Abstract
This study identified chemicals found on the eggs of two stink bug species, one native to western North America, Euschistus conspersus, and an invasive species from Asia, Halyomorpha halys. The responses of two scelionid egg parasitoids, Trissolcus erugatus and Telenomus podisi, toward natural stink bug egg volatiles, and synthetic reconstructions of egg volatiles, were tested in bioassays. A compound, methyl (2E,4Z)-2,4-decadienoate, previously identified as the major component of the male-produced aggregation pheromone of E. conspersus, was the major volatile identified from extracts of E. conspersus eggs. In contrast, for H. halys, the sesquiterpenoids that compose the male-produced aggregation pheromone of this species were not detected on eggs, whereas the presence of hexadecanal, octadecanal, and eicosanal was detected. In laboratory olfactometer tests, both Tr. erugatus and Te. podisi females were attracted to extracts of E. conspersus eggs, and to synthetic methyl (2E,4Z)-2,4-decadienoate. However, female Tr. erugatus and Te. podisi wasps were repelled, both by extracts of H. halys eggs and by a blend of the aldehydes identified from H. halys eggs. A follow-up field study, using hexane-washed and intact E. conspersus as sentinel eggs, showed that the parasitoids Trissolcus erugatus and Gryon obesum emerged from these eggs. Sentinel hexane-washed eggs treated with 3 ng of methyl (2E,4Z)-2,4-decadienoate were parasitized more by these two species than were hexane-washed or unwashed eggs, whereas hexane-washed eggs treated with a comparable dose of the C16,18,20 aldehyde mixture were avoided by these parasitoids. In a further field experiment, Trissolcus basalis was the primary parasitoid found in sticky traps baited with methyl (2E,4Z)-2,4-decadienoate, indicating that this species was attracted to, but either did not oviposit or develop in the E. conspersus sentinel eggs in the previous experiment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Complexes of stink bug species (Heteroptera: Pentatomidae) attack a multitude of agricultural crops worldwide, including cotton, corn, and soybeans (e.g., Tillman 2011) and, in California, tomatoes (Zalom et al. 1997). Invasive stink bug species that have established in California, such as the cosmopolitan pest Nezara viridula (L.) (Hoffmann et al. 1991), the crucifer specialist Bagrada hilaris (Burmeister) (Palumbo et al. 2016), and, most recently, the polyphagous brown marmorated stink bug, Halyomorpha halys (Stål) (Hoebeke and Carter 2003), are increasing in importance as pests of Californian crops. Stink bug populations are suppressed by a variety of generalist predators (e.g., Krupke and Brunner 2003; Tillman et al. 2015), but egg parasitoids likely provide the most effective biological control of stink bugs because of their ability to kill potential pentatomid pests before plant damage occurs (Conti and Colazza 2012; Sithanantham et al. 2013). Egg parasitoids are, in fact, the largest group of entomophagous insects associated with Heteroptera (Conti and Colazza 2012). In California, introduction of the scelionid egg parasitoid, Trissolcus basalis (Wollaston), from France, Italy, and Spain has been largely responsible for the decline of N. viridula to below economic threshold levels (Hoffmann et al. 1991).
Of the complex of native stink bugs that feed on tomatoes in California, the consperse stink bug, Euschistus conspersus Uhler (Heteroptera: Pentatomidae), is the most important (Cullen and Zalom 2005). Nymphs and adults of E. conspersus severely damage green and mature red fruit by injecting salivary enzymes as they feed (Zalom et al. 1997). Halyomorpha halys looms as a potentially damaging stink bug invader in California because of its wide host plant range (Joseph et al. 2015), and because its population and distribution are still expanding (StopBMSB.org 2015). In addition, brown marmorated stink bug adults are a nuisance due to their propensity to overwinter in buildings (Cira et al. 2016), sometimes in large numbers (Inkley 2012). In its native range in Asia, H. halys is an occasional or outbreak pest of numerous crops (Lee et al. 2013), but in the U.S., it has caused severe economic losses in the mid-Atlantic states on peaches, pears, apples, grapes, soybeans, sweet corn, green beans, eggplant, tomatoes, and other crops (Joseph et al. 2015; Rice et al. 2014). Moreover, H. halys is established and spreading in Canada (Fogain and Graff 2011) and Europe (Gariepy et al. 2015; Haye et al. 2015; Wermelinger et al. 2008), and is threatening to become a global pest (Zhu et al. 2012).
Parasitoid wasps in the subfamily Telenominae (Hymenoptera: Scelionidae) (Talamas and Buffington 2015) develop inside eggs of other insects, mainly those of Heteroptera, Lepidoptera, Diptera, and Neuroptera (Taekul et al. 2014). Based on molecular data, it appears that stink bugs (Pentatomidae) and seed bugs (Lygaeidae) are the ancestral hosts for the two telenomine core genera, Telenomus and Trissolcus (Taekul et al. 2014). Telenomus podisi Ashmead is a cosmopolitan parasitoid of eggs of many pentatomid species (e.g., Tillman 2010), particularly Euschistus eggs (Hoffmann et al. 1991; Tillman et al. 2010). In its native range, the most important egg parasitoids of H. halys are Trissolcus species (Lee et al. 2013; Rice et al. 2014). Although some North American and European Trissolcus wasps found fresh sentinel H. halys eggs in wooded field sites, few were able to develop successfully in these fresh H. halys eggs (Haye et al. 2015; Herlihy et al. 2016; Talamas et al. 2015a). Therefore, explorations for parasitoids in the Asian range of H. halys were undertaken, with two Trissolcus species from China, Tr. japonicus (Ashmead) and Tr. cultratus (Mayr), being identified as promising candidates for classical biological control of H. halys in regions where it has been introduced (Haye et al. 2015; Talamas et al. 2015a). Surprisingly, Tr. japonicus recently has been found established in both the eastern and western U.S., presumably by adventitious introductions (Herlihy et al. 2016; Lara et al. 2016; Talamas et al. 2015a).
Besides pupae, insect eggs are the most inconspicuous host stage attacked by parasitic wasps, yet chemicals directly associated with a host’s eggs are the ultimate cues triggering successful oviposition by egg parasitoids (Fatouros et al. 2008). This incongruence confronting foraging egg parasitoids is an example of the ‘reliability-detectability dilemma’ described by Vet and Dicke (1992) in their seminal effort to organize literature on insect parasitism. Potential foraging cues, such as host-plant volatiles, may be highly detectable at long distances but may not reliably predict the presence of hosts, whereas chemicals on or in host eggs are the most reliable cues for host recognition, but these compounds may be present in such low concentrations as to challenge detection by foraging parasitoids. This type of selection on eggs favors inconspicuousness.
