Abstract
Deciduous trees remobilize the nitrogen in leaves during the process of autumn coloration, thus providing a high quality food source for aphids preparing to lay over-wintering eggs. It has been suggested that aphids may use volatile organic compounds (VOCs) to: (a) select leaves where nutrient remobilization has started and induced defenses are reduced; and (b) detect the time of leaf abscission. We analyzed VOCs emitted by the foliage of Betula pendula Roth. during autumn coloration and from leaf litter just after leaf fall. We tested the hypothesis that costly, photosynthesis-related terpenes and other herbivore-induced VOCs related to attraction of aphid parasitoids and predators are reduced during the coloration process. We also investigated if the VOC emission profile of abscising leaves is different from that of early stage yellowing leaves. Enemy-luring compounds (E)-β-ocimene, linalool, and (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) were emitted only from the green foliage. Methyl salicylate (MeSa), known to recruit predatory bugs and attract migrant aphids, was emitted until the first stage of color change. Cis-3-hexenol, an indicator of cellular disintegration, became dominant in the emissions from abscising leaves and from fresh leaf litter. We discuss the ecological significance of the observed changes in birch leaf VOC profiles during the process of autumn senescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in the co-evolutionary role of autumn coloration of trees has increased since the publication of papers by Archetti (2000) and Hamilton and Brown (2001) that proposed that the brighter yellow and red autumn colors of individual trees are honest signals of more intense defensive chemical commitment and lower palatability to aphids. Aphids are important herbivores that rapidly respond to changes in host plant quality, particularly those indicated by leaf chlorosis or yellowing (Holopainen and Peltonen, 2002; White, 2003, 2009; Archetti, 2009; Cottrell et al., 2009; Holopainen et al., 2009), and they might have an increasing impact on vegetation under rapidly changing atmospheric conditions (Holopainen et al., 1991; Percy et al., 2002; Peltonen et al., 2006). Additionally, the co-evolution theory states that as chemical defense is costly it is likely that leaf color intensity and defenses also are correlated, with tree vigor being an indication of tree fitness (Archetti and Brown, 2006). Indeed, recent empirical evidence points toward plants with red leaf pigmentation in autumn being better defended against aphids and other herbivores than plants showing green or yellow autumn coloration (Archetti, 2009; Archetti et al., 2009).
It has recently been proposed that autumn leaf colors could indicate the status and development of indirect plant defense against aphids and other herbivores. Yamazaki (2008) suggested that autumn colors could signal tree quality to myrmecophilous specialist aphids, which attract aphid-tending ants that defend the trees from other herbivores. Another indirect defense hypothesis (Holopainen, 2008) states that green foliage, e.g., in Alnus before leaf fall, can continue to produce herbivore-induced plant volatiles and maintain volatile-based indirect plant defenses against aphids until leaf abscission, but in yellowing foliage indirect defenses become weaker, thus creating an enemy-free space for specialist aphids. This hypothesis is based on the fact that herbivore-damaged plants attract the natural enemies of the herbivorous insect by releasing herbivore-induced plant volatiles (Turlings et al., 1990; Kessler and Baldwin, 2001; Kappers et al., 2005; Mäntylä et al., 2008; Dicke and Baldwin, 2010; Holopainen and Gershenzon, 2010). The induction of the terpene volatiles, however, in particular is strongly photosynthesis dependent (Arimura et al., 2008). During leaf maturation and the start of senescence, photosynthesis declines and expression of photosynthesis- and carbon fixation-related genes decrease (Kontunen-Soppela et al., 2010). Furthermore, phenolic compounds, which are responsible for the leaf autumn coloration (Wilkinson et al., 2002), are formed through the shikimate pathway and have phosphoenolpyruvate (PEP) as a fundamental precursor. Isoprene and monoterpenes, produced through the chloroplastic MEP pathway, share this precursor (Rosenstiel et al., 2003; Magel et al., 2006). Under limited substrate conditions in autumn, PEP might be preferentially allocated to form phenolic compounds instead of isoprenoids (Fares et al., 2010). Therefore, in deciduous trees, a reduced proportion of photosynthesis-related VOCs could be expected during the advance of senescence and leaf coloration. Moreover, this should lead to nearly total loss of MEP dependent compounds after abscission, as these compounds are not released from storage pools (Ghirardo et al., 2010) as in needle litter of terpene-storing conifers (Kainulainen and Holopainen, 2002; Isidorov et al., 2010).
