Abstract
The bird cherry-oat aphid, Rhopalosiphum padi (L.), and the damson-hop aphid, Phorodon humuli (Schrank), migrate at the same time of year and colonize closely related Prunus spp. as primary hosts, but utilize (1R,4aS,7S,7aR)-nepetalactol and (1RS,4aR,7S,7aS)-nepetalactol, respectively, as sex pheromones. Interactions between these sex pheromones and benzaldehyde and methyl salicylate, plant volatiles common to primary hosts of both species, were investigated to assess whether they confer reproductive isolation between these species. Female autumn migrants (gynoparae) and males of these two species were caught in the field with water traps baited with their respective sex pheromones. Rhopalosiphum padi gynoparae and males also responded positively to benzaldehyde. Release of either benzaldehyde or methyl salicylate with the conspecific sex pheromone increased catches of both species of aphid. However, releasing both plant volatiles with the sex pheromone of R. padi increased catches of gynoparae and males, but reduced those with the sex pheromone of P. humuli. These results support the hypothesis that specific plant volatiles synergize responses of autumn migrating aphids to their sex pheromone. Because these interactions are species-specific, they may be important in allowing males to discriminate between conspecific sexual females (oviparae) and those of other aphid species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interaction between host-plant volatiles and sex pheromones of phytophagous insects, including aphids, is well established (Landolt and Phillips, 1997; Powell and Hardie, 2001). In many insect species, the response of males to a female-produced sex pheromone is directly influenced by host-plant volatiles. Host-plant volatiles may synergize the response to the sex pheromone, as has been shown for several species of Lepidoptera and Coleoptera with the simple addition of one or more green leaf volatiles (Light et al., 1993; Ruther et al., 2000, 2002; Reinecke et al., 2002a,b). Examples also exist where host-plant volatiles other than green leaf volatiles, or intact host plants, act synergistically with pheromones (Landolt and Phillips, 1997).
In contrast to the Lepidoptera and Coleoptera, for which many hundreds of species-specific sex pheromone components have been identified, of the 12 species of aphids for which sex pheromones are known, only three sex pheromone components have been identified (Hardie et al., 1999). There is also conflicting evidence as to whether male aphids can discriminate between the sex pheromone produced by conspecific sexually reproducing egg-laying females (oviparae) and those produced by oviparae of other species (Marsh, 1975; Eisenbach and Mittler, 1987; Steffan, 1990; Hardie et al., 1990, 1992).
The limited range of aphid sex pheromone components, and inconclusive evidence that aphids can discriminate between them, led Steffan (1987) and Guldemond (1990) to argue that the primary host plant may be important in promoting reproductive isolation. Subsequently, Powell and Hardie (2001) suggested that a more important role of host-plant volatiles may be to synergize the response of male aphids to sex pheromones. Gynoparae (female parthenogens that uniquely produce the sexually reproducing oviparae) of several species of aphid are known to respond to odors from primary host plants (Powell and Hardie, 2001) as do those and males also of the bird cherry-oat aphid, Rhopalosiphum padi (L.) and the damson-hop aphid, Phorodon humuli (Schrank). In olfactometer studies, gynoparae and males of R. padi responded positively to crushed buds of bird cherry, Prunus padus L., the primary host of this species (Pettersson, 1970). Subsequently, benzaldehyde was identified as an active component in the odor of bird cherry, eliciting positive behavioral responses on its own (Pettersson, 1970; Park et al., 2000). In contrast, in the field neither a steam distilled extract of bird cherry or benzaldehyde increased numbers of male R. padi caught in water traps (Hardie et al., 1994). However, when the steam distilled extract or benzaldehyde were released simultaneously with (1R,4aS,7S,7aR)-nepetalactol (I), the sex pheromone of this species, significantly more males were trapped than with the pheromone alone, indicating a significant interaction. Phorodon humuli males responded positively to leaf and twig material from myrobalan, Prunus cerasifera Ehrhart, one of its primary hosts, as well as a bark extract from the same host in an olfactometer and in the field (Campbell et al., 1990). In a second study, steam distilled extracts of two other primary hosts, sloe (Prunus spinosa L.) and plum (P. domestica L.), also increased catches of gynoparae, but not males, in clear water traps (Lösel et al., 1996b). In addition, release of extracts from these hosts increased catches of gynoparae and males to the sex pheromone produced by conspecific oviparae, (1RS,4aR,7S,7aS)-nepetalactol (III) (Campbell et al., 1990; Lösel et al., 1996a,b).