In their review, Conti and Colazza (2012) followed the conceptual approach of Vet and Dicke (1992) for the chemical ecology of egg parasitoids associated with true bugs. Foraging Telenomus and Trissolcus females exemplify this reliability/detectability spectrum enabling them to find their hosts quickly. In various species, this spectrum ranges from: 1) attraction of female wasps to male-produced aggregation pheromones of their host, followed by phoresy on the mated host females (Aldrich et al. 1984; Bruni et al. 2000); 2) attraction to male-produced pheromones (Borges et al. 1999; Krupke and Brunner 2003; Tognon et al. 2014) or to defensive secretions of adults (Laumann et al. 2009; Mattiacci et al. 1993) to arrive in the vicinity of potential host eggs; 3) attraction to plant volatiles induced by the combined feeding and oviposition by adult host females (Colazza et al. 2004); 4) intensified searching in areas where gravid females of potential hosts have left chemical ‘footprints’ (Colazza et al. 2007; Salerno et al. 2009); and 5) recognition of odors from adhesive secretions used to attach eggs to each other and the substrate (Bin et al. 1993; Borges et al. 1999).
Earlier research by Tognon et al. (2014) on the generalist pentatomid egg parasitoid Te. podisi from Brazil showed that female wasps from a colony maintained for many generations on eggs of the stink bug Euschistus heros (Fabricius) preferred to oviposit in eggs of E. heros rather than in eggs of the rice stink bug, Tibraca limbativentris Stål, whereas female wasps from T. limbativentris eggs collected in a rice field, preferred to oviposit in E. heros eggs after being reared for just one generation on the latter host. In contrast, Te. podisi reared directly from rice stink bug eggs did not exhibit a preference for eggs of T. limbativentris over those of E. heros. The present study is an extension of this earlier research on scelionid/pentatomid egg parasitism (Tognon et al. 2014) using eggs of E. conspersus and H. halys, and the native North American egg parasitoids Te. podisi and Trissolcus erugatus Johnson. The primary objective of this study was to identify the volatiles from the stink bug eggs, and test egg extracts and individual compounds and blends of compounds identified from the egg extracts, in both laboratory and field bioassays. Our results are similar to those of Tognon et al. (2014), and provide a chemical explanation for the divergent responses of scelionid parasitoids toward the eggs of different stink bug species, thus highlighting a little known level of chemical communication in Heteroptera.
Methods and Materials
Stink Bug Colonies
A colony of E. conspersus nymphs and adults was obtained from Paramount Farming in Shafter, Kern County, CA, USA in December 2014 from collections made in the southern San Joaquin Valley during the summer of 2014. This colony was maintained in a Percival Model I-36LL growth chamber (Perry, IA, USA; 26 ± 1 °C, 65 ± 10 % RH, 16 L:8D photoperiod) at the University of California at Davis (UCD). Adults were maintained in 19 × 25 × 19-cm cylindrical plastic containers (accumulated from Trader Joe’s grocery, Monrovia, CA, USA) with a 13 × 13-cm hole in the top fitted with organza material. Insects were fed organic green beans, sunflower seeds, and cherry tomatoes, with water provided from cotton-stoppered polypropylene shell vials (4.0 ml, 15 mm diam, 45 mm height; J. G Finneran Associates, Inc.,Vineland, NJ, USA). Each cage contained no more than 50 adults, and the food was replaced twice a week. Paper towel-lined cages served as an oviposition substrate. Eggs were collected from the cages daily, and maintained separately under the same conditions as above for colony maintenance, and used in bioassays.
Halyomorpha halys nymphs and adults were collected in 2015 from Fremont Community Garden in Sacramento County, CA, USA. A colony of H. halys was maintained as described above for E. conspersus, except that pumpkin seeds and slices of apple, plum or cherries also were provided.
A colony of the spined soldier bug, Podisus maculiventris Say (Heteroptera: Pentatomidae), was established from adults collected at the arboretum of the University of California at Santa Cruz (UCSC) in Rescue® Stink Bug Traps (Sterling International, Inc., Spokane, WA, USA) baited with the synthetic aggregation pheromone of P. maculiventris (Aldrich et al. 1984). The P. maculiventris colony was maintained as for the other stink bugs, except that insects were fed larvae and pupae of Tenebrio molitor L. (Coleoptera: Tenebrionidae) obtained commercially (Rainbow Mealworms, Compton, CA, USA).
Parasitoid Colonies
Trissolcus erugatus and Te. podisi were collected in the Student Farm at UCD and the UCSC arboretum using fresh sentinel egg masses of E. conspersus and P. maculiventris, respectively. Trissolcus erugatus was identified using the key of Talamas et al. (2015b); Te. podisi was identified using the key of Johnson (1984). Voucher specimens for each parasitoid species have been deposited in the UCD R. M. Bohart Museum of Entomology. Eggs were collected daily, and exposed in the field the same day by clipping masses onto leaves of garden plants for Tr. erugatus or onto coast live oaks (Quercus agrifolia) for Te. podisi. After 48 h, eggs were removed from the field, and held for possible parasitoid emergence. Both species were maintained in a Percival Model I-36LL growth chamber at 26 ± 1 °C, 65 ± 10 % RH, 14 L:10D photoperiod, using E. conspersus eggs; adult wasps were fed honey only.
Preparation of Egg Extracts
To collect eggs from E. conspersus and H. halys, mated females were separated from males and kept in different cages with food, water, and paper toweling. Eggs (12–24-h-old) were removed from the paper with forceps, while wearing unpowdered latex gloves, placed in glass vials (2 ml borosilicate; Waters Corp., Milford, MA, USA), and weighed. Enough tert-butyl methyl ether (99.8 %; Sigma-Aldrich, St. Louis, MO, USA) to cover ~1 g of eggs was added; after 5 min, the solvent was transferred by syringe to another clean glass vial. Six samples of egg extracts from each species were analyzed. Other samples used for laboratory bioassays were prepared similarly, but using hexane as the solvent (>99 %, Sigma-Aldrich); samples were kept at −4 °C until use. Tert-Butyl methyl ether was the solvent of choice for egg extracts for chemical analysis because of its greater purity in the range of early eluting volatiles, while hexane was used for egg extracts for bioassays because, unlike tert-butyl methyl ether, it lacks a strong odor.
Dissection of Male Stink Bugs
The genital capsules (terminal segment) of wild E. conspersus and H. halys males were dissected and extracted as follows. Soon after being captured in the field, adult males were killed by freezing at −4 °C for 20 min, and the genital capsule was clipped from the terminus of each male under a stereomicroscope using clean forceps and micro-scissors. Four or five capsules were clipped into 2 ml glass vials, and covered with tert-butyl methyl ether. After 5 min, the solvent was transferred by syringe to another clean glass vial. Five samples for each species were analyzed.