Ougham et al. (2005) proposed that biochemical reactions related to autumn color development and leaf senescence may affect volatiles released from plant leaves. They expected that peak emission of volatiles would coincide with the phase when the leaf was yellow or red but still alive, and that insect herbivores may respond to these volatiles, rather than to bright colors. Indeed, some plant species emit the sesquiterpene (E)-β-farnesene, which is the major component of the aphid alarm pheromone. Transgenic Arabidopsis plants that over-express (E)-β-farnesene synthase and emit (E)-β-farnesene are repellent to aphids and arrest aphid parasitoids; the olfactory (E)-β-farnesene cues override the yellow visual cues that normally are strongly attractive to Myzus persicae aphids (Beale et al., 2006). Blande et al., (2010) found that aphid feeding upon green foliage of Betula pendula and Alnus glutinosa induces emissions of methyl salicylate (MeSA) in both tree species, and intensity of emission is dependent on the duration of feeding. MeSA is known to act as both an aphid repellent in spring (Glinwood and Pettersson, 2000a) and an attractant of autumn migrants (Pope et al., 2007), but also an attractor of foraging predators and parasitoids (Zhu and Park, 2005; Pareja et al., 2009).
Many volatile organic compounds (VOCs), particularly terpenes (Gershenzon, 1994; Loreto and Schnitzler, 2010) are costly (in glucose units) to produce, and as much as 10% of fixed carbon could be released to the atmosphere as volatiles (Penuelas and Llusia, 2003). Given the high cost, we tested a hypothesis that disintegration of chloroplasts and chlorophyll during autumn senescence may reduce the plant’s ability to produce defensive VOCs.
Using a population of cloned Betula pendula that started autumn senescence in a field site, we addressed three specific questions. First, are the emissions of costly, photosynthesis-related terpenes and other herbivore-inducible VOCs lower in senescing (e.g., Bruggemann and Schnitzler, 2001) foliage than in green foliage? Second, are there emissions of specific aphid-repelling volatile compounds during the most active nutrient translocation period in yellowing leaves? Finally, does disintegration of chloroplasts and other cell organelles (Keskitalo et al., 2005) in the last stage of senescence lead to elevated emission of green leaf volatiles (GLVs), which could help aphids to avoid abscising leaves?
Methods and Materials
Plant Material
Ten 1-yr-old micropropagated silver birch (Betula pendula Roth) saplings, originating from naturally regenerated birch forest (mixed B. pendula and B. pubescens) in Punkaharju (southeastern Finland; 61°48′ N, 29°18′ E), were selected randomly. The potted saplings were grown at the Ruohoniemi experimental field at the Kuopio Campus Research Garden of the University of Eastern Finland (UEF) (62°13′ N, 27°13′ E, 80 m asl) in central Finland from May 2007 onwards (described e.g., in Blande et al., 2007 and Karnosky et al., 2007). Our surveys indicated that birch aphids did not appear on the saplings in this field site in summer and autumn 2007. Each sapling was healthy with green leaves, and no signs of senescence were visible prior to the start of the experiment (September 17) when they were brought from the field site to growth chambers. Some of the neighboring saplings already had distinctive foliage yellowing and were rejected during tree selection. However, we assumed that the autumn senescence process also had begun in our saplings, as the autumn senescence process, once initiated by environmental factors, seems to be a tightly controlled developmental program that is not significantly altered further by environmental factors (Keskitalo et al., 2005). The proportion of yellowing leaves in each sapling was monitored during the experiment. A leaf was considered yellow if one third or more of the leaf area was showing symptoms of yellowing. The proportion of green and yellow leaves was calculated on this basis.
All saplings were kept in two growth chambers under controlled conditions, which were based on the last 10 year mid-September weather and light conditions at the Ruohoniemi efield. The growth chambers were programmed with the following conditions; minimum temperature 8°C at 5:00, maximum 12°C from 15:00 to 17:00, relative humidity from 75% to 95%. Lights were on from 7:00 to 18:00, and the maximum light intensity (c.a. 350 μmol s−2 m−2) was maintained from 13:00 to 15:00 for 2 hr.