These results suggest that primary host-plant volatiles elicit positive responses, both on their own and through synergistic interactions with sex pheromones, in autumn migrants of R. padi and P. humuli. However, gynoparae and males of R. padi and P. humuli migrate to closely related species of Prunus, which may provide similar olfactory cues. In addition, because the migrations occur at similar times of the year, there is no temporal separation. Thus, these two species of aphid respond to different sex pheromones but are presented with similar olfactory cues from their respective host plants. The responses of gynoparae and males of these species to their respective sex pheromones presented together with plant volatiles common to their primary hosts may provide insight into how aphids are able to make species-specific responses with such a limited range of sex pheromone components.
The present study investigated responses of gynoparae and males of R. padi and P. humuli to combinations of I, III, benzaldehyde, and methyl salicylate. The headspace profiles of Prunus padus, the primary host of R. padi, and P. spinosa, P. domestica, and P. cerasifera, primary hosts of P. humuli, are rich in volatile compounds including benzaldehyde and methyl salicylate (Pettersson, 1970; Pettersson et al., 1994; Pope, unpublished data). Benzaldehyde alone, as outlined above, is known to elicit positive responses in R. padi autumn migrants and to synergize responses to I. Methyl salicylate has been shown to evoke negative behavioral responses in spring migrants of both R. padi and P. humuli, which suggests some role for these specific compounds in primary host plant recognition (Campbell et al., 1993; Pettersson et al., 1994; Lösel et al., 1996a; Ninkovic et al., 2003).
Methods and Materials
Chemicals
(4aS,7S,7aR)-Nepetalactol (I) was prepared from an extract of the catmint Nepeta cataria (L.) (see Dawson et al., 1987). A 7:3 mixture of the 1S- and 1R- diastereoisomers of (4aR,7S,7aS)-nepetalactol (III) was prepared from N. racemosa (syn. mussinii) (see Campbell et al., 1990, 2003). Benzaldehyde (B) and methyl salicylate (MS) were purchased from Sigma-Aldrich (Poole, UK) (purity 99% and 98%, respectively).
Field Experiments
Randomized block factorial experiments were conducted in plantations of dwarf hops growing on 2.4-m-high trellises at East Malling Research (EMR) in 1996, 2000, and 2001. The experimental design consisted of two, four, and two blocks in 1996, 2000, and 2001, respectively. Water traps were made from transparent plastic Petri dishes (14 cm diam × 2 cm deep) as described by Hardie et al. (1997). Traps were filled with a dilute solution of Lipsol, a nonionic detergent. Pheromones (10 mg) in 50 μl Et2O and B (50 mg) were placed into individual glass vials (08-CPV Chromacol), with a 1-mm hole drilled through the cap. The antioxidant butylatedhydroxytoluene (BHT, 5 mg), was added to vials containing 50 mg of B. MS (41 mg) was placed into closed polyethylene vials (WP/5, Fisons) with four 1-mm holes drilled through the cap. Control vials were added as necessary so that all traps presented an identical visual image. Vials were suspended 1 cm above the surface of the water. The traps were spaced 3 m apart and 60 cm above the ground and sited centrally in the 3-m-wide alleys between the rows of hops. Traps were emptied and their positions rerandomized daily. Trapped P. humuli and R. padi gynoparae and males were counted in the laboratory.