Chemical Identifications
Stink bug egg extracts or male genital capsule extracts were concentrated to 20 μl under N2, and analyzed by coupled gas chromatography/mass spectrometry (GC/MS) using an HP 6890 GC/5973 mass selective detector in the electron impact mode at 70 eV. An HP-5MS GC column (30 m × 0.25 mm × 0.25 μm film thickness; J & W Scientific, Folsom, CA, USA) was used for the analyses. Three microliters of each concentrated sample were injected into the GC/MS in splitless mode, with helium as carrier gas, and injector and detector temperatures of 250 and 300 °C, respectively. Column oven temperature was programmed from 50 °C (held for 1 min) to 240 °C at 10 °C.min−1, with a final hold of 10 min. Compounds of interest were tentatively identified using Wiley7N (Scientific Instrument Services, Inc., Ringoes, NJ, USA) and PAL 600 K (Palisade Corp., Ithaca, NY, USA) mass spectral libraries. Key compounds were fully identified by comparison of retention times and mass spectra to those of authentic standards. Fifty nanograms of octyl acetate (>99 % Sigma-Aldrich) was added as an internal standard (IS) for compound quantification to some unconcentrated samples before GC/MS analysis. The standard of the main pheromone component of Euschistus conspersus (Aldrich et al. 1991), methyl (2E,4Z)-2,4-decadienoate, was purchased commercially (>90 %; Bedoukian Research, Inc., Danbury, CT, USA). For identification of the aldehydes found on the eggs of H. halys, a crude mixture of C14–20 aldehydes was prepared by oxidation of the corresponding commercially available primary alcohols as described in detail below: C14,15,17:OHs (97, 97, 98 %, respectively; Sigma-Aldrich), C16,19:OHs (97 and 98 %, respectively; TCI America, Portland, OR, USA), C18:OH (97 %; Avocado Research Chemicals, Haysham, UK), and C20:OH (96 %; Lancaster Synthesis, Pelham, NH, USA). For laboratory olfactometer studies and field experiments, a purified synthetic mixture of C16,18,20 aldehydes (500 ng/μl hexane) was prepared at UC Riverside as follows. The C16,18,20 straight-chain aldehydes appeared to be present in a ratio of ~4.5:4.5:1 in extracts of H. halys eggs (see below), so the aldehydes were synthesized as a blend, rather than individually. Thus, hexadecanol (0.55 g, 2.25 mmol), octadecanol (0.61 g, 2.25 mmol), and eicosanol (0.15 g, 0.5 mmol) were dissolved in dry methylene chloride, and the solution was cooled to 0 °C. A mixture of pyridinium dichromate (2.65 g, 7 mmol) and 2.5 g powdered 4 Å molecular sieve was ground with a mortar and pestle, and then added in one portion to the solution of alcohols. The cooling bath was removed, and the mixture was warmed to room temperature and stirred for 2 h, then diluted with 100 ml of hexane and stirred 15 min. The resulting slurry was filtered through a celite pad. The filtrate was concentrated by rotary evaporation, then taken up in 10 ml of hexane and purified by vacuum flash chromatography on 40 g silica gel pre-wetted with hexane, eluting with 1 × 50 ml of hexane, and 6 × 50 ml of 5 % EtOAc in hexane. Fraction 4 containing the aldehydes was concentrated (~75 % overall yield), transferred to an ampoule, made up to 10 ml with hexane, and a small crystal of butylated hydroxytoluene was added as a stabilizer. The ampoule was sealed and shipped by overnight courier to UCD for bioassays. A subsample of the aldehyde mixture was sent to the Spokane laboratory for GC/MS analysis under the same conditions as for the natural product samples.
Laboratory Bioassays
The behavior of Te. podisi females reared from E. conspersus eggs was observed in a two-choice test using a horizontally positioned Y-tube olfactometer (1.4-cm diam), with a 16 cm basal arm that bifurcated at 60° into two 19-cm arms. Airflow was 0.8 l.min−1 provided by a vacuum pump connected to a flow meter and a humidifier. Before the experiment, each female was placed individually in a glass tube (5 ml), and provided with a drop of honey. The Y-olfactometer was surrounded by a paper wall to minimize possible cues from the room, and was illuminated by a white compact fluorescent bulb (9 W) located above the device. The temperature in the bioassay room was maintained at 22 °C. A piece of filter paper (1 × 2 cm, P5 Fisherbrand®, Fisher Scientific, Marshalltown, IA, USA) with 5 μl of a hexane solution of the test substance was placed in one arm of the olfactometer; the other arm contained tissue paper with 5 μl hexane. A single wasp was introduced into the Y-tube, and allowed to choose between the arm with the egg extracts of E. conspersus (10 egg equivalents; EE) and the hexane control arm. The same procedure was followed for egg extracts of H. halys (4.5 EE), and the synthetic compounds identified in egg extracts. The main pheromone component of E. conspersus males (methyl (2E,4Z)-2,4-decadienoate) was tested at two different concentrations (0.2 and 2 ng/μl hexane, or 1 and 10 ng total), and the mixture of C16,18,20 aldehydes tested at 0.02 ng/μl hexane (0.1 ng total); hexane was the control for all bioassays.
Each wasp was allowed 10 min to choose one of the olfactometer arms, and then was discarded whether or not it had made a choice. A choice was defined as when a wasp passed a line 3 cm into either arm of the Y-tube, and remained there for 1 min. Unresponsive females were those that did not move during the first 5 min or did not pass the 3 cm line in either of the two arms of the olfactometer in 10 min; these were excluded from the statistical analysis. The olfactometer was rotated 180° after every three trials, and washed after every nine trials with water and acetone, and dried at 100 °C in an oven. After this procedure, the tissue papers with test substances were renewed. Each treatment was tested at least 40 times.
Sentinel Egg Mass Bioassays
These experiments were conducted in two different staked organic tomato fields (Solanum lycopersicum L. var. “Big Beef”; flowering and early fruiting stages) at the UCD Student Farm during the summer of 2015. Both fields were surrounded by plantings of sweet alyssum [Lobularia maritima L. (Brassicaceae)] as a nectar source for natural enemies.
Euschistus conspersus sentinel egg masses were deployed in 12 randomized complete blocks, each consisting of 4 egg masses per treatment, as follows. Six 90 m row beds spaced on 2 m centers were selected for the study. Four E. conspersus sentinel egg masses <24 h old (total 50 eggs) from the laboratory colony were fastened onto 1 × 1.5-cm filter paper strips with double-sided tape, then clipped onto nearby plants approximately 1.1 m above the ground at a spacing of 20 m along each bed. Treatments in this experiment included: 1) hexane-extracted eggs with 30 μl of the synthetic aldehyde mixture (0.4 ng/μl, 12 ng total) added; 2) hexane-extracted eggs with 30 μl of methyl (2E,4Z)-2,4-decadienoate solution (0.1 ng/μl, 3 ng total) added; 3) hexane-extracted eggs; and 4) unextracted eggs. For egg extraction, masses were placed in a glass Petri dish, rinsed with 99 % hexane for 5 min, and air-dried. Synthetic test compounds were applied to the washed eggs in 30 μl of hexane with an electronic pipettor (Thermo Fisher Scientific, Waltham, MA, USA). After 24 h, eggs were removed from the field, placed in 7.5 × 1.3 cm glass vials containing a drop of honey, and sealed with parafilm, then kept in a laboratory growth chamber under the same conditions as described above. Parasitoid emergence and/or nymphal hatching were checked daily. There were 12 replications for each treatment, totaling 600 exposed eggs per treatment.
