selective for the trisubstituted double bond in the sidechain rather than the 1,2-disubstituted double bond in the cyclohexene ring.
The exact identification of the insect-produced compound was further complicated by the HSQC spectrum showing four, rather than two, signals from the protons on C9, as well as several very close, paired signals from carbons in the 1D 13C spectrum, suggesting that the molecule may exist as more than one conformer. It seems less likely that two isomers are present because of the clarity of the 1H signals in the 1D proton spectrum, and only the protons on a single carbon gave an HSQC signal that appeared anomalous.
Several interesting points emerged from the bioassays of the insect extracts and of the synthetic murgantiol mixtures. First, the insect fraction containing murgantiol was as attractive as crude extract, whereas the mixture of the remaining fractions was repellent. This suggests that the crude extract may contain defensive compounds or other repellent stimuli, the effects of which are overcome when murgantiol is present. Second, test bugs responded to the synthetic mixtures of murgantiol isomers, indicating that the bugs were not deterred by the presence of stereoisomeric impurities in the blends. Remarkably, the insects also responded to synthetic murgantiol mixtures prepared from either enantiomer of citronellal. Whether this result was an artifact of testing mixtures of stereoisomers, some of which are attractive, or indicative of a general response to all stereoisomers, will only be resolved when the pure stereoisomers are available for testing.
The structural similarity between murgantiol and a number of other sesquiterpenoid pheromones isolated from various pentatomid species is noteworthy. For example, epoxides of bisabolene constitute the male-produced pheromone components of Nezara viridula (Aldrich et al. 1987; Baker et al. 1987) and Acrosternum hilare (McBrien et al. 2001), and zingiberene and sesquiphellandrene form part of the pheromone blend of several Thyanta spp. More recently, zingiberenol 4 (the penultimate intermediate in our syntheses of murgantiol, Scheme 1), has been identified from males of the rice stalk stink bug, Tibraca limbativentris (Borges et al. 2006). As with murgantiol, the relative configuration of this compound, which has three chiral centers and hence eight possible stereoisomers, has yet to be determined. Thus, the bisabolane skeleton is emerging as a recurring structural motif among pheromones of stink bugs.
Although we do not know which stereoisomer of murgantiol is produced by male harlequin bugs, for practical purposes, it is convenient that the bugs respond to mixtures of the murgantiol isomers, which are relatively easy to synthesize. Thus, it should be possible to proceed to field trials with the impure “technical grade” murgantiol, to determine the potential for use of this compound in developing pheromone-based monitoring methods for this pest.
References
Aldrich, J. R., Oliver, J. E., Lusby, W. R., Kochansky, J. P., and Lockwood, J. A. 1987. Pheromone strains of the cosmopolitan pest Nezara viridula (Heteroptera: Pentatomidae). J. Exp. Zool. 244:171–175.
Aldrich, J. R., Hoffmann, M. P., Kochansky, J. P., Lusby, W. R., Eger, J. E., and Payne, J. A. 1991. Identification and attractiveness of a major pheromone component for Nearctic Euschistus spp. stink bugs (Heteroptera: Pentatomidae). Environ. Entomol. 20:477–483.
Aldrich, J. R., Avery, J. W., Lee, C., Graf, J. C., Harrison, D. J., and Bin, F. 1996. Semiochemistry of cabbage bugs (Heteroptera: Pentatomidae: Eurydema and Murgantia). J. Entomol. Sci. 31:172–182.
Aliabadi, A., Renwick, J. A., and Whitman, D. W. 2002. Sequestration of glucosinolates by harlequin bug Murgantia histrionica. J. Chem. Ecol. 28:1749–1762.
Amarasekare, P. 2000. Spatial dynamics in a host-multiparasitoid community. J. Anim. Ecol. 69:201–213.
Baker, R., Borges, M., Cooke, N. G., and Herbert, R. H. 1987. Identification and synthesis of (Z)-(1′S,3′R,4′)-(-)-2-(3′,4′-epoxy-4′-methylcyclohexyl)-6-methylhepta-2,5-diene, the sex pheromone of the southern green stink bug, Nezara viridula (L.). J. Chem. Soc. Chem. Commun. 1987:414–416.
Borges, M., Birkett, M., Aldrich, J. R., Oliver, J. E., Chiba, M., Murata, Y., Laumann, R. A., Barrigossi, J. A., Pickett, J. A., and Moraes, M. C. B. 2006. Sex attractant pheromone from the rice stalk stink bug, Tibraca limbativentris Stal. J. Chem. Ecol. 32:2749–2761.