Collection of Volatiles from Shoots and Litter
Emissions were collected four times from the main shoot of 9 saplings on September 18–19 (d 1), 21–22 (d 3), 24–25 (d 6), and 27–28 (d 9), 2007, until the fall of leaves began. After abscission, VOC emissions from the fresh litter were collected. Each shoot was enclosed in a pre-cleaned (oven-heated 120°C, 1 hr) multi-purpose polyethylene terephthalate (PET) cooking bag, (vol. 3 l, Look, Terinex Ltd., UK) (Ibrahim et al., 2010). The bags were fastened carefully to the shoot bark with gardening wire with care taken not to damage any foliage. One of the two outermost bag corners was cut and an air inlet tube inserted. Clean charcoal-filtered, and MnO2 scrubbed air was pumped through Teflon tubing and into the bag at 400 ml/min to flush the system, and then reduced to 230 ml min−1. The remaining bag corner was cut, and a stainless steel tube containing approximately 150 mg of Tenax TA-adsorbent (Supelco, mesh 60/80) was inserted and fastened into the opening. Air was pulled through the Tenax tube by battery-operated sampling pumps (Rietschle Thomas, Puchheim, Germany). The air flow through the Tenax tube was set to 200 ml min–1 with an M-5 bubble flowmeter (A.P. Buck, Orlando, FL, USA). Samples were collected between 9:00 and 15:00. After leaf abscission, emissions from leaf-free branches were sampled to detect possible bark emissions. Due to easy fall of leaves during the abscission phase, we sampled freshly abscised leaves to better evaluate emissions of the last stage. The newly generated litter was collected from these 5 saplings and stored separately in 5 pre-heated (1 h at 120°C to remove any adhered plant VOC from surfaces) and cooled 1.5 l glass containers in growth chambers. VOC emissions were sampled from these containers at +22°C by a similar method 6 day after the last sampling of foliar BVOCs. Each glass container held an average of 40 leaves. After VOC sampling, the leaves were dried in an oven at a temperature of 70°C until the dry weight was constant.
The VOC samples were analyzed with a gas chromatograph-mass spectrometer (Hewlett-Packard GC 6890, MSD 5973). Trapped compounds were desorbed with a thermal desorption unit (Perkin-Elmer ATD400 Automatic Thermal Desorption system) at 250°C for 10 min, cryofocused at −30°C, and injected onto an HP-5 capillary column (50 m × 0.2 mm i.d. × 0.5 μm film thickness, Hewlett-Packard) with helium as carrier gas. The temperature program was as follows: 40°C 1 min, 5°C min-1 to 210°C, 20°C min-1 to 250°C, 250°C 8 min. Compounds were identified and quantified by comparing the mass spectra to those of pure standards (one external standard mixture for terpenes and one for GLVs) and to spectra in the Wiley library (Vuorinen et al., 2004). Our terpene standard included pure compounds: α-pinene, sabinene, β-pinene, myrcene, (E)-β-ocimene, limonene, 1,8–cineole (eucalyptol), 1-chloro octan, γ-terpinene linalool, (E)-DMNT, alloocimene, α-copaene, longifolene, trans-β-farnesene, aromadendrene, α-humulene, δ-cadinene, and caryophyllene oxide. The GLV standard included: cis-3-hexen-1-ol, trans-2-hexenal, 1-hexanol, 1-octen-3-ol, cis-3-hexenyl acetate, 1-chloro octan, nonanal, cis-3-hexenyl butyrate, methyl salicylate, cis-3-hexenyl isovalerate, and cis-3-hexenyl tiglate. All compounds were purchased from Sigma-Aldrich (parent company of Sigma, Aldrich, Fluka and Supelco) except sabinene from CHEMOS GmbH, trans-β-farnesene and cis-3-hexenyl isovalerate from Bedoukian Research Inc. DMNT was synthesized in-house from commercial citral using methyltriphenylphosphonium bromide and butyl lithium (Ibrahim et al., 2008).
Data Analysis
The quantities of emitted compounds in the headspace of plants and litter leaves were calculated in nanograms per g dry weight per h. Emission rates for each sample were compared with one-way ANOVA or non-parametric Kendall’s W Test if data did not follow a normal distribution. The proportion of yellowing leaves was tested with Dunnet’s test. A principal component analysis (PCA) (SIMCA-P 11.5.0.0, Umetrics AB, Umeå, Sweden) was used to divide the VOCs on the basis of the composition and ordination of the saplings for each sampling date (eigenvalues over 1 were extracted, and the original data compressed to two principal components PC1 and PC2).
Results
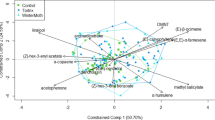
The proportion of green leaves in foliage was significantly affected (F 3,27 = 14.27, P < 0.001) during the monitoring period with the proportion significantly lower on days 6 and 9 (Dunnet’s test) than on the first day (Table 1). There were trends for total terpene emissions to decrease and total green leaf volatiles (GLVs) to increase (Fig. 1), but only total GLV emissions differed significantly among days (F 3,26 = 4,46, P = 0.012) being higher on day 6 than on day 1. The emissions of the common photosynthesis-related monoterpenes (1,8-cineole, α-pinene, β-myrcene, β-pinene, limonene, and sabinene) were low during the yellowing process, and did not differ among the days (Table 1). Inducible terpenes, monoterpenes (E)-β-ocimene and linalool, the homoterpene (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), and the sesquiterpene α-copaene, were emitted only from saplings with green foliage. The inducible aromatic compound, methyl salicylate (MeSA), was emitted by green foliage and by one sapling already showing yellowing symptoms, but not from leaves in the later phases of senescence. MeSa emissions differed significantly (W = 0.543, P = 0.043) (nonparametric Kendall’s W Test) among days. The GLVs cis-3-hexenol and cis-3-hexenyl acetate were emitted in minor amounts (17% of total emission) from green foliage. These compounds also were emitted from yellowing leaves and became more common volatiles just before leaf abscission. Principal component analysis (Fig. 2) showed that the emission of cis-3-hexen-1-ol was linked mostly to leaf senescence, and the emission was correlated positively with proportion of senescing leaves (r = 0.682, P < 0.01, N = 30). The ratio of cis-3-hexen-1-ol to cis-3-hexenyl acetate concentrations in emissions changed from 0.8 in green and yellowing foliage to 4.0 just before abscission, and it increased to 100 after leaf abscission, as cis-3-hexenyl acetate was not detectable in fresh litter samples.