Pheromone-Baited Traps for Parasitoids
The effect of synthetic methyl (2E,4Z)-2,4-decadienoate on attraction of egg parasitoids was evaluated using yellow sticky traps (13 × 8 cm; Olson Products, Inc., Medina, OH, USA) baited with treated or control rubber septa. Gray rubber septa (9 mm, Soxhlet-extracted overnight with methylene chloride; West Pharmaceutical Services, Kearney, NE, USA) were impregnated with methyl (2E,4Z)-2,4-decadienoate on the same day they were placed in the field. Initially, stock solutions of methyl (2E,4Z)-2,4-decadienoate were prepared by serial dilution at concentrations of 10 μg/μl hexane, 1 μg/μl, and 0.1 μg/μl, then 100 μl aliquots of the respective stock solutions were applied to prepare sets of septa with 1 mg, 0.1 mg, and 0.01 mg of methyl (2E,4Z)-2,4-decadienoate per septum; controls consisted of 100 μl of pure hexane/septum. The groups of loaded septa were stored by treatment, wrapped in aluminum foil in plastic freezer bags at −4 °C. Septa were transported to the field in Styrofoam boxes with ice packs. The sticky traps were hung from stakes ~1.3 m above the ground every 15 m along four 90 m row beds, each 2 m apart. Sticky traps and lures were replaced daily, with trap treatment positions randomly assigned. All traps were stored in a refrigerator so that the insects could be counted and identified at a later date. Samples of egg parasitoids were removed from sticky traps using diethyl ether (Sigma-Aldrich) and orange oil (100 % essential oil of Citrus sinensis, manufactured for Davis Food Co-op, Davis, CA, USA), transferred to vials with ethanol (96 %), and sent to Dr. Matthew Buffington (USDA-ARS, Systematic Entomology Laboratory, Washington, D.C., USA) for identification.
Statistical Analyses
Olfactometer data were analyzed by χ2-tests (P < 0.05). Field data means were compared with one-way ANOVA or by Kruskal-Wallis test, depending on the data normality (P < 0.05), using BioEstat® 5.0 (Ayres et al. 2007).
Results
Chemical Identifications
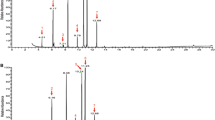
Methyl (2E,4Z)-2,4-decadienoate [key m/z 67, 79, 81, 111, 151, and 182 (M+)] was identified as the major volatile component in extracts of E. conspersus eggs, as confirmed by retention time and mass spectral matches with the synthetic standard (Fig. 1a). Based on the total ion chromatogram peak area and comparisons with the octyl acetate IS, the mean methyl (2E,4Z)-2,4-decadienoate concentration was 56 ng/g fresh weight of E. conspersus eggs (ranging from 20 to 128 ng/g eggs; N = 3). In addition, nonanal, (E)-2-decenal, (2E,4E)-2,4-decadienal, (E)-2-undecenal, and tridecane were tentatively identified as minor components (Fig. 1a). The common plasticizer, diethylphthalate, was present in all the E. conspersus egg extracts and was comsidered a contaminant of unknown origin. Palmitic and linoleic acids were abundant, non-volatile components in the E. conspersus egg extracts.
Total ion mass chromatograms for representative analyses of (a) Euschistus conspersus egg extract, and (b) Halyomorpha halys egg extract. Compounds labeled in bold (methyl (2E,4Z)-2,4-decadienoate for E. conspersus; hexadecanal, octadecanal, and eicosanal for H. halys) were identified by comparisons to authentic standards; other compounds were tentatively identified by comparisons to published mass spectra (* denotes contaminants)
The genital capsule extracts of E. conspersus males contained two major volatile components, methyl (2E,4Z)-2,4-decadienoate and tridecane, and a minor component, dodecane (Fig. 2a).
Total ion mass chromatograms for representative analyses of (a) male Euschistus conspersus genital capsule extract (N = 5), and (b) male Halyomorpha halys genital capsule extract (N = 5). Compounds labeled in bold (methyl (2E,4Z)-2,4-decadienoate for E. conspersus; hexadecanal, octadecanal, and eicosanal for H. halys) were identified by comparisons to authentic standards; other compounds were tentatively identified by comparisons to published mass spectra (* denotes contaminants)
Hexadecanal (63 ng/g), octadecanal (56 ng/g), and eicosanal (5 ng/g) were identified and quantified (via IS; N = 2) from H. halys egg extracts (Fig. 1b), and their identities confirmed using the synthetic aldehyde mixture. In addition to relatively large amounts of palmitic and linoleic acids and diethylphthalate, the same minor volatile aldehydes as found in the E. conspersus egg extracts [nonanal, (E)-2-decenal, (2E,4E)-2,4-decadienal, and (E)-2-undecenal] also were tentatively identified in H. halys egg extracts (Fig. 1b).
Analysis of the genital capsule extracts of H. halys males revealed the presence of the same three long-chain aldehydes (hexadecanal, octadecanal, and eicosanal) as identified from egg extracts (in a similar ratio) (Fig. 2b). Two other volatile components (tridecane and dodecane) also were detected, plus linoleic acid and some contaminants.
The GC/MS data of both H. halys egg extracts and male genital capsule extracts were also examined for selected ion chromatograms [m/z: 93, 71, 134, 165, and 220 (M+)] characteristic of the two 10,11-epoxy-1-bisabolen-3-ols identified as the male-produced aggregation pheromone of the brown marmorated stink bug (Khrimian et al. 2014). Neither of these pheromone components was detected in either egg or genital capsule extracts of H. halys.
Laboratory Bioassays
Females of Te. podisi and Tr. erugatus both were attracted to E. conspersus egg extracts over hexane controls in the olfactometer (P < 0.05) (Fig. 3). In contrast, females of Te. podisi and Tr. erugatus both were repelled by H. halys egg extracts compared to hexane controls (P < 0.05) (Fig. 4).
