Abstract
Epicuticular lipids are contact cues in intraspecific chemical communication in insects, both for aggregation and sexual behavior. Triatomine bugs are vectors of the parasite Trypanosoma cruzi, the cause of Chagas disease. In Triatoma infestans, the major epicuticular lipids are hydrocarbons, fatty alcohols, and free and esterified fatty acids. Previously, we found that epicuticular lipid extracts, or selected fatty acid components, trigger aggregation and arrestment behavior in this bug. Using headspace solid phase microextraction, we found no sexual dimorphism in epicuticular hydrocarbons, but found female-specific fatty alcohols (eicosanol and docosanol). The role of epicuticular lipids in T. infestans copulation behavior was tested by observing male responses to live or various treatments of freeze-killed females. We report that hexane-soluble contact cues on females trigger copulation by males. Freeze-killed intact females were attractive to males, but no response was observed when males were exposed to hexane-washed females. Responses were partially recovered when epicuticular extract was applied to the dorsal surface of dead, hexane-washed females. One female equivalent of docosanol, evoked similar responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triatoma infestans (Hemiptera: Reduviidae) is a major vector of Chagas disease in South America, especially in the 1.3 million km2 Gran Chaco geographic region, which has a mostly rural human population (Schofield and Kabayo, 2008). Late nymphs and adults are the major targets in vector control strategies, as they are more likely to transmit the disease. Attractants for this bug could help in the development of new control methods by using these chemicals as components of traps that monitor or “attract and kill”. Recently, a “trap and kill” device that used entomopathogenic fungi, was shown to be successful against T. infestans (Pedrini et al., 2009).
Epicuticular lipids are known to mediate mate recognition in many insect species (Schal et al., 1990; Ferveur et al., 1996; Ginzel et al., 2006; Barbour et al., 2007; Rutledge et al., 2009). Triatoma infestans and other triatomines exhibit characteristic aggregation (Juárez et al., 2008; Lorenzo Figueiras et al., 2009), although no evidence for a contact sex or aggregation pheromone has been described. The epicuticular lipids of T. infestans are a complex mixture of long-chain hydrocarbons and oxygenated components, mostly fatty alcohols, waxes, and free and esterified fatty acids, which protect insects against chemical and biological attack (Juárez, 1994, 1995; Juárez and Calderón-Fernández, 2007; Pedrini et al., 2009). Epicuticular hydrocarbons are sexual dimorphic in Triatoma sordida, although no sex difference was found in T. infestans (Juárez and Calderón-Fernández, 2007).
Here, we tested the hypothesis that contact pheromones mediate mate recognition in T. infestans. We examined the behavioral role of epicuticular lipids and show, for the first time, that these lipids elicit male mating attempts. We also report the identification of a female-specific fatty alcohol component that is capable of eliciting this behavior.
Methods and Materials
Insects
Triatoma infestans were from a colony at the INIBIOLP, reared at 28°C, 50–60% RH. Adults from a small field colony were collected and used in one experiment. Virgin adults were collected daily and maintained separately. Insects were fed on domestic chickens 2–3 d after last molt, and used 10–12 d thereafter. Chickens were housed and managed under standard laboratory conditions approved by INIBIOLP’s Animal Care and Use Committee.
Solvent Extraction and Head Space Solid Phase Microextraction (HS-SPME) Sampling
Epicuticular lipids were extracted from single-sex groups of three bugs in redistilled hexane (6 ml g−1, 3 times 5 min. each). The extract was concentrated under a stream of nitrogen. Hydrocarbons were fractionated by silica gel column chromatography, as described (Calderón-Fernández et al., 2011), using hexane as eluent.
For HS-SPME, hexane extracts of epicuticular lipids were deposited inside 10 ml crimp top vials (Supelco, Bellefonte, PA, USA), and the solvent was evaporated by a nitrogen stream. Vials were sealed and the residual lipids sampled at 150°C by HS-SPME for 20 min., using a 65 μm polydimethylsiloxane/divinylbenzene fiber (PDMS/DVB) (Supelco) inserted through the septum.