Breeden, D. C., and Coates, R. M. 1994. 7-Epizingiberene, a novel bisabolane sesquiterpene from wild tomato leaves. Tetrahedron 50:11123–11132.
Chavan, S. P., Dhondge, V. D., Patil, S. S., Rao, Y. T. S., and Govande, C. A. 1997. Enantiospecific total synthesis of (+)-laevigatin. Tetrahedron: Asymmetry 8:2517–2518.
Chittenden, F. H. 1920. Harlequin cabbage bug and its control. USDA Farmers Bulletin 1061:1–13.
Čokl, A., and Doberlet, M. V. 2003. Communication with substrate-borne signals in small plant-dwelling insects. Annu. Rev. Entomol. 48:29–50.
Čokl, A., Presern, J., Virant-Doberlet, M., Bagwell, G. J., and Millar, J. G. 2004. Vibratory signals of the harlequin bug and their transmission through plants. Physiol. Entomol. 29:372–380.
Fringuelli, F., Germani, R., Pizzo, F., Santielli, F., and Savelli, G. 1992. Regioselective epoxidation of allylic alcohols with monoperoxyphthallic acid in water. J. Org. Chem. 57:1198–1202.
Games, P. A., and Howell, J. F. 1976. Pairwise multiple comparison procedure with unequal n’s and/or variances: A Monte Carlo Study. J. Educ. Stat. 2:113–125.
Hagiwara, H., Okabe, T., Ono, H., Kamat, V. P., Hoshi, T., Suzuki, T., and Ando, M. 2002. Total synthesis of bisabolane sesquiterpenoids, α-bisabol-1-one, curcumene, curcuphenol, and elvirol: utility of catalytic enamine reaction in cyclohexenone synthesis. J. Chem. Soc., Perkin Trans. 1:895–900.
Hall, D. G., and Teetes, G. L. 1982. Yield loss-density relationships of 4 Species of panicle-feeding bugs, Oebalus pugnax F., Nezara viridula L., Chlorochroa ligata Say (Heteroptera: Pentatomidae), and Leptoglossus phyllopus L. (Heteroptera, Coreidae) in sorghum. Environ. Entomol. 11:738–741.
Harris, V. E., and Todd, J. E. 1980. Male-mediated aggregation of male, female and 5th-instar southern green stink bugs and concomitant attraction of a tachinid parasite, Trichopoda pennipes. Entomol. Exp. Appl. 27:117–126.
Ho, H. Y., and Millar, J. G. 2001a. Identification and synthesis of a male-produced sex pheromone from the stink bug Chlorochroa sayi. J. Chem. Ecol. 27:1177–1201.
Ho, H. Y., and Millar, J. G. 2001b. Identification and synthesis of male-produced sex pheromone components of the stink bugs Chlorochroa ligata and Chlorochroa uhleri. J. Chem. Ecol. 27:2067–2095.
Ho, H., Hsu, Y., Chuang, Y., and Chow, Y. 2005. Effect of rearing conditions on production of sternal gland secretion, and identification of minor components in the sternal gland secretion of the predatory stink bug Eocanthecona furcellata. J. Chem. Ecol. 31:29–37.
James, D. G., Mori, K., Aldrich, J. R., and Oliver, J. E. 1994. Flight-mediated attraction of Biprorulus bibax Breddin (Hemiptera: Pentatomidae) to natural and synthetic aggregation pheromone. J. Chem. Ecol. 20:71–80.
Kovats, E. 1965. Gas chromatographic comparison of organic substances in the retention index system. Adv. Chromatogr. 1:229–247.
Lucchi, A. 1994. The scent gland system of Nezara viridula (L.) (Heteroptera: Pentatomidae). III. Male ventral abdominal glands. Redia 77:1–10.
Ludwig, S. W., and Kok, L. T. 1998. Phenology and parasitism of harlequin bugs, Murgantia histrionica (Hahn) (Hemiptera: Pentatomidae), in Southwest Virginia. J. Entomol. Sci. 33:33–39.
Mcbrien, H. L., and Millar, J. G. 1999. Phytophagous bugs, pp. 277–304, in J. Hardie, and A. K. Minks (eds.). Pheromones of non-lepidopteran insects associated with agricultural crops. CABI Publishing, London, UK.
Mcbrien, H. L., Millar, J. G., Gottlieb, L., Chen, X., and Rice, R. E. 2001. Male-produced sex attractant pheromone of the green stink bug, Acrosternum hilare (Say). J. Chem. Ecol. 27:1821–1839.
Mcbrien, H. L., Millar, J. G., Rice, R. E., Mcelfresh, J. S., Cullen, E., and Zalom, F. G. 2002. Sex attractant pheromone of the red-shouldered stink bug Thyanta pallidovirens: A pheromone blend with multiple redundant components. J. Chem. Ecol. 28:1797–1818.
