Abstract
The pheromone composition of the Spanish population of the beet armyworm (BAW), Spodoptera exigua (Lepidoptera: Noctuidae), was identified. Analysis of female gland extracts showed the presence of compounds Z9,E12–14:Ac (1), Z9–14:Ac (2), Z11–16:Ac (3), Z9,E12–14:OH (4), Z9–14:OH (5), and Z11–16:OH (6) in a ratio of 26:11:1:22:31:9. The amount of compound per gland ranged from 2.08 ng for 5 to 0.09 ng for 3. However, analysis of female volatiles by SPME revealed only the presence of compounds, 1, 2, 3, and 5 in a 34:40:4:22 ratio. In electroantennogram assays, compound 1 elicited the highest response, and the C14 acetates evoked higher electrophysiological responses than the corresponding alcohols or C16 isomers. In a wind tunnel, no behavioral difference was observed between formulations based on the gland extracts and female volatiles. In both cases, males responded as when virgin females were used as the attractant source. Compound 1 alone elicited upwind flight by males, but required the presence of compound 5 in a 80:20 to 40:60 ratio for full activity. Ternary mixtures of 1, 5 and the minor components did not improve the performance of the 1+5 blend in a 60:40 ratio. In the field, the mixture 1+5+3 in a 56:37:7 ratio was the most attractive formulation, and is expected to be useful in future pest control strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The beet armyworm (BAW), Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), is a polyphagous pest widely distributed in Africa, Asia, Australia, North America, and southern Europe that attacks numerous crops and ornamental plants in the field and greenhouses. In southern Spain, the damage caused by this moth has increased considerably, particularly in greenhouses, possibly due to pesticide resistance. One alternative proposed to control this pest has been the use of a synthetic pheromone as a specific and environmentally-friendly management tool. The pheromone composition of BAW has been studied by numerous researchers, but with a great disparity among all existing data. Initial studies on pheromone gland extracts from American strains identified (Z,E)-9,12-tetradecadienyl acetate (Z9,E12–14:Ac, compound 1) as a pheromone compound, but the low efficiency of this acetate in field tests led to a reinvestigation of the pheromone complex (Brady and Ganyard 1972). Whereas Persoons et al. (1981) found compound 1, (Z,Z)-9,12-tetradecadienyl acetate (Z9,Z12–14:Ac), (Z)-11-tetradecenyl acetate (Z11–14:Ac), (Z)-9-tetradecenyl acetate (Z9–14:Ac, compound 2) and tetradecyl acetate (14:Ac) in pheromone gland extracts, Tumlinson et al. (1981, 1990) reported the presence of compounds 1, Z9,Z12–14:Ac, 2, (Z)-11-hexadecenyl acetate (Z11–16:Ac, compound 3) and (Z)-9-tetradecenol (Z9–14:OH, compound 5) in a 40:48:6:2:4 ratio in volatile collections. By contrast, Rogers and Underhill (1981) established that the blend 1+5 in 1:10 ratio was highly attractive to males whereas Mitchell et al. (1983) found the mixture 1+5 in 10:1 ratio to be the most attractive. Analysis of the strains present in other countries has also shown a disparity in pheromone composition. Thus, in Taiwan Cheng et al. (1985) proved that the latter blend also was highly effective but, in Japan, Mochizuki et al. (1994) reported a pheromone blend composed of compounds 1, 2, (Z,E)-9,12-tetradecadienol (Z9,E12–14:OH, compound 4), and 5 in a 98:60:86:100 ratio. In China, the pheromone composition of the insect was reported to be a mixture of these chemicals but in a 47:18:17:18 ratio (Dong and Du 2002). Therefore, it was not a surprise to discover that different commercial formulations of the pheromone were not sufficiently attractive in southern Spain to monitor and control the BAW in greenhouses. Consequently, the pheromone blend of the Spanish population was investigated.
Methods and Materials
Insects
S. exigua moths were reared on an artificial diet based on wheat (T. Cabello, personal communication). The initial larvae were sent by T. Cabello (University of Almería, Spain), allowed to pupate, and sexed. The pupae then were placed in plastic containers until emergence and maintained on a reversed 16:8 L:D cycle at 24 ± 1°C and 60% RH. The emerged adults were separated from pupae every day and fed with cotton balls soaked with a 10% sugar solution.