Chemical Analysis
Both hydrocarbon and epicuticular lipid samples were analyzed by coupled gas chromatography–mass spectrometry (GC-MS) using electron impact ionization (70 eV). A Hewlett-Packard 6890 GC, equipped with a DB-5MS capillary column (30 m × 0.25 mm × 0.25 μm film; J&W Scientific, Folsom, CA, USA), interfaced to an Agilent 5975 mass selective detector, was used for the analyses. Samples were injected either splitlessly or directly (SPME), with helium as carrier gas. The column oven was programmed from 50–180°C (at 20°C.min−1), then to 310°C (at 3°C.min−1). Transfer line and quadrupole were held at 320°C and 150°C, respectively. Peak areas were calculated and expressed as a percentage of total peak area. Interpretation of hydrocarbon mass spectra was performed as described in Juárez et al. (2001). Spectra of other lipids were compared to data from MS libraries (NIST/EPA/NIH, NIST 05 and ADAMS 07). Fatty alcohols were identified by interpretation of their mass spectral fragmentation and by a close match of their mass spectra and retention times with those of authentic standards. Absolute amounts of fatty alcohols were quantified by flame ionization detection and calculated relative to eicosanol as an external standard; response was linear in the concentration range tested.
Bioassays
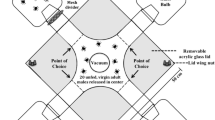
Bioassays were performed in the late evening in plastic Petri dishes (13 cm diam. × 12 cm height), generally using paired virgin males and females (alive or dead). Live males and females (N = 68 each sex) were used to characterize the mating behavior of adults. A further experiment, using live females, tested the role of antennal contact in mating by covering the antennae of males with eyelash gel.
To test whether a contact pheromone was involved in mate recognition, females were freeze-killed (−20°C, 30 min) and allowed to warm to room temperature. These dead females were tested to determine whether recognition cues remained unchanged after killing, and therefore independent of female behavior. Next, epicuticular lipids were extracted from a dead female, as above, and the female was air-dried for ~30 min. The responses of individual males, were tested to the solvent-washed female. Finally, we loaded 1 or 3 female equivalents (FE) of lipid extract (in 20 μl hexane) onto a dead female. Each dead female was presented to 1–5 males (N = 84 males and 48 females). The response of a male was observed by eye or recorded with a video camera over 30 min. A trial was scored as a “positive response” if the male, after antennal contact, stopped walking (arrest behavior), aligned with the female, and mounted and grasped her (copulation attempt). If the male showed no response after antennal contact, it was scored as “no response”. Male responses were tested to live females, dead and intact females, dead and hexane-washed females, and dead, washed and lipid-reconstituted females.
To test the role of female-specific components in mate recognition, hexane solutions of 1 FE of docosanol and eicosanol (Sigma Aldrich, St. Louis, MO, USA) either individually, or combined, were placed on 2 cm long, polypropylene half cylinders, cut from 1 ml micropipette tips. Males were exposed to treated tips or control tips (hexane), in a arena similar to that described. A positive response was scored when a male mounted the tip.
The responses to treatments were tested by Fisher’s exact test (Sokal and Rohlf, 2001). Differences were considered to be significant if the probability of their occurrence by chance was less than 5%.
Results
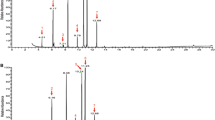
Hexane-extracts of epicuticular lipids of mature virgin T. infestans sampled by HS-SPME had a high abundance of saturated hydrocarbon components, mostly straight chain from 21 to 33 carbons, together with straight chain fatty alcohols of 20–30 carbons. Other minor components were fatty acids and fatty aldehydes (Fig. 1 and Table 1). No evident sexual dimorphism was observed for these components, except female extracts contained more than 7% of eicosanol and docosanol, whereas male extract had only trace amounts of these compounds. One equivalent of docosanol and eicosanol in a female corresponded to 14.1 ± 3.9 ng and 6.1 ± 0.8 ng, respectively. Females also had higher proportions of tetracosanol, which coeluted with n- heptacosane (Fig. 1 and Table 1).