Mcpherson, J. E. 1982. The Pentatomoidea (Hemiptera) of northeastern North America with emphasis on the fauna of Illinois, pp. 1–240, The Pentatomoidea. University Press, Carbondale, IL, USA.
Millar, J. G. 1997. Methyl (2E,4Z,6Z)-deca-2,4,6-trienoate, a thermally unstable, sex-specific compound from the stink bug Thyanta pallidovirens. Tetrahedron Lett. 38:7971–7972.
Millar, J. G. 2005. Pheromones of true bugs, pp. 37–84, in S. Schulz (ed.). Topics in Current Chemistry 240. The Chemistry of Pheromones and Other Semiochemicals. Springer, Berlin.
Millar, J. G., and Sims, J. J. 1998. Preparation, cleanup, and preliminary fractionation of extracts, pp. 1–37, in J. G. Millar, and K. F. Haynes (eds.). Methods in Chemical Ecology. Chapman and Hall, NY.
Millar, J. G., Rice, R. E., Steffan, S. A., Daane, K. M., Cullen, E., and Zalom, F. G. 2001. Attraction of female digger wasps, Astata occidentalis Cresson (Hymenoptera: Sphecidae) to the sex pheromone of the stink bug Thyanta pallidovirens (Hemiptera: Pentatomidae). Pan-Pac. Entomol. 77:244–248.
Mitchell, W. C., and Mau, R. F. L. 1971. Response of female southern green stink bug Hemiptera-Pentatomidae and its parasite, Trichopoda pennipes, to male stink bug pheromones. J. Econ. Entomol. 64:856–859.
Moriya, S., and Shiga, M. 1984. Attraction of the male brown-winged green bug, Plautia stali Scott (Heteroptera: Pentatomidae) for males and females of the same species. Appl. Entomol. Zool. 19:317–322.
Nuessly, G. S., Hentz, M. G., Beiriger, R., and Scully, B. T. 2004. Insects associated with faba bean, Vicia faba (Fabales: Fabaceae), in southern Florida. Fla. Entomol. 87:204–211.
Numata, H. 2004. Environmental factors that determine the seasonal onset and termination of reproduction in seed-sucking bugs (Heteroptera) in Japan. Appl. Entomol. Zool. 39:565–573.
Panizzi, A. R., Niva, C. C., and Hirose, E. 1995. Feeding preference by stink bugs (Heteroptera: Pentatomidae) for seeds within soybean pods. J. Entomol. Sci. 30:333–341.
Pavis, C., and Malosse, C. 1986. A sex pheromone produced by mature males in the southern green stink bug, Nezara viridula (L.). C. R. Acad. Sci. Ser. 3 Sci. Vie 303:273–276.
Sugie, H., Yoshida, M., Kawasaki, K., Noguchi, H., Moriya, S., Takagi, K., Fukuda, H., Fujiie, A., Yamanaka, M., Ohira, Y., Tsutsumi, T., Tsuda, K., Fukumoto, K., Yamashita, M., and Suzuki, H. 1996. Identification of the aggregation pheromone of the brown-winged green bug, Plautia stali Scott (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 31:427–431.
Wang, Q., and Millar, J. G. 1997. Reproductive behavior of Thyanta pallidovirens (Heteroptera: Pentatomidae). Ann. Entomol. Soc. Am. 90:380–388.
Young, D. C., Vouros, P., and Holich, M. F. 1990. Gas chromatography-mass spectrometry of conjugated dienes by derivatization with 4-methyl-1,2,4-triazoline-3,5-dione. J. Chromatogr. 522:295–302.
Zahn, D. K., Girling, R. D., Mcelfresh, J. S., Cardé, R. T., and Millar, J. G. 2007. Biology and reproductive behavior of Murgantia histrionica (Heteroptera: Pentatomidae). Ann. Ent. Soc. Am., in press.
Zalom, F. G., Smilanick, J. M., and Ehler, L. E. 1997. Fruit damage by stink bugs (Hemiptera: Pentatomidae) in bush-type tomatoes. J. Econ. Entomol. 90:1300–1306.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10886-012-0061-6
Rights and permissions
About this article
Cite this article
Zahn, D.K., Moreira, J.A. & Millar, J.G. Identification, Synthesis, and Bioassay of a Male-Specific Aggregation Pheromone from the Harlequin Bug, Murgantia histrionica . J Chem Ecol 34, 238–251 (2008). https://doi.org/10.1007/s10886-007-9415-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9415-x