Olfactometer responses of Telenomus podisi and Trissolcus erugatus females (reared from Euschistus conspersus eggs) to volatiles from E. conspersus egg extracts (10 egg equivalents; EE) vs. to hexane (numbers of insects are in parentheses; values followed by an asterisk are different by χ2 at P < 0.05 from the hexane control)
Olfactometer responses of Telenomus podisi and Trissolcus erugatus females (reared from Euschistus conspersus eggs) to volatiles from Halyomorpha halys egg extracts (4.5 egg equivalents; EE) vs. to hexane (numbers of insects are in parentheses; values followed by an asterisk are different by χ2 at P < 0.05 from the hexane control)
When the synthetic compounds were tested in the olfactometer, females of Te. podisi and Tr. erugatus both were attracted to synthetic methyl (2E,4Z)-2,4-decadienoate at high (10 ng) and low (1 ng) doses (Figs. 5 and 6). At the high dose of synthetic methyl (2E,4Z)-2,4-decadienoate, 71 and 23 % of Te. podisi females responded to the treatment and the control, respectively (χ2 = 20.00; df = 1; P < 0.001), while 64 and 28 % of Tr. erugatus females responded to the treatment and the control, respectively (χ2 = 11.54; df = 1; P = 0.0015) (Fig. 5). At the low dose of synthetic methyl (2E,4Z)-2,4-decadienoate, 76 and 19 % of Te. podisi females responded to the treatment and the control, respectively (χ2 = 28.80; df = 1; P < 0.001), while 56 and 28 % of Tr. erugatus females responded to the treatment and the control, respectively (χ2 = 8.00; df = 1; P = 0.0095) (Fig. 6). In contrast, when female wasps were tested in the olfactometer to low doses (0.1 ng) of the synthetic C16,18,20 aldehyde blend, females of both species were repelled (P < 0.05) (Fig. 7).
Olfactometer responses of Telenomus podisi and Trissolcus erugatus females (reared from Euschistus conspersus eggs) to methyl (2E,4Z)-2,4-decadienoate (10 ng) vs. to hexane (numbers of insects are in parentheses; values followed by an asterisk are different by χ2 at P < 0.05 from the hexane control)
Olfactometer responses of Telenomus podisi and Trissolcus erugatus females (reared from Euschistus conspersus eggs) to the synthetic C16,18,20 aldehyde blend (0.1 ng) vs. to hexane (numbers of insects are in parentheses; values followed by an asterisk are different by χ2 at P < 0.05) from the hexane control)
Sentinel Egg Mass Bioassays
Two scelionid parasitoid species, Gryon obesum Masner and Tr. erugatus (Hymenoptera: Scelionidae), were obtained from E. conspersus sentinel eggs exposed in tomato fields (Table 1). The eggs that were hexane-washed and treated with methyl (2E,4Z)-2,4-decadienoate had higher rates of parasitization (94.2 %) than the other three treatments (P < 0.001). In contrast, only 0.2 % of washed eggs treated with the C16,18,20 aldehyde mixture were parasitized (Table 1, treatment 2), and this level of parasitism was not different from that for the washed eggs plus hexane control (9.7 %; Table 1, treatment 3); parasitism rates for both these treatments were lower than that of unwashed eggs (31.8 %, Table 1, treatment 4) (Table 1, P < 0.001).
Pheromone-Baited Traps for Parasitoids
Two parasitoid species, Tr. basalis (Hymenoptera: Scelionidae) and Polynema sp. (Hymenoptera: Mymaridae), were captured on sticky traps baited with methyl (2E,4Z)-2,4-decadienoate or with hexane controls. Parasitoid captures decreased daily over the five days of the test. The mean numbers of parasitoids collected in traps baited with 1 mg and 0.1 mg of methyl (2E,4Z)-2,4-decadienoate were not different from each other (H = 2.5, df = 3, P = 0.50), but were greater than the mean numbers of parasitoids in traps baited with 0.01 mg methyl (2E,4Z)-2,4-decadienoate or the hexane control (P < 0.05). There was no difference in catches between the latter two treatments (H = 0.9, df = 3, P = 0.81) (Table 2).
Discussion
The most surprising result of the present study is that the main male-produced aggregation pheromone component of Euschistus conspersus, methyl (2E,4Z)-2,4-decadienoate (Aldrich et al. 1991), is the principal volatile on E. conspersus eggs, and that females of Telenomus podisi and Trissolcus erugatus are attracted in olfactometer tests to egg extracts containing low (nanogram levels) of this chemical and to comparable levels of the synthetic compound. Females of E. conspersus do not produce methyl (2E,4Z)-2,4-decadienoate (Aldrich et al. 1991). Analyses of extracts of the genital capsules from E. conspersus males verified the presence of nanogram levels of methyl (2E,4Z)-2,4-decadienoate. These results suggest that methyl (2E,4Z)-2,4-decadienoate is transferred to conspecific females during mating, and that the surfaces of eggs are contaminated subsequently with this pheromone compound during oviposition, with the parasitoids, thus exploiting this compound as a host-egg finding kairomone.
In contrast, eggs of the brown marmorated stink bug, Halyomorpha halys, completely lack the sesquiterpenoid pheromone compounds identified from males of this species (Khrimian et al. 2014). However, C16,18,20 aldehydes are present on H. halys eggs, and the synthetic mixture of these aldehydes proved to be repellent to Te. podisi and Tr. erugatus females in olfactometer tests. Furthermore, the same aldehydes were present in extracts of male H. halys genital capsules. Comparable analyses were not performed on conspecific females, so the possibility that H. halys females themselves are the source of the C16,18,20 aldehydes cannot yet be excluded. More experiments are needed, ideally including Trissolcus japonicus, to clarify the semiochemistry of H. halys egg parasitism.
Results of field tests using E. conspersus sentinel egg masses treated with various extracts or compounds, as well as trials testing traps baited with methyl (2E,4Z)-2,4-decadienoate, substantiated and extended the results of the laboratory olfactometer tests. Trissolcus erugatus and another generalist egg parasitoid, Gryon obesum Masner (Krupke and Brunner 2003; Rider 2016), were particularly attracted to hexane-washed sentinel eggs of E. conspersus that had been treated with methyl (2E,4Z)-2,4-decadienoate. By contrast, hexane-washed egg masses of E. conspersus that were treated with the mixture of aldehydes found on H. halys eggs were avoided by these parasitoids in sentinel egg field bioassays, suggesting that these compounds serve a defensive role. In addition, sticky traps baited with methyl (2E,4Z)-2,4-decadienoate and deployed in staked tomatoes caught mainly Trissolcus basalis, demonstrating that females of this exotic generalist, introduced for biological control of Nezara viridula in 1986 (Hoffmann et al. 1991), recognized the key pheromone component of E. conspersus (Weber et al. 1996) even though they did not oviposit in, or were unable to survive in E. conspersus sentinel eggs.