Total ion mass chromatograms obtained by solid phase microextraction of epicuticular lipids of virgin female (top) and male (bottom) Triatoma infestans. Eicosanol and docosanol (marked with asterisk) were found in large abundance only in females. The identities and relative proportions of major components are given in Table 1
Male T. infestans only attempted to mate (57.6% of total males tested attempted mating, N = 66) with live females after contacting them with their antennae. After initial wandering in the container, males stopped, turned their antennae toward the female and contacted her with them, usually on the abdominal dorsal surface and the thorax (step 1). As described (Manrique and Lazzari, 1994), males aligned their body to the female, mounted and grasped her (step 2). After a male assumed a dorsolateral position and exposed his genitalia, copulation commenced (step 3). Most (98.5%) males that reached step 2 also completed copulation. The time to first antennal contact, varied from 0.16 to 5 min (\( {\hbox{mean}} = {1}.{25}\pm {1}.{31}\,{ \min } \), N = 13), and to mating attempt, from 0.33 to 12 min (4.27 ± 4.63 min, N = 10). The percentage of mating attempts by previously mated males (62.5%, N = 8, unmated for 2 d) was not different from that of virgin males. No males (N = 10) that had their antennae covered with an eyelash gel attempted mating
Freeze-killed females elicited 61.0% of males to attempt mating (N = 41) (Fig. 2), not different (P = 0.836) to the percentage elicited by live females, suggesting that active female behavioral cues were not involved. Hexane-washed female bodies (N = 42) elicited no response from males, different from that elicited by intact, freeze-killed females (P < 0.001). When epicuticular lipids (1FE) were loaded onto dead, washed females, 41.2% of males responded, differently (P = 0.001, N = 17) from that to dead, washed females only. At a higher dose (3 FE) of extract, 53.8% (N = 26) of males responded, not different (P = 0.536) from the percentage that responded to the lower dose (Fig. 2). Responses to dead and intact or reconstituted freeze-killed females were not different at either of the 2 doses tested (3 FE, P = 0.246; 1 FE, P = 0.616).
Responses of Triatoma infestans males to females with different treatments: freeze-killed, extracted with solvent, or addition of 1 (white) or 3 female equivalents (FE; gray) of epicuticular lipid extract following extraction with solvent (reconsitution). Forty one males and 27 females were used both in the freeze-killed and solvent-extracted treatments, and 43 males and 21 females in the reconstitution treatment. Different letters indicate differences (P ≤ 0.05) among treatments by Fisher’s exact test. The letter atop the reconstitution bar applies to both 1 and 3 FE
Triatoma infestans from a small field colony were also tested. Freeze-killed females elicited mating attempts from 100% of males tested (N = 10), solvent-washed females evoked no males (N = 9) to respond, and epicuticular lipid-reconstituted females (1 FE) elicited mating attempts from 57.1% of males (N = 14). One FE of docosanol elicited 46.7% of males (N = 30) to attempt copulation with the treated pipette tip. This percentage was different (P = 0.025) from that observed by using 1 FE of eicosanol (16.7% of males, N = 30) (Fig. 3). The percentage responses of males to docosanol (1 FE) were similar to those to docosanol plus eicosanol (1 FE each), either on a polypropylene tip (50%, P = 0.811, N = 38) (Fig. 3), or on hexane-washed females (61.1%, P = 0.334, N = 18). Control tips elicited no response from males, differing from responses to eicosanol (P = 0.008), docosanol (P < 0.001), and eicosanol plus docosanol (P < 0.001).
Responses of Triatoma infestans males to polypropylene pipette tips treated with 1 female equivalent of eicosanol (plain), docosanol (lines), or 1 equivalent each of eicosanol plus docosanol (dots). Thirty males were tested to each individual alcohol treatment and 38 to the mixture. Different letters indicate differences (P ≤ 0.05) among treatments by Fisher’s exact test
Discussion
Previous analyses of cuticular hydrocarbons of T. infestans found saturated, mostly odd-numbered, straight chain compounds of 23–33 carbons, together with methyl-branched compounds of 29 or more carbons (Juárez and Blomquist, 1993). No evident sexual dimorphism in epicuticular hydrocarbons was detected in bugs of unknown mating status (Juárez and Brenner, 1985). In the present study, our analyses of extracts of virgin males and females of the same age also found no sexual dimorphism in the hydrocarbon fraction. However, our HS-SPME sampling allowed us to detect various oxygenated compounds in the epicuticular lipid extracts of T. infestans. A whole series of even-numbered fatty aldehydes of 22 to 30 carbons were novel, and present in both sexes. Of particular note were two female-specific fatty alcohols, docosanol and eicosanol. Previous studies, using solvent extracts, showed that triacontanol and dotriacontanol were the major fatty alcohol components in both females and males (Juárez and Calderón-Fernández, 2007).
Free fatty alcohols are not commonly found in epicuticular lipids of insects, although high molecular weight alcohols have been reported in honeybees (Blomquist et al., 1980) and triatomines (Juárez and Calderón-Fernández, 2007). High molecular weight alcohols also have been reported in the defensive secretions of scale insects (Buckner, 1993; Byrne and Hardley, 1988). Typically, insects more commonly produce lower molecular weight alcohols. Honeybees produce alcohols of 17–22 carbons, which induce arrestment in parasitic varroa mites (Donze et al., 1998). A mixture of short chain alcohols, their acetate derivatives, and (Z)-11-eicosenol are components of bee alarm pheromones (Pickett et al., 1982). In moths, a major class of female sex pheromone is unsaturated compounds of 10–18 carbons with oxygenated functional groups, mostly alcohols, their acetate esters or aldehydes (Bjostad et al., 1987; Arn et al., 1998).
