Abstract
Analysis of extracts of sex pheromone glands of grapevine moth females Lobesia botrana showed three previously unidentified compounds, (E)-7-dodecenyl acetate and the (E,E)- and (Z,E)-isomers of 7,9,11-dodecatrienyl acetate. This is the first account of a triply unsaturated pheromone component in a tortricid moth. The monoenic acetate (E)-7-dodecenyl acetate and the trienic acetate (7Z,9E,11)-dodecatrienyl acetate significantly enhanced responses of males to the main pheromone compound, (7E,9Z)-7,9-dodecadienyl acetate, in the wind tunnel. The identification of sex pheromone synergists in L. botrana may be of practical importance for the development of integrated pest management systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The grapevine moth Lobesia botrana Denis and Schiffermüller (Lepidoptera, Tortricidae) is among the economically most important insects in Europe. It occurs throughout the wine-growing area of ca. 4 million hectares and completes two to four generations per year. The larvae feed on grapes, where Botrytis cinerea and other fungi rapidly develop, causing the entire grape cluster to rot (Fermaud and Giboulot, 1992). Pheromone-based control of L. botrana has been successfully adopted in wine-growing areas in Germany, Italy, and Switzerland. However, the cost effectiveness of the mating disruption technique needs to be improved (Arn and Louis, 1996).
The sex pheromone of L. botrana, which has been studied since the 1970s, consists of the main compound (7E,9Z)-7,9-dodecadienyl acetate (E7,Z9-12Ac) plus at least four other compounds: (7E,9Z)-7,9-dodecadien-1-ol (E7,Z9-12OH), (Z)-9-dodecenyl acetate (Z9-12Ac), (E)-9-dodecenyl acetate (Z9-12Ac), and 11-dodecenyl acetate (11-12Ac; Roelofs et al., 1973; Arn et al., 1988; El-Sayed et al., 1999). Here, we show that L. botrana pheromone glands contain further compounds, which are of potential interest for the development of integrated pest management systems.
Methods and Materials
Insects
Insects were collected near Fribourg (Germany), San Michele (Italy), and La Rioja (Spain) and were reared in the laboratory on an artificial diet under a 16-hr light/8-hr dark photoperiod. Pupae were separated by sex, and adult moths were kept in 36-l Plexiglas cages.
Chemical Analysis
Excised glands of 2- to 3-d-old calling females were extracted for 1 min in 7 μl of redistilled hexane (Labscan, Malmö, Sweden). These extracts were analyzed on a Hewlett-Packard (HP) 5970B mass spectrometer (MS) with electron impact ionization, which was interfaced with a HP 5890 gas chromatograph (GC), using a polar DB-Wax column (30 m × 0.25 mm; J&W Scientific, Folsom, CA, USA). Gland extracts were further studied on an HP 6890 GC, using a DB-Wax column and a nonpolar SE-54 column (25 m × 0.32 mm; Kupper, Bonaduz, Switzerland). The oven temperature was programmed from 60°C/2 min, then at 10°C/min to 100°C, at 1.5°C/min to 150°C, and at 10°C/min to 230°C.
Gland extracts were also injected on an HP 6890 GC equipped with an HP-INNOWax capillary column (30 m × 0.25 mm), which was coupled to an electroantennographic detector (GC-EAD; Syntech, Hilversum, The Netherlands). The oven was programmed from 50°C (2 min hold) at 10°C/min to 230°C. The column outlet was split in a 1:1 ratio for simultaneous recordings by EAD and the flame ionization detector of the GC. Excised antennae were mounted in a holder, which was 0.5 cm away from the GC outlet.