A principal component bi-plot, showing the variation of volatile organic compound (VOC) composition and ordination of the Betula pendula saplings for each sampling date (day 1, 3, 6 and 9), on the basis of foliar VOC emission profile (1–4). Sapling symbols: ● = day 1, ▽ = day 3, ☓ = day 6, and ο = day 9. Compounds are marked with the symbol ▲ and the symbol △ marks the proportion of yellow leaves
No volatile emissions were detected from the leafless shoots. Analysis of VOC emissions from abscised leaves in fresh leaf litter indicated that GLV compounds constituted more than 75% of the emission of birch leaves at the early decomposition stage (Fig. 3). Some emissions of monoterpenes and MeSa were detected, but the dominating photosynthesis related monoterpene α-pinene was not detected, while linalool, β-myrcene, and limonene were detected.
Discussion
VOC Emissions during Senescence
There were sequential changes in the emission profiles during senescence of birch foliage, and principal component analysis was able to characterize and group the saplings separately on day 1 and 9 on the basis of the emission composition during the yellowing process. Inducible terpenes and photosynthesis-related monoterpenes were typical of green foliage emission profiles, and GLVs typical of senescing foliage. Our first hypothesis that a reduced proportion of costly photosynthesis-related VOCs will be emitted by senescing foliage is supported by our results, which show that VOCs known to be induced by herbivores (Vuorinen et al., 2007; Blande et al., 2010) or abiotic stress (Ibrahim et al., 2010) like DMNT, linalool, and (E)-β-ocimene are emitted by green leaves, but not by mostly yellow foliage. These inducible compounds can be found in small amounts (emission rates below 50 ng g−1 DW h−1), in the emissions of actively growing intact B. pendula saplings (Vuorinen et al., 2005). Fungal pathogen infection, however, may increase (E)-β-ocimene emission by 30-fold, and chewing herbivore feeding increase the emission of these compounds by more than 10-fold (Vuorinen et al., 2007). We did not find the sesquiterpene (E,E)-α-farnesene in the emission profile, although during summer it is emitted abundantly by many B. pendula genotypes (Vuorinen et al., 2005, 2007; Ibrahim et al., 2010) and is a typical herbivore-induced VOC in several plant species (e.g., Blande et al., 2007; Pinto et al., 2007). However, α-pinene and β-pinene—light and photosynthesis-related monoterpenes (Hakola et al., 2001; Loreto et al., 2004)—were emitted from leaves in the last stage of senescence, which is against our hypothesis. Explanation for this could be that there were still 20% greenish leaves on the same shoot among senescent leaves, and these may be responsible for monoterpene emissions when analyzing emissions on a shoot or branch basis. Alternatively, α-pinene and β-pinene may have still unknown functions relating to degeneration of photosynthesis.
Against our second hypothesis, we did not find emission of the sesquiterpene (E)-β-farnesene—a known aphid alarm pheromone released by some plant species in nature (Beale et al., 2006)—or any other aphid repellent volatile that might have been expected according to the co-evolution hypothesis (Hamilton and Brown, 2001). MeSa is an aphid-induced compound in B. pendula (Blande et al., 2010); it is less reactive than most of the terpenes in the atmosphere, and emissions are induced even by abiotic factors such as ozone (Pinto et al., 2007). It is known to repel certain migrating aphids in spring (Glinwood and Petersson, 2000a), but mostly it is an attractant of autumn migrants (Pope et al., 2007). The cessation of MeSA emissions from leaves starting to change color together with the color signals could inform aphids of nutrient remobilization in leaves.