The release rates of B and MS were determined by suspending vials within a fume cupboard with an air flow of 0.6 m sec−1 at 18 ± 2°C. Weight changes in vials were recorded over a 7-d period and the release rates were calculated (mean ± SE) as 1.03 ± 0.02 mg day−1 for B, and 0.81 ± 0.16 mg day−1 for MS.
The experiments were conducted from October 8 to 25, 1996, from October 14 to 30, 2000, and from September 22 to October 26, 2001. A complete factorial design was used in 1996 with each chemical at two levels (present and absent). The treatments were (1) III, (2) B, (3) MS, (4) III + B, (5) III + MS, (6) B + MS, (7) III + B + MS, and (8) control. Incomplete factorial designs were used in 2000 and 2001; all treatments that combined both sex pheromones were omitted. Therefore, the treatments in both years were (1) I, (2) III, (3) B, (4) MS, (5) I + B, (6) I + MS, (7) III + B, (8) III + MS, (9) B + MS, (10) I + B + MS, (11) III + B + MS, and (12) control.
Data Analysis
Data were analyzed by analysis of variance (ANOVA). To stabilize variances, daily counts were transformed to square roots (n 0.5) and summed for the whole sampling period. A two-way classification of treatments within blocks was used for first-step analyses, with differences between means compared against the least significant difference (LSD) only if the overall treatment terms were significant. The data were then reanalyzed with the variance partitioned into factorial contrasts. Separate terms for block-to-block variance, main effects of each chemical individually, two- and three-chemical interactions, and residual variance for the incomplete factorial designs in 2000 and 2001 were accumulated into analysis of variance from regressions using GenStat 5 (Payne, 2000).
Results
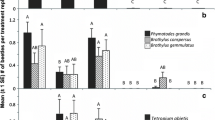
A total of 2120 P. humuli gynoparae and 9764 males were caught in the 1996 experiment (Table 1). P. humuli gynoparae and males were more numerous in traps baited with III than in traps without pheromone. Furthermore, traps baited with III together with either B or MS caught significantly more gynoparae and males (1.9–2.1 and 2.5–2.7 times as many respectively) than did traps baited with III alone. The factorial ANOVA of these data showed that P. humuli gynoparae and males responded significantly to III (gynoparae, F 1,7 = 71.57, P < 0.001; males, F 1,7 = 162.93, P < 0.001). The three-way interactions between III, B, and MS were also significant for both morphs (gynoparae, F 1,7 = 5.73, P < 0.05; males, F 1,7 = 11.27, P < 0.05). Both morphs responded positively to the plant volatiles, but only in the presence of III (III + B vs. III, gynoparae, t = 2.95, P < 0.05, males, t = 3.69, P < 0.01; III + MS vs. III, gynoparae, t = 3.11, P < 0.05, males, t = 3.98, P < 0.01), but the response was negated when both plant volatiles were presented with III (III + B + MS vs. III gynoparae, t = 1.33, males, t = 0.82).
Few gynoparae of R. padi and P. humuli were caught in the year 2000 experiment (29 and 1, respectively), which was conducted after the peak migration period for that morph. Totals of 441 R. padi and 368 P. humuli males were caught (Table 2). Males of R. padi responded significantly to their own pheromone (I) irrespective of the presence of the two plant volatiles, and more males responded to I released with B ± MS (1.6–2.3 times as many) than to I alone. In contrast, catches of males in traps releasing III were no different from controls. The factorial ANOVA confirmed that R. padi males responded to I compared with either no pheromone (t = 7.48, P < 0.001) or III (t = 6.24, P < 0.001), as did gynoparae (I vs. control, t = 2.16, P < 0.05; I vs. III, t = 2.27, P < 0.05; III vs. control, t = 0.11; data not shown). Rhopalosiphum padi males also responded to B (F = 9.45, P < 0.05) but not to MS. Although the two- and three-way interactions between pheromones, B, and MS were not significant at the 5% probability level, the two-way interaction between pheromones and B were close for both morphs (males, F = 2.90, P = 0.069; gynoparae F = 2.63, P = 0.087). Although these results must be treated with caution owing to the borderline significance of the F ratios, almost twice as many males responded to B when released with I compared with I alone (t = 3.32, P < 0.01) and a similar proportion to B alone compared with control (t = 2.04, P < 0.05). Gynoparae also responded to B when released together with I, as compared with I alone (t = 2.58, P < 0.05), but not otherwise.