Chemicals
Compound 1 was obtained from Sigma-Aldrich (St Louis, MO, USA), compound 2 from Acros-Organics (Geel, Belgium), and compounds 3, 4, 5, and 6 were provided by SEDQ, S.A. (Barcelona, Spain). All compounds were >95% pure by GC or GC-MS analysis. Dimethyl disulfide was purchased from Sigma-Aldrich Química (Madrid, Spain). The solvents used (hexane, ether) were of analytical quality.
Pheromone Extracts
Before proceeding to the gland excision, virgin females of different ages were observed to determine the moment of maximum calling activity. Groups of 10 virgin females were placed in plastic boxes under a red lamp in the dark, and their behavior was monitored every 30 min. During the calling period, the pheromone glands were excised and immersed in hexane. After 1 h of extraction, the supernatant was collected and stored at −80°C. A total of 28 extracts of 2–8 glands per extract were studied.
Volatiles Collection
Five virgin females were selected and placed for 16 h (overnight) in a 40 ml vial (29 × 81 mm) containing a polydimethylsiloxane fiber (100 µm) for solid phase microextraction (SPME) (Matich et al. 1996). The fiber was inserted into the injection port of a GC-MS system for 5 min for analysis. A total of eight volatile collections were performed.
Derivatization with Dimethyl Disulfide
(DMDS) Location of the double bonds in the monounsaturated compounds of the pheromone gland extracts was determined by DMDS derivatization, as previously described (Buser et al. 1983). Briefly, the pheromone gland extract (ca. 20 female equivalent) in 100 µl hexane was reacted with 100 µl DMDS and 5 µl iodine solution (60 mg/ml in ether) at 40°C for 24 h. The mixture was cooled, diluted with 200 µl hexane, and the iodine was removed by treatment with 100 µl of 5% aq. soln. of Na2S2O3. The organic phase was concentrated to ca. 10 µl and kept at −80°C until GC-MS analysis.
Chemical Analysis
Gland extracts and synthetic compounds were analyzed on a Fisons GC 8000 series coupled to a MD 800 mass spectrometer (ThermoFisher Scientific, Madrid, Spain) using helium (1 ml/min) as the carrier gas. The column used for analysis was a 30 m × 0.25 mm i.d. × 0.25 μm HP-5MS fused silica capillary column (Agilent Technologies, Madrid, Spain) under the following chromatographic conditions: injection at 70°C for 1 min, then program of 5°C/min up to 120°C, and 3°C/min up to 300°C, which was maintained for a further 10 min. Electron impact mass spectra were recorded at 70 eV in the range of m/z 40–400. The extracts were carefully concentrated to 1–2 µl, and then the entire extract was injected in splitless mode. For SPME analysis, a 30 m × 0.25 mm i.d. × 0.25 μm SPB-20 column (Supelco, Bellefonte, PA, USA) was used under the following conditions: injection at 100°C for 1 min, then program of 10°C/min up to 120°C, 3°C/min up to 250°C, and 5°C/min up to 260°C, which was kept for a further 10 min. Injections were made in splitless mode, and the mass range was also 40–400.
Electroantennogram (EAG) Assays and Coupled GC-EAD
The EAG apparatus was commercially available from Syntech (Hilversum, The Netherlands). In brief, male antennae were excised, cut on both ends, and fixed to both electrodes with conducting gel Spectra 360 (Parker Lab. Inc., Hellendoorn, The Netherlands). A flow of humidified pure air (1000 ml/min) was directed continuously over the male antenna through the main branch of a glass tube (7 cm long × 5 mm diam). Test stimulations were carried out by giving puffs of air (300 ml/min) for 100 ms through a Pasteur pipette with the aid of a stimulus controller CS-01 (Syntech). The pipette contained a small piece of filter paper (1.5 cm diam) on which the gland extracts or the synthetic compounds 1–6 (10, 100, and 1000 ng diluted in hexane) had been deposited. The solvent was allowed to evaporate before the tests. Test compounds were applied at intervals of 60 sec on 10 antennae, and three times on each antenna. The antennae of at least 10 insects were used for the experiments. Control puffs with a piece of paper containing only solvent (hexane) were also intercalated between two consecutive stimuli to determine the baseline depolarization of the antennae. The signals were amplified (100 ×) and filtered (DC to 1 kHz) with an IDAC-2 interface (Syntech), digitized on a PC, and analyzed with the EAG Pro program. Depolarization means were compared for significance using analysis of variance (ANOVA) followed by LSD tests (P < 0.05).