In accordance with our third expectation, GLVs dominated the VOC profile in the later stages of senescence, particularly in abscised leaves. GLVs commonly are emitted from mechanically damaged plant material (Fall et al., 1999; Vuorinen et al., 2005; D’Auria et al., 2007;), including plant material damaged by chewing and piercing herbivores (Pinto et al., 2007). Some GLVs, for example cis-3-hexen-1-ol, can increase the predation rate of generalist predators (Kessler and Baldwin, 2001). Our saplings did not have any direct herbivore or mechanical damage, and the emissions of GLVs were rather low. Emissions of hexenol, hexanal, cis-3-hexen-1-ol, and trans-2-hexenal have been found to be associated with the senescence of grass crops (Karl et al., 2005). Physical stress leading to senescence strongly affects emission rates of GLVs (Fall et al., 1999). Our results suggest that these compounds might be related to the disintegration of cell organelles and dying cells (Keskitalo et al., 2005) during formation of necrotic leaf spots a few days before abscission. The dominance of cis-3-hexen-1-ol and concomitant decrease of cis-3-hexenyl acetate in leaf emissions in the last phase of senescence could be the olfactory cue, which female aphids might rely on to avoid falling from foliage with abscising leaves (Glinwood and Petersson, 2000b).
VOC Emissions after Leaf Fall
The most common photosynthesis-related monoterpene (α-pinene) was not emitted from the fresh birch litter, which indicates that the emission of this highly volatile compound depends on photosynthesizing leaves in deciduous species that do not store monoterpenes. However, some other monoterpenes like β-myrcene, linalool, and (E)-β-ocimene were found in litter emissions. This could be due partly to emissions from aqueous and lipid phase stores (Noe et al., 2006) in abscised leaves due to limited volatility in cold autumn conditions. Furthermore, as the litter leaves were incubated for a few days in the same container, emissions per unit of leaf dry mass are not quantitatively comparable to foliage emissions due to possible adsorption and desorption of compounds onto the container surfaces (Schaub et al., 2010) and loss of leaf weight due to decomposition. The condensation and accumulation of terpenes on leaf and container surfaces could be an explanation for the appearance of linalool in litter samples, although it was detectable only in green foliage of saplings.
The dominance of GLV compounds in the VOC emission profile of fresh litter, and particularly the compound cis-3-hexen-1-ol, was most obvious and could be a result of the increasing proportion of necrotic leaf tissues during microbial decomposition. High concentrations of GLVs have been found in the forest atmosphere in autumn when senescing and litter leaves thaw after freezing spells of weather and emit GLVs (Karl et al., 2001). Fall et al. (1999) demonstrated with aspen, beech, and clover that after leaf detachment, emissions of GLVs were not dependent on light, and emissions were enhanced greatly as detached leaves dried out. Our results show that GLVs dominate in the emissions from senescing leaves and fresh leaf litter, although leaves did not undergo thawing or drying. Substantial cis-3-hexen-1-ol and cis-3-hexenyl acetate emissions from birch foliage and birch litter in autumn could have significant atmospheric and ecological impacts, as both compounds participate strongly in secondary organic aerosol formation in reactions with tropospheric ozone (Pinto et al., 2007; Hamilton et al., 2009) and hydroxyl radicals (Hamilton et al., 2009).
VOCs and the Coevolutionary Theory of Autumn Colors
Handicap signals are strategic signals that indicate that an individual has possession of a great deal of some resources for costly investments (Zahavi, 1975). If the signal is not costly, then other individuals might mimic strong defense and thus diminish signal reliability (Hamilton and Brown, 2001). Green foliage is known to have high light use efficiency and photosynthetic capacity (Evain et al., 2004). There is a strong coupling of photosynthesis and phytogenic VOC emissions (Penuelas and Lusia, 2003), and among other functions these compounds are effective cues for predators and parasitoids toward herbivore-attacked plants (Holopainen, 2008; Dicke and Baldwin, 2010; Holopainen and Gershenzon, 2010). Our results indicate that particularly costly terpenes (Gershenzon, 1994; Loreto and Schnitzler, 2010) are diminished in volatile emission of yellowing birch leaves. This observation suggests that green foliage is capable of facilitating induced indirect defense based on volatile terpenes. It should be tested in behavioral experiments whether the color-changing senescing leaves are able to produce sufficiently volatile terpenes as odor signals for natural enemies of aphids and other herbivorous insects. Further research also should elucidate whether increasing GLV emissions from senescing foliage distinguishes herbivore-damaged leaves from undamaged leaves and whether carnivores can respond to this difference. Behavioral tests could give support for the hypothesis (Holopainen, 2008) that honest signals of herbivore damage for natural enemies of herbivorous insects are produced only by green foliage, and hence senescing foliage could provide an enemy-free space for aphids.
References
Archetti, M. 2000. The origin of autumn colours by coevolution. J. Theor. Biol. 205:625–630.
Archetti, M. 2009. Evidence from the domestication of apple for the maintenance of autumn colours by coevolution. Proc. Royal Soc. London. Series B, Biol. Sci. 276:2575–2580.