The results for P. humuli males in 2000 were similar to those in 1996. They responded to III ± B and III ± MS, but the release of both plant volatiles with III halved the response. Males did not respond to I. The factorial ANOVA of these data confirmed that male P. humuli responded to III (III vs. control, t = 7.25, P < 0.001; III vs. I, t = 7.22, P < 0.001). Although the three-way interaction was not significant, an examination of the means showed that release of plant volatiles together with III reduced responses of males (III vs. III + B + MS, t = 3.09, P < 0.01).
Totals of 629 R. padi gynoparae and 606 males together with 104 P. humuli gynoparae and 597 males were caught in the 2001 experiment (Table 3). Owing to a shortage of pheromone, only two blocks of the experiment were deployed, and the distribution of P. humuli was skewed positionally with 94% of males and 80% of gynoparae trapped in one experimental block. This positional disparity increased the error variance and contributed to large differences in numbers trapped between treatments being not statistically significant. No such disparity in distribution was observed with R. padi. In comparison with I alone, 1.6–4.7 times as many gynoparae and 20–45 times as many males responded to I released with plant volatiles. The factorial ANOVA of these data confirmed that R. padi gynoparae responded significantly to I compared either with no pheromone (t = 4.29, P < 0.01) or with III (t = 3.72, P < 0.01). Rhopalosiphum padi gynoparae responded to B as in previous trials (F = 10.54, P < 0.01), but not to MS. The factorial ANOVA showed that males of R. padi also responded to I compared either with no pheromone (t = 6.72, P < 0.001) or with III (t = 6.97, P < 0.001), but did not respond to III. In contrast to the 2000 experiments, R. padi males also responded to MS in 2001 (F = 9.17, P < 0.05), but not to B. The significant two-way interaction between pheromones and MS (F = 10.49, P < 0.01) showed that males responded to MS only when it was released with I (I + MS vs. I, t = 5.49, P < 0.001; I + MS vs. MS, t = 7.42, P < 0.001). The three-way interaction between pheromones and plant volatiles was not significant.
Gynoparae of P. humuli responded only to III in 2001 (Table 3). The factorial ANOVA of these data confirmed that gynoparae responded to III irrespective of the plant volatiles (III vs. no pheromone, t = 2.58, P < 0.05; III vs. I, t = 2.50, P < 0.05). Trap catches were unaffected by the presence of I. The two- and three-way interactions between pheromones, B, and M were not significant. Although the response of males to III + B was the only treatment combination greater than the control by two-way ANOVA, the factorial analysis showed that significantly more males were trapped in response to III than either to no pheromone (t = 2.81, P < 0.05) or to I (t = 2.67, P < 0.05). Gynoparae did not respond to either B or MS.
Discussion
Autumn migrants of both R. padi and P. humuli responded to the plant volatiles when the latter were released individually with their conspecific sex pheromone. The strength of these interactions differed between aphid species, with benzaldehyde inducing a stronger effect on R. padi than P. humuli. Rhopalosiphum padi males and gynoparae responded significantly to the release of benzaldehyde in 2000 and 2001, and catches of both morphs were enhanced by the release of benzaldehyde with I. These results confirm those of Hardie et al. (1994), where extracts from bird cherry synergistically increased the number of males caught in the field in response to I, as did the release of benzaldehyde with I.
In contrast, P. humuli autumn migrants responded to benzaldehyde only when it was released with III, and then only in one of three years. Interestingly, in 1996, when responses to benzaldehyde were recorded, far more P. humuli were trapped in the 12.2 m Rothamsted Insect Survey suction trap at Wye (approximately 48 km from EMR) than in the two subsequent years of this study. The suction trap results showed that catches of P. humuli in 1996 were approximately 30 times greater than in 2000 or 2001.