Synthesis
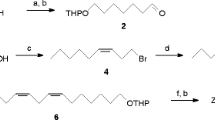
The syntheses of all compounds are described in detail in the online supplement (Appendix) (Electronic Supplementary Material is available forthis article at http://dx.doi.org/10.1007/s10886-005-8404-1 and is accessible for authorized users). 3-Octyn-1-ol (Aldrich) was isomerized to 7-octyn-1-ol using the “acetylene zipper” protocol (Abrams and Shaw, 1988). The obtained octynol served as the starting material for the syntheses of (7Z,9E,11)-dodecatrienyl acetate (Scheme 1) as well as for the (7E,9Z,11) and the (7E,9E,11) isomers (Scheme 2). Preparation of the latter two acetates proceeded similarly to the synthesis of the corresponding 9,11,13-tetradecatrienyl acetates (Tellier, 1991; Tellier et al., 1991), whereas the approach to (7Z,9E,11)-dodecatrienyl acetate was essentially the same as the sequences described for (9Z,11E,13)-tetradecatrienal (Millar, 1990) and for (11Z,13E,15)-hexadecatrienyl acetate (Gries etal., 2004).
a) Amberlyst 15/isobutene–heptane → 9; b) DIBAH/I2; c) Ac2O/FeCl3/Et2O; d) 3-butyn-1-ol, Pd (PPh3)4, n-C3H7NH2, CuI/toluene; e) TsCl/pyridine; f) K–O tBu, crowne 6/toluene (Dehmlow and Lissel, 1979); g) KOH/MeOH; h) Zn/MeOH; i) Ac2O/pyridine; k) 1: TMS/(1.3 eq)/CCl4 rt, 2: MeOH; l) as in (d) but using propynol; m) LiAlH4/THF; n) MnO2/CH2Cl2; o) Ph3PCH3Br, BuLi/THF, −78°C.
Hydrogenation of conjugated triple bonds with activated zinc in methanol produced (Z)-double bonds (Boland et al., 1987), whereas LiAlH4 reduction yielded (E)-double bonds (Porter et al., 1985). All reactions were carried out under argon. Intermediates were purified by distillation in vacuo or by column chromatography on silica gel. In all three syntheses, the alcohols corresponding to the target acetates were carefully recrystallized from hexane/toluene at −25°C to furnish products with stereochemical purity >98%.
For the synthesis of (7Z,9E,11)-dodecatrienyl acetate, 7-octyn-1-ol was chain-elongated with (E)-1,2-dichloroethane (Sonogashira, 1991). The resulting ω-chloroalcohol was protected and, again, chain-elongated with vinyl magnesium bromide (Millar, 1990). The C14 chain obtained was easily transformed into the final product.
For the synthesis of the two (7E)-configured trienylacetates, 7-octyn-1-ol (1) was transformed in two steps into (E)-1-t-butoxy-8-iodo-7-octene (Alexakis et al., 1988; Alexakis and Duffault, 1988). Chain elongation of this key intermediate used Pd°-catalyzed alkynyl coupling (Sonogashira, 1991 and references cited therein). The syntheses of the target acetates were completed by conventional steps (Scheme 2).
Details of the syntheses are provided in the online supplement.
Wind Tunnel
The wind tunnel hardware and the test protocol have been described (Witzgall et al., 2001). The wind tunnel with a flight section of 63 × 90 × 200 cm was lit diffusely at 6.5 lx. Charcoal-filtered air was blown by a horizontal fan through the tunnel at 30 cm/sec, temperature was 23 ± 2°C, and relative humidity was 40–60%. The outcoming air was aspired by another fan and cleaned by two sets of charcoal filters.
Synthetic pheromone components in redistilled ethanol were released from a piezoelectric sprayer (El-Sayed et al., 1999; Gödde et al., 1999). The solution was delivered at a rate of 10 μl/min through Teflon tubing to a 20-μl glass capillary tube with a drawn-out tip. The capillary was vibrated by a piezo-ceramic disk at ca. 100 kHz to produce an aerosol, which evaporated within a few centimeters from its tip. This “sprayer” allows application of pheromone chemicals at a constant rate and known chemical purity. Isomeric purity of the test compounds was ≥99%, except for 7E,9E,11-12Ac (98%) and 7E,9Z,11-12Ac (95%). Single females were held in glass tubes (2.5 × 5 cm) covered with gauze on both sides. Females were kept in the wind tunnel for at least 30 min before experiments until they showed the typical calling behavior.
Wind tunnel tests were carried out from 1 to 4 hr after onset of the scotophase. Four batches of 15 2- to 3-d-old males (N = 60) were tested per pheromone blend on four different days. Single males were placed in glass tubes (2.5 × 12.5 cm), which were stoppered with gauze on both sides ca. 15 min before testing. Males were tested individually and were given 2 min to respond. The number of males landing at the source, following upwind-oriented flight, in each batch of 15 males was transformed to log(x + 1) and submitted to a one-way ANOVA followed by Duncan's multiple range test (P < 0.05).