Archetti, M. and Brown, S.P. 2006. Putting ‘red alerts’ in an ecological and evolutionary context. BioEssays 28:959–959.
Archetti, M., Döring, T.F., Hagen, S.B., Hughes, N.M., Leather, S.R., Lee, D.W., Lev-Yadun, S., Manetas, Y., Ougham, H.J., Schaberg, P.G. et al. 2009. Unravelling the evolution of autumn colours: an interdisciplinary approach. Trends Ecol. Evol. 24:166–173.
Arimura, G.I., Kopke, S., Kunert, M., Volpe, V., David, A., Brand, P., Dabrowska, P., Maffei, M.E., and Boland, W. 2008. Effects of feeding Spodoptera littoralis on lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol. 146:965–973.
Beale, M.H., Birkett, M.A., Bruce, T.J.A., Chamberlain, K., Field, L.M., Huttly. A.K., Martin, J.L., Parker, R., Phillips, A.L., Pickett, J.A., Prosser, I.M., Shewry, P.R., Smart, L.E., Wadhams, L.J., Woodcock, C.M., and Zhang, Y.H. 2006. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc. Nat. Acad. Sci. U. S. A. 103:10509–10513.
Blande, J.D., Korjus, M., and Holopainen, J.K. 2010. Foliar methyl salicylate emissions indicate prolonged aphid infestation on silver birch and black alder. Tree Physiol. 30:404–416.
Blande, J.D., Tiiva, P., Oksanen, E., and Holopainen, J.K. 2007. The emission of herbivore induced volatile terpenoids from two hybrid aspen (Populus tremula x tremuloides) clones under ambient and elevated ozone concentrations in the field. Global Change Biol. 13: 2538–2550.
Bruggemann, N. and Schnitzler, J.P. 2001. Influence of powdery mildew (Microsphaera alphitoides) on isoprene biosynthesis and emission of pedunculate oak (Quercus robur L.) leaves. J. Appl. Bot. 75:91–96.
Cottrell, T.E., Wood, B.W., and Ni, X. 2009. Chlorotic feeding injury by the black pecan aphid (Hemiptera: Aphididae) to pecan foliage promotes aphid settling and nymphal development. Environ. Entomol. 38:411–416.
D’auria, J.C., Pichersky, E., Schaub, A., Hansel, A., and Gershenzon J. 2007. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 49:194–207.
Dicke M. and Baldwin I.T. 2010. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci. 15:167–175.
Evain, S., Flexas, J., and Moya, I. 2004. A new instrument for passive remote sensing: 2. Measurement of leaf and canopy reflectance changes at 531 nm and their relationship with photosynthesis and chlorophyll fluorescence. Rem. Sens. Environ. 91:175–185.
Fall, R., Karl, T., Hansel, A., Jordan, A., and Lindinger W. 1999. Volatile organic compounds emitted after leaf wounding: On-line analysis by proton-transfer-reaction mass spectrometry. J. Geophys. Res. Atmos. 104(D13):15963–15974.
Fares, S., Oksanen, E., Lännenpää, M., Julkunen-Tiitto, R., and Loreto, F. 2010. Volatile emissions and phenolic compound concentrations along a vertical profile of Populus nigra leaves exposed to realistic ozone concentrations. Photosynth. Res. 104:61–74.
Gershenzon, J. 1994. Metabolic costs of terpenoid accumulation in higher-plants. J. Chem. Ecol. 20:1281–1328.
Ghirardo, A., Koch, K., Taipale, R., Zimmer, I., Schnitzler J.P., and Rinne J. 2010. Determination of de novo and pool emissions of terpenes from four common boreal/alpine trees by 13CO(2) labelling and PTR-MS analysis. Plant Cell Environ. 33:781–792.
Glinwood, R.T. and Pettersson. J. 2000a. Change in response of Rhopalosiphum padi spring migrants to the repellent winter host component methyl salicylate. Entomol. Exp. Appl. 94:325–330.
Glinwood R and Pettersson J. 2000b. Movement by mating females of a host alternating aphid: a response to leaf fall. Oikos 90:43–49.
Hakola, H., Laurila. T., Lindfors. V., Hellen, H., Gaman, A., and Rinne, J. 2001. Variation of the VOC emission rates of birch species during the growing season. Boreal Environ. Res. 6:237–249.
Hamilton, J.F., Lewis, A.C., Carey, T.J., Wenger, J.C., Garcia, E.B.I., and Munoz, A. 2009. Reactive oxidation products promote secondary organic aerosol formation from green leaf volatiles. Atmos. Chem. Phys. 9:3815–3823.
Hamilton, W.D. and Brown, S.P. 2001. Autumn tree colours as a handicap signal. Proc. Royal Soc. London. Series B, Biol. Sci. 268:1489–1493.