To establish the optimum conditions for performing the EAG experiments, the activity of different aged males (1–4 d-old) at different times of the photoperiod (3rd, 5th, 7th, and 8th h into the scotophase), 1 h before the onset of the scotophase and 1–2 h at the beginning of the photophase, was studied.
GC-EAD analysis was carried out on the pheromone glands of virgin females to which Hez-PBAN (pheromone biosynthesis-activating-neuropeptide, 50 pmol) had been applied 45 min before excision. Hexane extracts of the glands were analyzed on a Focus GC (Thermo Instruments, Barcelona, Spain) equipped with an FID detector, a split/splitless injector, and a second make up gas (nitrogen). Helium was the carrier gas (1–2 ml/min), and the column was a 30 m × 0.25 mm i.d. × 0.25 μm HP-5 fused silica capillary column (Agilent Technologies, Madrid, Spain). The effluent from the column was split 50:50, and branches to the FID and EAD were from a deactivated fused silica capillary column (35 cm long × 0.25 mm i.d.). The transfer tube to the EAG preparation was heated to 230°C, and the GC conditions were the same as for the GC-MS.
Wind Tunnel Assays
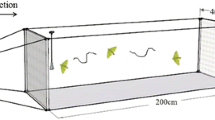
Assays were conducted in a glass tunnel of 180 × 50 × 50 cm as previously described (Quero et al. 1995). The wind was pushed through the tunnel by a 30 cm diam fan at 20 cm/s. The tunnel was illuminated with two red light fluorescent tubes dimmed to 1 lux. The temperature was maintained at 25 ± 2°C, and the relative humidity was 43 ± 10%. BAW males were acclimatized to the experimental conditions of the tunnel for 30 min, and individually released into the tunnel between the 6th and the 8th h of the scotophase. Before the tests, the insects were placed on filter paper in a Petri dish, then introduced into the tunnel at a height of 20 cm and a distance of 125 cm from the emission source. After a 30 sec acclimatization period, the behavior of the males was recorded for 5 min. For each responding insect, the following four types of behavior were recorded: taking flight; halfway: oriented upwind flight and arrival to the middle of the tunnel; final: arrival to the proximity of the lure; and contact: landing and contact with the source. In each treatment, a minimum of 40 virgin males of 1–3 d-old were used, and each insect was tested only once. The attractant source consisted of filter paper loaded with 1 μg of the most active EAG component (compound 1) plus the corresponding amount of the other components to test the desired formulation. For statistical analyses, a χ2 homogeneity test (P < 0.05) was performed for every treatment.
Field Trials
Field tests were conducted in greenhouses at two locations (Campo de Cartagena and Lorca) in the province of Murcia (southern Spain) from 6 July to 13 October 2006. In 2008, two more trials were conducted in the same province at the locations of Torre Pacheco from 1 July to 14 November, and Mazarrón from 21 July to 11 November. All tests were implemented by Kenogard, S.A. Three formulations were tested in 2006: F1: compounds 1+5 (91:9 ratio, 182 μg of 1 + 18 μg of 5); F2: compounds 1+5+3 (87:2.5:10.5 ratio, 174 μg of 1 + 5 μg of 5 + 21 μg of 3); and F3: compounds 1+5+2+4 (31:31:16:22 ratio, 62 μg of 1 + 62 μg of 5, +32 μg of 2 + 44 μg of 4), along with a commercial formulation (C1=Pherocon® BAW, Trécé, Adair, OK, USA) (two replicates per formulation in each location). In 2008, five formulations were assayed: the same F2 as above; F4: 1+5 (60:40 ratio, 174 μg of 1 + 116 μg of 5); F5: 1+5 (80:20 ratio, 174 μg of 1 + 43.5 μg of 5); F6: 1+5+2 (35:23:42 ratio, 174 μg of 1 + 116 μg of 5 + 209 μg of 2); and F7: 1+5+3 (56:37:7 ratio, 174 μg of 1 + 116 μg of 5 + 23 μg of 3), along with two different commercial lures: C1 as above, and C2 (Econex, Econex S.L., Murcia, Spain) (two replicates per formulation in each location). The synthetic chemicals were dissolved in hexane, and the required amounts were deposited onto red rubber septa (Aldrich, Milwaukee, WI, USA). Delta traps containing the septa were suspended at a height of 120–150 cm, and the distance between traps was about 10–15 m. The number of males caught per trap was recorded every week, the traps were emptied, and the septa were replaced every 3 wk. For statistical analyses, the number of moths captured per trap was converted to √(x + 0.5) and subjected to ANOVA followed by LSD test (P < 0.05).