Methyl salicylate elicited responses from R. padi and P. humuli only when released with the conspecific sex pheromone. In addition, responses were inconsistent, with methyl salicylate significantly increasing catches of R. padi males in 2001, and P. humuli gynoparae and males in 1996. Despite this, the responses of autumn migrants to methyl salicylate in the present study contrasts with the lack of response of spring migrants recorded previously (Campbell et al., 1993; Pettersson et al., 1994; Lösel et al., 1996a; Ninkovic et al., 2003). Therefore, our results offer moderate support for the suggested role of methyl salicylate as a cue allowing R. padi migrants to discriminate between primary and secondary hosts.
In contrast to the positive responses of the two aphid species to individual plant volatiles, responses of P. humuli males were reduced by the release of both plant volatiles with the conspecific sex pheromone. The same pattern was also evident for males in 2001. In addition, this response appeared to be species-specific because similar numbers of R. padi responded to the three-way combination and each two-way combination.
It is interesting to note that in spring, production of methyl salicylate by hop plants was increased from plants infested with P. humuli spring migrants (Campbell et al., 1993). Methyl salicylate acted as an antiaggregant, negating previously identified positive behavioral responses to (E)-2-hexenal and (−)-β-caryophyllene. Similarly, primary hosts of P. humuli also produced more methyl salicylate when infested with gynoparae (Wadhams, unpublished data.). Therefore, methyl salicylate may have a similar antiaggregation role in the response of P. humuli autumn migrants to III as does, for example, verbenone as an antiaggregant for the spruce bark beetle, Ips typographus (L.) (Zhang, 2003; Zhang and Schlyter, 2003).
The effect on catches of P. humuli males of releasing the plant volatiles with III depended upon whether benzaldehyde and methyl salicylate were released individually or together. Both of these plant volatiles are produced by primary hosts of P. humuli, and extracts of these hosts enhanced catches of gynoparae and males to III (Campbell et al., 1990; Lösel et al., 1996a,b). Therefore, the importance of other factors such as the ratio of compounds must be considered. Indeed, there is some evidence to suggest that aphids are able to discriminate between different ratios of I and (4aS,7S,7aR)-nepetalactone (Marsh, 1975; Eisenbach and Mittler, 1987; Steffan, 1990; Hardie et al., 1990, 1992). Aphids also discriminate between ratios of their common alarm pheromone, (E)-β-farnesene (EBF), and specific plant volatiles (Dawson et al., 1984). Dawson et al. (1984) noted that although hop plants also produce EBF, they are readily colonized by aphids, and that it was the corelease of (−)-β-caryophyllene by hop plants that allowed several aphid species, including P. humuli, to discriminate the source of EBF.
Rhopalosiphum padi and P. humuli are, to date, the only aphid species for which host-plant volatiles have been found to increase responses to sex pheromones (Campbell et al., 1990; Hardie et al., 1994; Lösel et al., 1996a,b). The host-plant extracts used in those studies would have contained many of the same volatiles, given that the hosts are closely related species of Prunus, and likely included both methyl salicylate and benzaldehyde. The results presented here indicate that both benzaldehyde and methyl salicylate can affect the responses of R. padi and P. humuli autumn migrants to their respective sex pheromones, but show also that these responses are species-specific. Our results also provide some support for assertions that host plant volatiles and their interactions with aphid sex pheromones may be important in promoting reproductive isolation (Steffan, 1987; Guldemond, 1990; Powell and Hardie, 2001).
References
Campbell, C. A. M., Dawson, G. W., Griffiths, D. C., Pettersson, J., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1990. Sex attractant pheromone of the damson-hop aphid Phorodon humuli (Homoptera: Aphididae). J. Chem. Ecol. 16:3455–3465.
Campbell, C. A. M., Pettersson, J., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1993. Spring migration of the damson-hop aphid Phorodon humuli (Homoptera: Aphididae) and summer host plant-derived semiochemicals released on feeding. J. Chem. Ecol. 19:1569–1576.