Both Telenomus and Trissolcus species are known to exploit the attractant pheromones of their heteropteran hosts as cues (i.e., as kairomones) to guide their long-range search for potential host eggs (Conti and Colazza 2012; Tognon et al. 2014). In particular, Te. podisi females were attracted to traps baited with racemic methyl 2,6,10-trimethyltridecanoate (Silva et al. 2006), the main male-produced aggregation pheromone component of E. heros (Aldrich et al. 1994; Borges and Aldrich 1994). The study of Borges et al. (1999) is particularly illuminating for Te. podisi parasitism of E. heros eggs, because the attractiveness of both fertile and unfertilized host eggs to female wasps was studied; E. heros females eventually lay eggs if not allowed to mate, which precludes the possibility of male-produced semiochemicals being transferred to these eggs. Borges et al. found that unfertilized E. heros eggs were less attractive to, and stimulatory for oviposition by, Te. podisi females than were fertilized eggs. Furthermore, egg-sized glass beads (3 beads/replicate) treated with an extract of fertile eggs, an extract of sexually mature males, or 0.1 ng of synthetic racemic methyl 2,6,10-trimethyltridecanoate were as attractive and stimulatory to the wasps as fertile eggs; higher doses of synthetic pheromone were less attractive (Borges et al. 1999). These findings are consistent with our interpretation that stink bug methyl ester pheromones are produced in the genital capsule of males, transferred to females during mating, and eventually transferred to eggs during oviposition. Nevertheless, Borges et al. (1999) failed to detect methyl 2,6,10-trimethyltridecanoate in extracts of E. heros fertile or infertile eggs, and the same negative result was reported recently by the same group in a study on T. podisi parasitism of E. heros (Michereff et al. 2016). The discrepancy between the latter researchers’ results and our data may be due to the fact that we extracted gram quantities of E. conspersus eggs vs. their extraction of only 20 E. heros eggs per sample. Future research is needed to clarify this discrepancy. Be that as it may, it appears that the long-range pheromone from Euschistus males may be a relatively easily detectable, but imprecise, cue leading to the location of potential host eggs, whereas the presence of pheromone methyl esters on host eggs themselves may be a faint, but precise indicator of acceptability (Aldrich et al. 1994).
We suggest that our current findings provide an explanation for the earlier results of Tognon et al. (2014) showing that female wasps reared from eggs of the rice stink bug, T. limbativentris, prefer to oviposit in eggs of E. heros after just one generation on the latter host. We hypothesize that the preference switch to E. heros eggs is due to the imprinting of wasps during eclosion to methyl ester pheromone components from males that are transferred to the surface of E. heros eggs via mated females. Inherent in this explanation is that emerging wasps are able to learn to prefer odors on the surface of eggs, which was demonstrated by Tognon et al. (2013) using lemongrass extract applied to the surface of washed host eggs. This hypothesis is reminiscent and pertinent to a modern interpretation (Barron 2001; Corbet 1985) of Hopkins’ host selection principle: that many phytophagous and parasitic insects have a preference for the host species on which they developed (Hopkins 1917).
In concluding, it is worthwhile to consider the known distributions of methyl ester vs. sesquiterpenoid pheromones in pentatomids, and the potential ramifications of our current findings for future research on the mediation of egg parasitoid behaviors by heteropteran semiochemicals. Male-produced attractant pheromones have been identified for species in several genera of phytophagous stink bugs, primarily species that are agricultural pests, and many of these pentatomids produce pheromones consisting of methyl esters and/or sesquiterpenoids (Khrimian et al. 2014; Millar 2005; Weber et al. 2014). Studies of egg parasitoids of several species of the “sesquiterpenoid-type” pheromone species have been conducted, especially for the Nezara viridula/Tr. basalis host/parasitoid pair (Conti and Colazza 2012), yet there is no evidence from any of these species that sesquiterpenoids are transferred to the surface of eggs via mating. In N. viridula, males are believed to produce and release their bisabolene epoxide-type sesquiterpenoid pheromone components from single-celled epidermal glands on the abdominal sternum (Cribb et al. 2006), which would explain why their sesquiterpenoid pheromone components are not transferred to conspecific eggs during mating. Our inability to detect the bisabolene-type sesquiterpenes of H. halys males in their genital capsules or on the surface of conspecific eggs is consistent with pheromone biosynthesis taking place in the cuticle of the abdominal sternum, as in Nezara males. If methyl ester pheromones of other species besides Euschistus spp. prove to come from the genital capsules of males, whereas the sesquiterpenoid type pheromones come from the abdominal sternum of males (in species producing those compounds), then our proposed hypothesis for the kairomonal dichotomies discovered for Telenomus and Trissolcus parasitoids and Euschistus, Tibraca, and Halyomorpha host eggs can be tested further. Moreover, analogous host/egg parasitoid predictions can also be proposed and tested in species in the related families Coreidae and Alydidae, some of which possess so-called ventral abdominal glands positioned such that their secretions are likely to be transferred to females during mating (Aldrich 1988; Millar 2005).
Finally, one may wonder what type of pheromone system evolved first in the Pentatomidae, and why? As noted by Blum (1974), the semiochemical category known as “kairomones”, signals defined as maladaptive to the producing individuals, is an evolutionarily artificial category because nothing can evolve if it is solely disadvantageous to the emitter. Therefore, there must be some positive function associated with methyl ester pheromones being transferred to females during mating that, at least in evolutionary history, outweighed the disadvantage of attracting egg parasitoids. One such advantage may be that marking females as mated might limit subsequent matings by conspecific males or the same male himself. However, over time, as parasitoids evolved to exploit this female and egg-marking vulnerability, selection led to the evolution of the alternative sesquiterpenoid pheromone system, possibly through a stage in which species utilized both methyl ester and sesquiterpenoid compounds (e.g., McBrien et al. 2002) in their pheromone blends. In this scenario, the sesquiterpenoid pheromone system would be more derived than the methyl ester pheromone system. Our finding, that instead of transferring sesquiterpene pheromone compounds to females, males of the brown marmorated stink bug produce aldehydes in the genital capsule that may defend against North American egg parasitoids once transferred via mating to conspecific eggs [but not against Tr. japonicus with which it coevolved (Herlihy et al. 2016)]. This suggests that evolution toward an “enemy free space” (Jeffries and Lawton 1984) has been a powerful force in heteropteran evolution. Modern phylogenetics may soon provide an answer as to which pheromone type came first in the Pentatomidae, with research on stink bug phylogenetics in progress (Dr. Jocelia Grazia, Universidade Federal do Rio Grande do Sul, Brazil; pers. Comm.). In the meantime, the current semiochemical knowledge should be useful in guiding biological control of heteropteran pests.