Results
Pheromone Composition
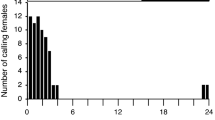
Females of all ages showed a maximum calling behavior during the last 3 h of the scotophase (data not shown). Thus, young females (1st–3rd scotophase) started to call 6 h after the onset of the scotophase, with the number of calling females gradually increasing until the end of the dark period. Females of 3rd–5th scotophase reached peak calling activity during the 6–7 h into the scotophase.
Analysis of the pheromone glands excised at the maximum calling period showed the presence of the previously identified compounds 1–5 by their GC retention times on polar and non-polar columns, and mass spectra in comparison with those of authentic samples (Fig. 1). In addition, Z11–16:OH (6) also was detected in very low amounts (0.58 ng/gland).
Gas chromatography (GC) analyses of a blend of synthetic compounds 1-5 and internal standard (7), b gland extract of four Spodoptera exigua females. Identities of compounds are as follows: Z9,E12–14:Ac (1), Z9–14:Ac (2), Z11–16:Ac (3), Z9,E12–14:OH (4), Z9–14:OH (5) and 12:Ac (IS, 7). Compound in b at retention time 26.2 min corresponds to Z11–16:OH (6) as shown independently by GC-MS analysis of the DMDS adduct
To confirm the presence of this previously unidentified compound in any of the BAW strains studied, DMDS derivatization of the extract produced an adduct with a molecular ion of m/z (%) 334 (16) and diagnostic ions at m/z 117 (72) and 217 (100). The molecular ion corresponded to the addition of DMDS to one double bond and the other ions were assigned to fragments [CH3(CH2)3CH(SCH3)+] and [(CH3S)CH(CH2)10OH]+, confirming the structure of the new product. The presence of compounds 2, 3, and 5 was also verified by DMDS adducts. The final composition of the extract was a mixture of compounds 1–6 in a 26:11:1:22:31:9 ratio, and the amount of compound per gland ranged from 2.08 ng for 5 to 0.09 ng for 3 (Fig. 2). However, analysis of female volatiles using the SPME technique revealed only the presence of compounds 1, 2, 3, and 5 in a 34:40:4:22 ratio (Fig. 2).
GC-EAD analysis of PBAN-treated pheromone gland extracts confirmed the presence of compounds 1, 2, 4, 5, and 6 but in a different ratio from those found in non-treated glands (Fig. 3). Compound 1 elicited the highest response followed by the 14-carbon compounds 2, 4, and 5. Compound 6, in turn, induced a very small EAG depolarization.
EAG Assays
Males of 1st–2nd scotophase elicited higher electrophysiological responses (2.0 mV on average) than older males (1.6 mV of 2nd–3rd scotophase, 1.5 mV of 3rd–4th scotophase) (data not shown). In addition, males displayed increasing EAG responses with time, reaching a maximum at the end of the scotophase (values rose from 1.6 to 2.2 mV, and dropped to 1.6 mV after the scotophase). When the pheromone compounds 1–6 were tested at several doses (10, 100, and 1000 ng), the highest responses were displayed by compound 1 (Fig. 4). Depolarizations induced by compounds 2–6 were dose-dependent, with the responses induced by the C16 compounds being lowest (Fig. 4). The relative activity of the compounds followed the order 1 > 4 ≅ 2 > 5 > 3 ≅ 6.