Results and Discussion
Chemical analysis of sex pheromone gland extracts of L. botrana females confirmed the presence of previously identified compounds. The main pheromone compound is E7,Z9-12Ac. The analogous E7,Z9-12OH and two saturated alcohols were present, in addition to several saturated, mono-, and diunsaturated acetates (Table 1; Arn et al., 1988; El-Sayed et al., 1999
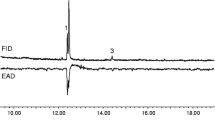
GC-EAD recordings (Figure 1) showed an additional, active compound eluting after E7,Z9-12OH. GC-MS studies indicated a conjugated trienylacetate because of characteristic fragments and its retention time. Co-occurrence of Δ7, Δ9-12Ac and 11-12Ac in L. botrana pheromone glands made Δ7, Δ9,11-12Ac a likely candidate. Synthesis of 7,9,11-12Ac isomers confirmed the presence of E7,E9,11-12Ac in glands of Italian females (Table 1). The EZ and ZE isomers were found in smaller amounts. These three trienyl acetates also were present in gland extracts of females from Germany and Spain (not shown in Table 1). This is the first report of a triply unsaturated compound in a tortricid moth (Arn et al., 2000). The extremely unstable (7Z,9Z,11)-dodecatrienyl acetate or corresponding products of its rearrangements (Näf et al., 1975; Rockach et al., 1980) was not detected. No attempts were made to synthesize this isomer.
Another compound that elicited antennal responses, which could not be associated with known L. botrana pheromone compounds, eluted shortly before E- and Z9-12Ac. This new compound was shown to be E7-12Ac. It was present in gland extracts of females from Italy (Table 1; Figure 1) and from Spain. However, E7-12Ac was not detected in insects from Germany (this study, data not shown), which corroborates analysis of insects from Switzerland (Arn et al., 1988). This suggests the occurrence of L. botrana pheromone dialects north and south of the Alps.
Wind tunnel tests were performed with the newly identified compounds, E7-12Ac, and the EE, EZ, and ZE isomers of 7,9,11-12Ac (Table 2). These compounds were tested in two-component blends with the main compound, using males of a lab population originating from northern Italy. A 10% addition of Z7,E9,11-12Ac significantly increased attraction followed by landing at the source from 24% of the test males with the main compound alone to 65% (N = 60; Table 2). The two other isomers, E7,E9,11-12Ac and E7,Z9,11-12Ac, were less active. A four-component blend of the main compound plus 10% of each of the three triene isomers attracted 61% of males to the source. The trienic E7,Z9,11-12Ac may mimic the main pheromone compound E7,Z9-12Ac because some males were attracted to this compound as a single component.
E7-12Ac had a similar effect as Z7,E9,11-12Ac, with addition of 10% increasing landings to 67% (N = 60; Table 2). Male attraction to the best synthetic blends, at a release rate of 10 pg/min of the main compound, was not different from attraction to calling females. The female release rate is on the order of 10 pg/min (El-Sayed et al., 1999).
The monoene acetates E9-12Ac, Z9-12Ac, and 11-12Ac, and the alcohol corresponding to the main compound, E7,Z9-12OH, are known pheromone synergists of L. botrana (Arn et al., 1988; El-Sayed et al., 1999). Here, we show that E7-12Ac and Z7,E9,11-12Ac are additional, strong pheromone synergists. Partial blends are as attractive as calling females (Table 2; El-Sayed et al., 1999), and this may indicate redundancy in the pheromone signal of L. botrana, as described for the cabbage looper moth Trichoplusia ni (Linn et al., 1984).
Availability of new pheromone synergists is nonetheless of practical importance. Mating disruption of L. botrana by aerial dissemination of pheromone can be effective, but there are many reports of unexpected failures (Arn and Louis, 1996). For example, geographic pheromone races may contribute to inconsistent results. Studies of L. botrana sex pheromone have all relied on insects from Switzerland (Roelofs et al., 1973; Arn et al., 1988; El-Sayed et al., 1999), whereas pheromone-mediated mating disruption is used in vineyards both north and south of the Alps (Kast, 2001; Louis and Schirra, 2001; Varner etal., 2001; Zingg, 2001). The availability of a behavioral synergist, E7-12Ac, which can be made economically, merits a reinvestigation of the effect of pheromone synergists on L. botrana mating disruption.