Holopainen, J.K. 2008. Importance of olfactory and visual signals of autumn leaves in the coevolution of aphids and trees. BioEssays 30:889–896.
Holopainen, J.K. and Gershenzon, J. 2010. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci.15:176–184.
Holopainen, J.K., Kainulainen, E., Oksanen, J., Wulff, A., and Kärenlampi, L. 1991. Effect of exposure to fluoride, nitrogen compounds and SO2 on the numbers of Spruce shoot aphids on Norway spruce seedlings. Oecologia 86:51–56.
Holopainen, J.K. and Peltonen, P. 2002. Bright autumn colours of deciduous trees attract aphids: nutrient retranslocation hypothesis. Oikos 99:184–188.
Holopainen, J.K., Semiz, G., and Blande, J.D. 2009. Life-history strategies affect aphid preference for yellowing leaves. Biol. Lett. 5:603–605.
Ibrahim, M.A., Mäenpää, M., Hassinen, V., Kontunen-Soppela, S., Malec, L., Rousi, M., Pietikäinen, L., Tervahauta, A., Kärenlampi, S., Holopainen, J.K. et al. 2010. Elevation of night-time temperature increases terpenoid emissions from Betula pendula and Populus tremula. J. Exp. Bot. 61:1583–1595.
Ibrahim, M.A., Stewart-Jones, A., Pulkkinen, J., Poppy, G.M., and Holopainen, J.K. 2008. The influence of different nutrient levels on insect-induced plant volatiles in Bt and non-Bt oilseed rape plants. Plant Biol. 10: 97–107.
Isidorov, V.A., Smolewska, M. Purzynska-Pugacewicz, A., and Tyszkiewicz, Z. 2010. Chemical composition of volatile and extractive compounds of pine and spruce leaf litter in the initial stages of decomposition. Biogeosciences Discuss. 7:1727–1750.
Kainulainen, P. and Holopainen, J.K. 2002. Concentrations of secondary compounds in Scots pine needles at different stages of decomposition. Soil Biol. Biochem. 34:37–42.
Karl, T., Fall, R., Crutzen, P. J., Jordan, A., and Lindinger,W. 2001. High concentrations of reactive biogenic VOCs at a high altitude site in late autumn, Geophys. Res. Lett. 28:507–510.
Karl, T., Harren, F., Warneke, C., De Gouw, J., Grayless, C., and Fall, R. 2005. Senescing grass crops as regional sources of reactive volatile organic compounds. J. Geophys. Res. Atmos. 110 (D15), Art. No. D15302
Karnosky, D.F., Werner, H., Holopainen, T., Percy, K., Oksanen, T., Oksanen, E., Heerdt, C., Fabian, P., Nagy, J., Heilman, W., Cox, R., Nelson, N., and Matyssek, R. 2007. Free-air exposure systems to scale up ozone research to mature trees. Plant Biol. 9:181–190.
Kappers, I.F., Aharoni, A., Van Herpen, T.W.J.M., Luckerhof,F L.L.P., Dicke, M., and Bouwmeester, H.J. 2005. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 309:2070–2072.
Keskitalo, J., Bergquist, G., Gardeström, P., and Jansson S. 2005. A cellular timetable of autumn senescence. Plant Physiol. 139:1635–1648.
Kessler, A. and Baldwin, I.T. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144.
Kontunen-Soppela, S., Parviainen, J., Ruhanen, H., Brosche, M., Keinänen, M., Thakur, R.C., Kolehmainen, M., Kangasjärvi, J., Oksanen, E,. Karnosky, D.F., and Vapaavuori E. 2010. Gene expression responses of paper birch (Betula papyrifera) to elevated CO2 and O3 during leaf maturation and senescence. Environ. Poll. 158:959–968.
Loreto, F., Pinelli, P., Manes, F., and Kollist, H. 2004. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol. 24:361–367
Loreto, F. and Schnitzler, J.P. 2010. Abiotic stresses and induced BVOCs. Trends Plant Sci. 15:54–166.
Magel, E., Mayrhofer, S., Muller, A., Zimmer, I., Hampp, R., and Schnitzler, J.P. 2006. Photosynthesis and substrate supply for isoprene biosynthesis in poplar leaves. Atmos. Environ. 40:S138–S151.
Mäntylä, E., Alessio, G.A., Blande, J.D., Heijari, J., Holopainen, J.K., Laaksonen, T., Piirtola, P., and Klemola T. 2008. From plants to birds: higher avian predation rates in trees responding to insect herbivory. Plos One 3:e2832
Noe, S.M., Ciccioli, P., Brancaleoni, E., Loreto, F., and Niinemets, U. 2006. Emissions of monoterpenes linalool and ocimene respond differently to environmental changes due to differences in physico-chemical characteristics. Atmos. Environ. 40:4649–4662.