Campbell, C. A. M., Cook, F. J., Pickett, J. A., Pope, T. W., Wadhams, L. J., and Woodcock, C. M. 2003. Responses of the aphids Phorodon humuli and Rhopalosiphum padi to sex pheromone stereochemistry in the field. J. Chem. Ecol. 29:2225–2234.
Dawson, G. W., Griffiths, D. C., Pickett, J. A., Smith, M. C., and Woodcock, C. M. 1984. Natural inhibition of the aphid alarm pheromone. Entomol. Exp. Appl. 36:197–199.
Dawson, G. W., Griffiths, D. C., Janes, N. F., Mudd, A., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1987. Identification of an aphid sex pheromone. Nature 325:614–616.
Eisenbach, J. and Mittler, T. E. 1987. Sex pheromone discrimination by male aphids of a biotype of Schizaphis graminum. Entomol. Exp. Appl. 43:181–182.
Guldemond, J. A. 1990. Choice of host plant as a factor in reproductive isolation of the aphid genus Cryptomyzus (Homoptera, Aphididae). Ecol. Entomol. 15:43–51.
Hardie, J., Holyoak, M., Nicholas, J., Nottingham, S. F., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1990. Aphid sex pheromone components: Age dependent release by females and species-specific male response. Chemoecology 1:63–68.
Hardie, J., Nottingham, S. F., Dawson, G. W., Harrington, R., Merritt, L. A., Pickett, J. A., and Wadhams, L. J. 1992. Attraction of field-flying aphid males to synthetic sex pheromone. Chemoecology 3:113–117.
Hardie, J., Storer, R., Nottingham, S. F., Peace, L., Harrington, R., Merritt, L. A., Wadhams, L. J., and Wood, D. K. 1994. The interaction of sex pheromone and plant volatiles for field attraction of male bird-cherry aphid, Rhopalosiphum padi. Brighton Crop Prot. Conf. Pests Dis. 1994:1223–1230.
Hardie, J., Peace, L., Pickett, J. A., Smiley, D. W. M., Storer, J. R., and Wadhams, L. J. 1997. Sex pheromone stereochemistry and purity affect field catches of male aphids. J. Chem. Ecol. 23:2547–2554.
Hardie, J., Pickett, J. A., Pow, E. M., and Smiley, D. W. M. 1999. Aphids, pp. 227–250, in J. Hardie and A. K. Minks (eds.). Pheromones of Non-Lepidopteran Insects Associated with Agricultural Plants. CAB International, Wallingford, UK.
Landolt, P. J. and Phillips, T. W. 1997. Host plant influences on sex pheromone behavior of phytophagous insects. Annu. Rev. Entomol. 42:371–391.
Light, D. M., Flath, R. A., Buttery, R. G., Zalom, F. G., Rice, R. E., Dickens, J. C., and Jang, E. B. 1993. Host-plant green-leaf volatiles synergize the synthetic sex pheromones of the corn earworm and codling moth (Lepidoptera). Chemoecology 4:145–152.
Lösel, P. M., Lindemann, M., Scherkenbeck, J., Maier, J., Engelhard, B., Campbell, C. A. M., Hardie, J., Pickett, J. A., Wadhams, L. J., Elbert, A., and Thielking, G. 1996a. The potential of semiochemicals for control of Phorodon humuli (Homoptera: Aphididae). Pestic. Sci. 48:293–303.
Lösel, P. M., Lindemann, M., Scherkenbeck, J., Campbell, C. A. M., Hardie, J., Pickett, J. A., and Wadhams, L. J. 1996b. Effect of primary-host kairomones on the attractiveness of the hop-aphid sex pheromone to Phorodon humuli males and gynoparae. Entomol. Exp. Appl. 80:79–82.
Marsh, D. 1975. Responses of male aphids to the female sex pheromone in Megoura viciae Buckton. J. Entomol. 50:43–64.