There have been no previous reports of eicosanol and docosanol being involved in insect sexual communication. Although eicosanol was found in the sex pheromone blend of the grapevine moth Lobesia botrana, the actual pheromone components are unsaturated acetates of 12-carbon alcohols (Witzgall et al., 2005). Although volatile chemicals, which elicit electroantennogram responses, are released by T. infestans, this occurs during, and not before, copulation (Gonzales Audino et al., 2007; Crespo and Manrique, 2007). Our results show that mating attempts by a T. infestans male requires contact of the male’s antennae with the female, and that epicuticular lipids trigger this response. Female-specific docosanol seems to be an important chemical that mediates this behavior. The characteristic triatomine aggregation behavior in small nests probably facilitates contact, and consequently recognition, of mates. Further studies on pheromone components may help the development of new control tools (Juárez et al., 2008; Pedrini et al., 2009) against Chagas disease vectors.
References
Arn, H., Rauscher, S., Guerin, P., and Buser, H. R. 1998. Sex pheromone blends of three tortricid pests in European vineyards. Agric. Ecosyst. Environ 21:111–117.
Barbour, J. D., Lacey, E. S., and Hanks, L. M. 2007. Cuticular hydrocarbons mediate mate recognition in a species of longhorned beetle (Coleoptera: Cerambycidae) of the primitive subfamily Prioninae. Ann. Entomol. Soc. Am. 100:333–338.
Bjostad, L. B., Wolf, W. A., and Roelofs, W. L. 1987. Pheromone biosynthesis in lepidopterans: Desaturation and chain-shortening, pp. 77–120, in G. D. Prestwich and G. J. Blomquist (eds.). Pheromone Biochemistry. Academic Press, Orlando, Florida.
Blomquist, G. J., Chu, A. J., and Remaley, S. 1980. Biosynthesis of wax in the honey bee, Apis mellifera L. Insect Biochem. 10:313–321.
Buckner, J. S. 1993. Cuticular polar lipids of insects, pp. 227–270, in D. W. Stanley-Samuelson and D. R. Nelson (eds.). Insect Lipids: Chemistry, Biochemistry and Biology. University of Nebraska Press, Lincoln, Nebraska.
Byrne, D. N. and Hardley, N. F. 1988. Particulate surface waxes of whiteflies: morphology, compositions and waxing behaviour. Physiol. Entomol. 13:267–276.
Calderón-Fernández, G. M., Girotti, J. R., and Juárez M. P. 2011. Cuticular hydrocarbons of Triatoma dimidiata (Hemiptera: Reduviidae): intraspecific variation and chemotaxonomy. J. Med. Entomol. 48: in press.
Crespo, J. G. and Manrique, G. 2007. Mating behavior of the hematophagous bug Triatoma infestans: role of Brindley’s and metaesternal glands. J. insect Physiol. 53:708–714.
Donze, G., Schnyder-Candrian, S., Bogdanov, S., Diehl, P. A., Guerin, P. M., Kilchenmann, V., and Monachon, F. 1998. Aliphatic alcohols and aldehydes of the honey bee cocoon induce arrestment behavior in Varroa jacobsoni (Acari: Mesostigmata), an ectoparasite of Apis mellifera. Arch. Insect Biochem. Physiol. 37:129–145.
Ferveur, J. F., Cobb, M., Boukella, H., and Jallon, J. M. 1996. World-wide variation in Drosophila melanogaster sex pheromone: behavioural effects, genetic bases and potential evolutionary consequences. Genetica 97:73–80.
Ginzel, M. D., Moreira, J. A., Ray, A. M., Millar, J. G., and Hanks, L. M. 2006. (Z)-9-Nonacosene major component of the contact sex pheromone of the beetle, Megacyllene caryae. J. Chem. Ecol. 32:435–451.
Gonzales Audino, P., Alzogaray, R. A., Vassena, C., Masuh, H., Fontan, A., Gatti, P., Martinez, A., Camps, F., Cork, A., and Zerba, E. 2007. Volatile compounds secreted by Brindley’s glands of adult Triatoma infestans: identification and biological activity of previously unidentified compounds. J. Vect. Ecol. 32:75–82.