Ougham, H.J., Morris, P., and Thomas, H. 2005. The colours of autumn leaves as symptoms of cellular recycling and defenses against environmental stresses. Curr. Top. Devel. Biol. 66:135–160.
Pareja, M., Mohib, A., Birkett, M.A., Dufour, S., and R.T. Glinwood. 2009. Multivariate statistics coupled to generalized linear models reveal complex use of chemical cues by a parasitoid. Anim. Behav. 77:901–909.
Peltonen, P.A., Vapaavuori, E., Julkunen-Tiitto, R., and Holopainen, J.K. 2006. Effects of elevated carbon dioxide and ozone on aphid oviposition preference and birch bud exudate phenolics. Glob. Change Biol. 12:1670–1679.
Penuelas, J. and Llusia, J. 2003. BVOCs: plant defense against climate warming? Trends Plant Sci. 8:105–109.
Percy, K.E., Awmack, C.S., Lindroth, R.L., Kubiske, M.E., Kopper, B.J., Isebrands, J.G., Pregitzer, K.S., Hendrey, G.R., Dickson, R.E., Zak, D.R., Oksanen, E., Sober, J., Harrington, R., and Karnosky, D.F. 2002. Altered performance of forest pests under atmospheres enriched by CO2 and O3. Nature 420:403–407.
Pinto, D.M., Blande, J.D., Dong, W.X., Nerg, A.M., and Holopainen, J.K. 2007. Ozone degrades common herbivore-induced plant volatiles: does this affect herbivore prey location by predators and parasitoids? J. Chem. Ecol. 33:683–694.
Pope, T.W., Campbell, C.A.M., Hardie, J., Pickett, J.A., and Wadhams, L.J. 2007. Interactions between host-plant volatiles and the sex pheromones of the bird cherry-oat aphid, Rhopalosiphum padi and the damson-hop aphid, Phorodon humuli. J. Chem. Ecol. 33:157–165.
Rosenstiel, T.N., Potosnak, M.J., Griffin, K.L., Fall, R., and Monson, R.K. 2003. Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 421:256–259.
Schaub, A., Blande, J.D., Graus, M., Oksanen, E., Holopainen, J.K., and Hansel, A. 2010. Real-time monitoring of herbivore induced volatile emissions in the field. Physiol. Plantarum 138:123–133.
Turlings, T.C.J., Tumlinson, J.H., and Lewis, W.J. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253.
Vuorinen, T., Nerg, A.M., Ibrahim, M.A., Reddy, G.V.P., and Holopainen, J.K. 2004. Emission of Plutella xylostella-induced compounds from cabbage grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiol. 135:1984–1992.
Vuorinen, T., Nerg, A.M., Syrjälä, L., Peltonen, P., and Holopainen, J.K. 2007. Epirrita autumnata induced VOC emission of Silver birch differ from emission induced by leaf fungal pathogen. Arthropod-Plant Interactions 1:159–165.
Vuorinen, T., Nerg, A.-M., Vapaavuori, E., and Holopainen, J.K. 2005. VOC emissions from silver birch (Betula pendula) grown under elevated CO2 and O3 concentrations. Atmos. Environ. 39:1185–1197.
White, T.C.R. 2003. Nutrient retranslocation hypothesis, a subset of the flush feeding/senescence feeding hypothesis. Oikos 103:217.
White T.C.R. 2009. Catching a red herring: autumn colours and aphids. Oikos 118:1610–1612.
Wilkinson, D.M., Sherratt, T.N., Phillip, D.M., Wratten, S.D., Dixon, A.F.G., and Young, A.J. 2002. The adaptive significance of autumn leaf colours. Oikos 99:402–407.
Yamazaki, K. 2008. Autumn leaf colouration: a new hypothesis involving plant ant mutualism via aphids. Naturwissenschaften 95:671–676.
Zahavi, A. 1975. Mate selection—a selection for a handicap. J. Theor. Biol. 53:205–214.
Zhu, J.W. and Park, K.C. 2005. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J. Chem. Ecol. 31:1733–1746.
Acknowledgements
We thank James Blande and Robert Glinwood for comments on an earlier draft of the manuscript. This study was financially supported by the Academy of Finland (project no. 111543, J.K.H, and J.H., project no. 109933, E.O.), European Commission (ISONET, MRTN-CT-2003-504720, J.K.H) and the European Science Foundation, (VOCBAS programme, G.A.A.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holopainen, J.K., Heijari, J., Oksanen, E. et al. Leaf Volatile Emissions of Betula pendula during Autumn Coloration and Leaf Fall. J Chem Ecol 36, 1068–1075 (2010). https://doi.org/10.1007/s10886-010-9857-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9857-4