Ninkovic, V., Ahmed, E., Glinwood, R., and Pettersson, J. 2003. Effects of two types of semiochemical on population development of the bird cherry oat aphid Rhopalosiphum padi in a barley crop. Agric. For. Entomol. 5:27–33.
Park, K. C., Elias, D., Donato, B., and Hardie, J. 2000. Electroantennogram and behavioural responses of different forms of the bird cherry-oat aphid, Rhopalosiphum padi, to sex pheromone and a plant volatile. J. Insect Physiol. 46:597–604.
Payne, R. W. 2000. The Guide to Genstat. Part 2: Statistics. VSN International, Oxford.
Pettersson, J. 1970. Studies on Rhopalosiphum padi (L.) I. Laboratory studies on olfactometric responses to the winter host, Prunus padus L. Lantbrukshoegsk. Ann. 36:381–399.
Pettersson, J., Pickett, J. A., Pye, B. J., Quiroz, A., Smart, L. E., Wadhams, L. J., and Woodcock, C. M. 1994. Winter host component reduces colonisation by bird-cherry oat aphid, Rhopalosiphum padi (L.) (Homoptera, Aphididae), and other aphids in cereal fields. J. Chem. Ecol. 20:2565–2574.
Powell, G. and Hardie, J. 2001. The chemical ecology of aphid host alternation: How do return migrants find the primary host plant? Appl. Entomol. Zool. 36:259–267.
Reinecke, A., Ruther, J., and Hilker, M. 2002a. The scent of food and defence: Green leaf volatiles and toluquinone as sex attractant mediate mate finding in the European cockchafer. Melolontha melolontha. Ecol. Lett. 5:257–263.
Reinecke, A., Ruther, J., Tolasch, T., Francke, W., and Hilker, M. 2002b. Alcoholism in cockchafers: Orientation of male Melolontha melolontha towards green leaf alcohols. Naturwissenschaften 89:265–269.
Ruther, J., Reinecke, A., Thiemann, K., Tolasch, T., Francke, W., and Hilker, M. 2000. Mate finding in the forest cockchafer, Melolontha hippocastani, mediated by volatiles from plants and females. Physiol. Entomol. 25:172–179.
Ruther, J., Reinecke, A., and Hilker, M. 2002. Plant volatiles in the sexual communication of Melolontha hippocastani: Response towards time-dependent bouquets and novel function of (Z)-3-hexen-1-ol as a sexual kairomone. Ecol. Entomol. 27:76–83.
Steffan, A. W. 1987. Fern- und Nahorientierung geflügelter Gynoparae und Sexualis-Männchen bei Blattläusen (Homoptera: Aphidinea: Aphididae). Entomol. Gen. 12:235–258.
Steffan, A. W. 1990. Courtship behaviour and possible pheromone spread by hindleg raising in sexual females in aphids (Homoptera: Aphidinea). Entomol. Gen. 15:33–49.
Zhang, Q.-H. 2003. Interruption of aggregation pheromone in Ips typographus (L.) (Col. Scolytidae) by non-host bark volatiles. Agric. For. Entomol. 5:145–153.
Zhang, Q.-H. and Schlyter, F. 2003. Redundancy, synergism, and active inhibitory range of non-host volatiles in reducing pheromone attraction in European spruce bark beetle. Ips typographus. Oikos 101:299–310.
Acknowledgement
T.W.P. was funded by the East Malling Trust for Horticultural Research and C.A. M.C. by the Department for Environment, Food and Rural Affairs. J.H. was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom. Rothamsted receives grant-aided support from the BBSRC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pope, T.W., Campbell, C.A.M., Hardie, J. et al. Interactions Between Host-plant Volatiles and the Sex Pheromones of the Bird Cherry-oat Aphid, Rhopalosiphum padi and the Damson-hop Aphid, Phorodon humuli . J Chem Ecol 33, 157–165 (2007). https://doi.org/10.1007/s10886-006-9199-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9199-4