References
S. R. Abrams A. C. Shaw (1988) ArticleTitleTriple bond isomerizations: 2- to 9-decyn-1-ol Org. Synth. 66 127–130 Occurrence Handle1:CAS:528:DyaL1MXlslegtrc%3D
A. Alexakis J. M. Duffault (1988) ArticleTitleThe hydroalumination of ω-tertbutoxy alkynes, an easy access to ω-hydroxy alkenyl iodides. Application to the synthesis of dienic insect pheromones Tetrahedron Lett. 29 6243–6246 Occurrence Handle1:CAS:528:DyaL1MXksFOgsbw%3D
A. Alexakis M. Gardette S. Coli (1988) ArticleTitleMild protection and deprotection of alcohols as tert.-butyl ethers in the field of pheromone synthesis Tetrahedron Lett. 29 2951–2954 Occurrence Handle1:CAS:528:DyaL1MXhsFeqt7Y%3D
H. Arn F. Louis (1996) Mating disruption in European vineyards R. T. Cardé A. K. Minks (Eds) Pheromone Research—New Directions Chapman and Hall New York 377–382
H. Arn S. Rauscher P. Guerin H.-R. Buser (1988) ArticleTitleSex pheromone blends of three tortricid pests in European vineyards Agric. Ecosyst. Environ. 21 111–117 Occurrence Handle1:CAS:528:DyaL1MXjs1yltA%3D%3D
Arn, H., Tóth, M., and Priesner, E. 2000. The Pherolist. Internet Edition: http://www.pherolist.slu.se.
W. Boland N. Schroer C. Sieler M. Feigel (1987) ArticleTitleStereospecific syntheses and properties of isomeric 2,4,6,10-undecatetraenes. New hydrocarbons from the marine brown algae Giffordia mitchellae Helv. Chim. Acta 70 1025–1029 Occurrence Handle10.1002/hlca.19870700415 Occurrence Handle1:CAS:528:DyaL1cXhvVKns7o%3D
E. V. Dehmlow M. Lissel (1979) ArticleTitleAnwendungen der Phasentransferkatalyse; 9. Neue phasentransferkatalytische Herstellung von Alkenen aus Alkylhalogeniden Synthesis 1979 372–374 Occurrence Handle10.1055/s-1979-28686
A. El-Sayed J. Gödde P. Witzgall H. Arn (1999) ArticleTitleCharacterization of pheromone blend for grapevine moth, Lobesia botrana by using flight track recording J. Chem. Ecol. 25 389–400 Occurrence Handle10.1023/A:1020811200054 Occurrence Handle1:CAS:528:DyaK1MXht1CjtL4%3D
M. Fermaud A. Giboulot (1992) ArticleTitleInfluence of Lobesia botrana larvae on field severity of Botrytis rot of grape berries Plant Dis. 76 404–409 Occurrence Handle10.1094/PD-76-0404
J. Gödde H. Arn A. El-sayed (1999) ArticleTitleThe pheromone sprayer: new technology in stimulus application IOBC/WPRS Bull. 225 49–56
R. Gries A. Reckziegel H. Bogenschütz H.-G. Kontzog C. Schlegel W. Francke J. G. Millar G. Gries (2004) ArticleTitle(Z,Z)-11,13-Hexadecadienyl acetate and (Z,E)-11,13,14-hexadecatrienyl acetate: synergistic sex pheromone components of oak processionary moth, Thaumetopoea processionea (Lepidoptera: Thaumetopoeidae) Chemoecology 14 95–100 Occurrence Handle10.1007/s00049-003-0266-9 Occurrence Handle1:CAS:528:DC%2BD2cXjslers7k%3D
W. K. Kast (2001) ArticleTitleTwelve years of practical experience using mating disruption against Eupoecilia ambiguella and Lobesia botrana in vineyards of the Wuerttemberg region, Germany IOBC/WPRS Bull. 24 IssueID2 71–73
C. E. Linn SuffixJr. L. B. Bjostad J. W. Du W. L. Roelofs (1984) ArticleTitleRedundancy in a chemical signal: Behavioral responses of male Trichoplusia ni to a 6-component sex pheromone blend J. Chem. Ecol. 10 1635–1658 Occurrence Handle10.1007/BF00988431 Occurrence Handle1:CAS:528:DyaL2MXlvFKrsw%3D%3D