References
Aldrich JR (1988) Chemical ecology of the Heteroptera. Annu Rev Entomol 33:211–238
Aldrich JR, Kochansky JP, Abrams CB (1984) Attractant for a beneficial insect and its parasitoids: pheromone of the predatory spined soldier bug, Podisus maculiventris (Hemiptera: Pentatomidae. Environ Entomol 13:1031–1036
Aldrich JR, Hoffmann MP, Kochansky JP, Lusby WR, Eger JE, Payne JA (1991) Identification and attractiveness of a major pheromone component for Nearctic Euschistus spp. stink bugs (Heteroptera: Pentatomidae. Environ Entomol 20:477–483
Aldrich JR, Oliver JE, Lusby WR, Kochansky JP, Borges M (1994) Identification of male-specific volatiles from Nearctic and Neotropical stink bugs (Heteroptera: Pentatomidae. J Chem Ecol 20:1103–1111
Ayres M, Ayres M Jr, Ayres D, Santos A (2007) BioEstat 5.0 Aplicações Estatísticas nas Áreas da Ciências Bio-médicas. Belém, Sociedade Civil Mamirauá. 339 p
Barron AB (2001) The life and death of Hopkins’ host-selection principle. J Insect Behav 14:725–737
Bin F, Vinson SB, Strand MR, Colazza S, Jones WA (1993) Source of an egg kairomone for Trissolcus basalis, a parasitoid of Nezara viridula. Physiol Entomol 18:7–15
Blum MS (1974) Deciphering the communicative Rosetta stone. Bull Entomol Soc Amer 20:30–35
Borges M, Aldrich JR (1994) Attractant pheromone for Nearctic stink bug, Euschistus obscurus (Heteroptera: Pentatomidae): insight into a Neotropical relative. J Chem Ecol 20:1095–1102
Borges M, Costa MLM, Sujii E, Cavalcanti MDG, Redigolo G, Resck I, Vilela E (1999) Semiochemical and physical stimuli involved in host recognition by Telenomus podisi (hymenoptera: Scelionidae) toward Euschistus heros (Heteroptera: Pentatomidae. Physiol Entomol 24:227–233
Bruni R, Sant’Ana J, Aldrich JR, Bin F (2000) Influence of host pheromone on egg parasitism by scelionid wasps: Comparison of phoretic and non-phoretic parasitoids. J Insect Behavior 13:165–172
Cira TM, Venette RC, Aigner J, Kuhar T, Mullins DE, Gabbert SE, Hutchison W (2016) Cold tolerance of Halyomorpha halys (Hemiptera: Pentatomidae) across geographic and temporal scales. Environ Entomol 45:484–491
Colazza S, McElfresh JS, Millar JG (2004) Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J Chem Ecol 30:945–964
Colazza S, Aquila G, De Pasquale C, Peri E, Millar JG (2007) The egg parasitoid Trissolcus basalis uses n-nonadecane, a cuticular hydrocarbon from its stink bug host Nezara viridula, to discriminate between female and male hosts. J Chem Ecol 33:1405–1420
Conti E, Colazza S (2012) Chemical Ecology of egg parasitoids associated with true bugs. Psyche 2012:11. doi:10.1155/2012/651015
Corbet SA (1985) Insect chemosensory responses: a chemical legacy hypothesis. Ecol Entomol 10:143–153
Cribb BW, Siriwardana KN, Walter GH (2006) Unicellular pheromone glands of the pentatomid bug Nezara viridula (Heteroptera: Insecta): ultrastructure, classification, and proposed function. J Morphol 267:831–840
Cullen EM, Zalom FG (2005) Relationship between Euschistus conspersus (hem., Pentatomidae) pheromone trap catch and canopy samples in processing tomatoes. J Appl Entomol 129:505–514
Fatouros NE, Dicke M, Mumm R, Meiners T, Hilker M (2008) Foraging behavior of egg parasitoids exploiting chemical information. Behav Ecol 19:677–689
Fogain R, Graff S (2011) First records of the invasive pest, Halyomorpha halys (Hemiptera: Pentatomidae), in Ontario and Quebec. J Entomol Soc Ontario 142:45–48
Gariepy T, Bruin A, Haye T, Milonas P, Vétek G (2015) Occurrence and genetic diversity of new populations of Halyomorpha halys in Europe. J Pest Sci 88:451–460
Haye T, Fischer S, Zhang J, Gariepy T (2015) Can native egg parasitoids adopt the invasive brown marmorated stink bug, Halyomorpha halys (Heteroptera: Pentatomidae), in Europe? J Pest Sci 88:693–705
Herlihy MV, Talamas EJ, Weber DC (2016) Attack and success of native and exotic parasitoids on eggs of Halyomorpha halys in three Maryland habitats. PLoS One 11:e0150275
Hoebeke ER, Carter ME (2003) Halyomorpha halys (Stal) (Heteroptera: Pentatomidae): a polyphagous plant pest from Asia newly detected in North America. Proc Entomol Soc Wash 105:225–237
Hoffmann M, Davidson N, Wilson L, Ehler L, Jones W, Zalom F (1991) Imported wasp helps control southern green stink bug. Calif Agric 45:20–22
Hopkins AD (1917) A discussion of C. G. Hewitt’s paper on “insect behaviour”. J Econ Entomol 10:92–93
Inkley DB (2012) Characteristics of home invasion by the brown marmorated stink bug (Hemiptera: Pentatomidae. J Entomol Sci 47:125–130
Jeffries M, Lawton J (1984) Enemy free space and the structure of ecological communities. Biol J Linn Soc 23:269–286
Johnson NF (1984) Systematics of nearctic Telenomus: classification and revisions of the podisi and phymatae species groups (Hymenoptera: Scelionidae). Bull Ohio Biol Survey 6:1–113
Joseph SV, Nita M, Leskey TC, Bergh JC (2015) Temporal effects on the incidence and severity of brown marmorated stink bug (Hemiptera: Pentatomidae) feeding injury to peaches and apples during the fruiting period in Virginia. J Econ Entomol 108:592–599
Khrimian A, Zhang A, Weber DC, Ho H-Y, Aldrich JR, Vermillion KE, Siegler MA, Shirali S, Guzman F, Leskey TC (2014) Discovery of the aggregation pheromone of the brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1-bisabolen-3-ols. J Nat Prod 77:1708–1717
Krupke CH, Brunner JF (2003) Parasitoids of the consperse stink bug (Hemiptera: Pentatomidae) in north Central Washington and attractiveness of a host-produced pheromone component. J Entomol Sci 38:84–92
Lara J, Pickett C, Ingels C, Haviland D, Grafton-Cardwell E, Doll D, Bethke J, Faber B, Dara S, Hoddle M (2016) Biological control program is being developed for brown marmorated stink bug. Calif Agric 70:15–23
Laumann RA, Aquino MF, Moraes MC, Mn P, Borges M (2009) Response of the egg parasitoids Trissolcus basalis and Telenomus podisi to compounds from defensive secretions of stink bugs. J Chem Ecol 35:8–19