Juárez, M. P. 1994. Inhibition of cuticular lipid synthesis and its effect on insect survival. Arch. Insect Biochem. Physiol. 25:177–191.
Juárez, M. P. 1995. The effect of sublethal doses of insecticides on Triatoma infestans lipid synthesis. Pest. Biochem. Physiol. 52:81–89.
Juárez, M. P. and Blomquist, G. J. 1993. Cuticular hydrocarbons of Triatoma infestans and T. mazzotti. Comp. Biochem. & Physiol. B 106:667–674.
Juárez, M. P. and Brenner, R. R. 1985. The epicuticular lipids of Triatoma infestans, II. Hydrocarbon dynamics. Comp. Biochem. Physiol. 82:793–803.
Juárez, M. P. and Calderón-Fernández, G. 2007. Cuticular hydrocarbons of triatomines. (Review). Comp. Biochem. Physiol. A 147:711–730.
Juárez, M. P., Blomquist, G. J., and Schofield, C. J. 2001. Hydrocarbons of Rhodnius prolixus, a Chagas disease vector. Comp. Biochem. Physiol. B 129:133–146.
Juárez, M. P., Pedrini, N., Girotti, J. R., Mijailovsky, S. J., and Lorenzo Figueiras, A. N. 2008. Trampa para insectos hematófagos, método de control y método de detección de dichos insectos (Triatoma infestans). Patent pending, Argentina. AR068790 INPI Boletín 568:5.
Lorenzo Figueiras, A. L., Girotti, J. R., Mijailovsky, S. J., and Juárez, M. P. 2009. Epicuticular lipids induce aggregation in Chagas disease vectors. Parasit. Vect. 2:8.
Manrique, G. and Lazzari, C. R. 1994. Sexual behaviour and stridulation during mating in Triatoma infestans (Hemiptera: Reduviidae). Mem. Inst. Oswaldo Cruz 89:629–633.
Pedrini, N., Mijailovsky, S. J., Girotti, J. R., Stariolo, R., Cardozo, R. M., Gentile, A., and Juárez, M. P. 2009. Control of pyrethroid-resistant Chagas disease vectors with entomopathogenic fungi. PLoS Negl. Trop. Dis. 3:e434.
Pickett, J. A., Williams, I. H., and Martin, A. P. 1982. (Z)-11-eicosen-1-ol, an important new pheromonal component from the sting of the honey bee, Apis mellifera L. (Hymenoptera, Apidae.). J. Chem. Ecol. 8:163–175.
Rutledge, C. E., Millar, J. G., Romero, C. M., and Hanks, L. M. 2009. Identification of an Important Component of the Contact Sex Pheromone of Callidiellum rufipenne (Coleoptera: Cerambycidae). Environ. Entomol. 38:1267–1275.
Schal, C., Burns, E. L., Jurenka, R. A., and Blomquist, G. J. 1990. A new component of the female sex pheromone of Blattella germanica (L.) (Dictyoptera: Blattellidae), and interaction with other pheromone components. J. Chem. Ecol. 16:1997–2008.
Schofield, C. J., and Kabayo, J. P. 2008. Trypanosomiasis vector control in Africa and Latin America. Parasit. Vect. 1:24.
Sokal, R. R. and Rohlf, F. J. 2001. Biometry. Freeman, New York.
Witzgall, P., Tasin, M., Buser, H. R., Wegner-KIß, G., Mancebon, V. S. M., Ioratti, C., Bäckman, A. C., Bengtsson, M., Lehmann, L., and Francke, W. 2005. New Pheromone Components of the Grapevine Moth Lobesia botrana. J. Chem. Ecol. 31:2923–2932.
Acknowledgements
We thank Raúl Stariolo for providing insects, and Facundo Bozzolo and Cecilia Fuse for rearing insects. The research was supported in part by grants from the Agencia Nacional para la Promocion de la Ciencia y Tecnología, Argentina (PICT 2004–25479 and PICT 2007–01503) to MPJ. Both MPJ and GMCF are members of the Consejo Nacional de Investigaciones Científicas y Técnicas Researcher’s Career.
Author information
Authors and Affiliations
Corresponding author
Additional information
Luciana M. Cocchiararo-Bastias and Sergio J. Mijailovsky contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cocchiararo-Bastias, L.M., Mijailovsky, S.J., Calderon-Fernández, G.M. et al. Epicuticle Lipids Mediate Mate Recognition in Triatoma infestans . J Chem Ecol 37, 246–252 (2011). https://doi.org/10.1007/s10886-011-9927-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-9927-2