F. Louis K.-J. Schirra (2001) ArticleTitleMating disruption of Lobesia botrana (Lepidoptera: Tortricidae) in vineyards with very high population densities IOBC/WPRS Bull. 24 IssueID2 75–79
J. G. Millar (1990) ArticleTitleSynthesis of 9Z,11E,13-tetradecatrienal, the major component of the sex pheromone of the carob moth, Ectomyelois ceratoniae (Lepidoptera; Pyralidae) Agric. Biol. Chem. 54 2473–2476 Occurrence Handle1:CAS:528:DyaK3MXitlGrsg%3D%3D
F. Näf R. Decarzant W. Thommen W. Willhelm G. Rohloff (1975) ArticleTitleThe four 1,3,5-undecatrienes. Synthesis and configurational assignment Helv. Chim. Acta 58 1016–1037
N. A. Porter C. B. Ziegler SuffixJr. F. F. Khouri D. H. Roberts (1985) ArticleTitleGeneral synthesis of polyunsaturated fatty acid hydroperoxides involving a novel vinylcyclopropyl bromide ring opening J. Org. Chem. 50 2252–2258 Occurrence Handle1:CAS:528:DyaL2MXlt1aqt7Y%3D
J. Rockach Y. Girard Y. Guindon J. Atkinson M. Larue R. N. Young P. Masson G. Holme (1980) ArticleTitleThe synthesis of a leucotriene with SRS-like activity Tetrahedron Lett. 21 1485–1488
W. L. Roelofs J. Kochansky R. T. Cardé H. Arn S. Rauscher (1973) ArticleTitleSex attractant of the grapevine moth, Lobesia botrana Mitt. Schweiz. Entomol. Ges. 46 71–73
K. Sonogashira (1991) Coupling reactions between sp2 and sp carbon centers B. M. Trost I. Fleming (Eds) Comprehensive Organic Synthesis 73 Pergamon Press New York, USA 521–550
F. Tellier (1991) ArticleTitleStereospecific synthesis of (E,E)-9,11,13-tetradecatrienyl acetate and aldehyde (geometrical isomers of the major components of two sex pheromones of Lepidoptera) Synth. Commun. 21 2294–2298
F. Tellier C. Descoins R. Sauvêtre (1991) ArticleTitleStereospecific synthesis of 1,5-dien-3-ynes and 1,3,5-trienes: Application to the stereochemical identification of trienic sex pheromones Tetrahedron 47 7767–7774 Occurrence Handle10.1016/S0040-4020(01)81934-7 Occurrence Handle1:CAS:528:DyaK38XlvFSjtw%3D%3D
M. Varner R. Lucin L. Mattedi F. Forno (2001) ArticleTitleExperience with mating disruption technique to control grape berry moth, Lobesia botrana, in Trentino IOBC/WPRS Bull. 24 IssueID2 81–88
P. Witzgall M. Bengtsson S. Rauscher I. Liblikas A.-C. Bäckman M. Coracini P. Anderson J. Löfqvist (2001) ArticleTitleIdentification of further sex pheromone synergists in the codling moth, Cydia pomonella Entomol. Exp. Appl. 101 131–141 Occurrence Handle10.1023/A:1019270515505 Occurrence Handle1:CAS:528:DC%2BD38Xit1WnsLs%3D
D. Zingg (2001) ArticleTitleMating disruption in Switzerland IOBC/WPRS Bull. 24 IssueID2 65–69
Acknowledgments
This study was supported by the Agribio Project (Trento, Italy), MISTRA (Sweden), and the Fonds der chemischen Industrie (Germany).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Witzgall, P., Tasin, M., Buser, HR. et al. New Pheromone Components of the Grapevine Moth Lobesia botrana . J Chem Ecol 31, 2923–2932 (2005). https://doi.org/10.1007/s10886-005-8404-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-8404-1