Lee D-H, Short BD, Joseph SV, Bergh JC, Leskey TC (2013) Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ Entomol 42:627–641
Mattiacci L, Vinson SB, Williams HJ, Aldrich JR, Bin F (1993) A long-range attractant kairomone for egg parasitoid Trissolcus basalis, isolated from defensive secretion of its host, Nezara viridula. J Chem Ecol 19:1167–1181
McBrien HL, Millar JG, Rice RE, McElfresh JS, Cullen E, Zalom FG (2002) Sex attractant pheromone of the red-shouldered stink bug Thyanta pallidovirens: a pheromone blend with multiple redundant components. J Chem Ecol 28:1797–1818
Michereff M, Borges M, Aquino M, Laumann R, Mendes GA, Blassioli-Moraes M (2016) The influence of volatile semiochemicals from stink bug eggs and oviposition-damaged plants on the foraging behaviour of the egg parasitoid Telenomus podisi. Bull Entomol Res. doi:10.1017/S0007485316000419
Millar JG (2005) Pheromones of true bugs. Topics Current Chem 240:37–84
Palumbo JC, Perring TM, Millar JG, Reed DA (2016) Biology, ecology, and management of an invasive stink bug, Bagrada hilaris, in North America. Annu Rev Entomol 61:453–473
Rice KB, Bergh CJ, Bergmann EJ, Biddinger DJ, Dieckhoff C, Dively G, Fraser H, Gariepy T, Hamilton G, Haye T, Herbert A, Hoelmer K, Hooks CR, Jones A, Krawczyk G, Kuhar T, Martinson H, Mitchell W, Nielsen AL, Pfeiffer DG, Raupp MJ, Rodriguez-Saona C, Shearer P, Shrewsbury P, Venugopal PD, Whalen J, Wiman NG, Leskey TC, Tooker JF (2014) Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J Integr Pest Manage 5:A1–A13. doi:10.1603/IPM14002
Rider D (2016) Pentatomoidea Home Page https://www.ndsu.edu/ndsu/rider/Pentatomoidea/.
Salerno G, Frati F, Conti E, De Pasquale C, Peri E, Colazza S (2009) A finely tuned strategy adopted by an egg parasitoid to exploit chemical traces from host adults. J Exp Biol 212:1825–1831
Silva CC, Moraes MCB, Laumann RA, Borges M (2006) Sensory response of the egg parasitoid Telenomus podisi to stimuli from the bug Euschistus heros. Pesq Agropec Bras 41:1093–1098
Sithanantham S, Ballal CR, Jalali S, Bakthavatsalam N (2013) Biological control of insect pests using egg parasitoids. Springer, New Delhi
StopBMSB.org (2015) Where is BMSB? http://www.stopbmsb.org/where-is-bmsb/.
Taekul C, Valerio AA, Austin AD, Klompen H, Johnson NF (2014) Molecular phylogeny of telenomine egg parasitoids (Hymenoptera: Platygastridae s.l.: Telenominae): evolution of host shifts and implications for classification. Syst Entomol 39:24–35
Talamas E, Buffington M (2015) Fossil Platygastroidea in the National Museum of Natural History, Smithsonian Institution. J Hymenop Res 47:1–52
Talamas EJ, Herlihy MV, Dieckhoff C, Hoelmer KA, Buffington M, Bon M-C, Weber DC (2015a) Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J Hymenop Res 43:119–128
Talamas EJ, Johnson NF, Buffington M (2015b) Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae). J Hymenop Res 43:45–110
Tillman P (2010) Parasitism and predation of stink bug (Heteroptera: Pentatomidae) eggs in Georgia corn fields. Environ Entomol 39:1184–1194
Tillman PG (2011) Natural biological control of stink bug (Heteroptera: Pentatomidae) eggs in corn, peanut, and cotton farmscapes in Georgia. Environ Entomol 40:303–314
Tillman PG, Aldrich JR, Khrimian A, Cottrell TE (2010) Pheromone attraction and cross-attraction of Nezara, Acrosternum, and Euschistus spp. stink bugs (Heteroptera: Pentatomidae) in the field. Environ Entomol 39:610–617
Tillman PG, Greenstone MH, JS H (2015) Predation of stink bugs (Hemiptera: Pentatomidae) by a complex of predators in cotton and adjoining soybean habitats in Georgia, USA. Fla Entomol 98:1114–1126
Tognon R, Sant’Ana J, Jahnke SM (2013) Aprendizagem e memória de Telenomus podisi (Hymenoptera, Platygastridae). Iheringia, Série Zoologia, Porto Alegre 103:266–271
Tognon R, Sant’Ana J, Jahnke S (2014) Influence of original host on chemotaxic behaviour and parasitism in Telenomus podisi Ashmead (hymenoptera: Platygastridae. Bull Entomol Res 104:781–787
Vet LE, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Weber CA, Smilanick JM, Ehler LE, Zalom FG (1996) Ovipositional behavior and host discrimination in three scelionid egg parasitoids of stink bugs. Biol Control 6:245–252
Weber DC, Walsh GC, DiMeglio AS, Athanas MM, Leskey TC, Khrimian A (2014) Attractiveness of harlequin bug, Murgantia histrionica, aggregation pheromone: field response to isomers, ratios, and dose. J Chem Ecol 40:1251–1259
Wermelinger B, Wyniger D, Forster B (2008) First records of an invasive bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Mitteliungen der Schweizerischen Entomologischen Gesellschaft 81:1–8
Zalom FG, Smilanick JM, Ehler L (1997) Fruit damage by stink bugs (Hemiptera: Pentatomidae) in bush-type tomatoes. J Econ Entomol 90:1300–1306
Zhu G, Bu W, Gao Y, Liu G (2012) Potential geographic distribution of brown marmorated stink bug invasion (Halyomorpha halys). PLoS One 7:31246
Acknowledgments
We thank Matthew Buffington, Elijah Talamas, and Michael Gates, USDA-ARS Systematic Entomology Laboratory, Washington, D.C., USA, for identifying the parasitoids. We also are grateful to Dr. Jocelia Grazia, Universidade Federal do Rio Grande do Sul, Brazil, for discussions on phylogenetics of the Pentatomidae. RT thanks the Coordination for the Improvement of Higher Education Personnel (CAPES) from Brazil for financial support, and JGM acknowledges support from Hatch Act project CA-R*-ENT-5181-H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tognon, R., Sant’Ana, J., Zhang, QH. et al. Volatiles Mediating Parasitism of Euschistus conspersus and Halyomorpha halys Eggs by Telenomus podisi and Trissolcus erugatus . J Chem Ecol 42, 1016–1027 (2016). https://doi.org/10.1007/s10886-016-0754-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0754-3