EAG responses of antennae from male Spodoptera exigua (N = 10) to different doses of compounds Z9,E12–14:Ac (1), Z9–14:Ac (2), Z11–16:Ac (3), Z9,E12–14:OH (4), Z9–14:OH (5), and Z11–16:OH (6) identified in female gland extracts. Bars (± SE) with the same letters within the same dose are not significantly different (ANOVA, LSD test, P < 0.05)
Wind Tunnel Assays
Blends of synthetic compounds in a ratio similar to that found in gland extracts and volatile collections elicited similar responses by males as did five calling females (data not shown). No significant differences were observed at any stage of the courtship sequence, although the number of contacts elicited by virgin females was slightly higher (63%) than the formulations of gland extracts (43%) and volatiles (51%).
When tested alone, compound 1 evoked a remarkable long-range attraction (71% of males reached the proximity of the lure) but no insect landed at the source (Fig. 5). Binary mixtures of 1 with compounds 2, 3, and 5 also induced upwind flight on males, but only blend A (1+5 in a 60:40 ratio) induced males to significantly contact the source (71%). Other 1+5 binary mixtures ranging from 80:20 to 40:60 did not significantly differ in activity in comparison to the 60:40 blend, but higher relative amounts of 5, such as 20:80 or 0:100 of the 1+5 blend dramatically affected the number of males arriving in the vicinity of the source (data not shown).
Percentage of response of Spodoptera exigua males (N = 40–48) in wind tunnel to 1 µg of Z9,E12–14:Ac (1) and to blends A: 1+5 (60:40), B: 1+2 (46:54), C: 1+3 (90:10), D: 1+5+2 (35:23:42), E: 1+5+3 (56:37:7), F: 1+5+3 (87:2.5:10.5). Bars with the same letters within the same behavior are not significantly different (Chi square test of homogeneity, P < 0.05)
Binary blends of 1 with 2 or 3 induced close approach to the source in 45–55% of males but none of them made contact (Fig. 5). When both 2 and 3 were added to blend A, in the same proportion as in female volatiles, the resulting blends D (1+5+2 in 35:23:42 ratio) and E (1+5+3 in 56:37:7 ratio) also elicited the complete behavioral sequence in males. The slightly lower number of insects contacting the source in comparison to blend A was not significant (Fig. 5). Lowering the relative amount of compound 5 to 2.5% in the 1+5+3 blend (formulation F) resulted in a clearly less active formulation.
When compounds 4 and 6 were added to blend A, they evoked dissimilar effects. Compound 4 had no effect on the number of males contacting the source when added to blend A at two different doses (blend A+10% of 4: 68% contacts, blend A+50% of 4: 66% contacts). In contrast, the presence of compound 6 markedly decreased the number of males flying upwind and contacting the source (blend A: 71% of contacts, blend A+10% of 6: 52%, blend A+50% of 6: 40%) (Fig. 6).
Percentage of response of Spodoptera exigua males (N = 41–69) in wind tunnel to blend A (1+5, 60:40) and mixtures with compounds 4 and 6 in different ratios (1: Z9,E12–14:Ac, 4: Z9,E12–14:OH 5: Z9–14:OH, 6: Z11–16:OH). Bars with the same letters within the same behavior are not significantly different (Chi square test of homogeneity, P < 0.05)
Field Assays
In the 2006 trials, a mixture of compounds 1+5 in a 91:9 ratio (formulation F1, Fig. 7) (Mitchell et al. 1983) resulted in a remarkably attractive lure (95.5 ± 15.5 and 51 ± 21 males per trap in each field). When compound 3 was added to this mixture to produce a similar composition to the one described by Tumlinson et al. (1990), the resulting formulation F2 attracted more males than F1, but the difference was significant in only one of the fields tested (149 ± 55 males/trap caught in F2 vs. 51 ± 21 in F1). By contrast, the mixture 1+5+2+4 in a 31:31:16:22 ratio (formulation F3), an initial pheromone composition found in gland extracts, displayed attractant activity similar to that of F1 (Fig. 7). In one field, all new formulations were superior to the commercial lure C1, whereas in the other field only F2 was more effective.
Mean number of catches (± SE) of Spodoptera exigua males in traps baited with various pheromone formulations in two different fields in 2006: C1 (commercial bait), F1: 1+5 (91:9), F2: 1+5+3 (87:2.5:10.5), F3: 1+5+2+4 (31:31:16:22) (1: Z9,E12–14:Ac, 2: Z9–14:Ac, 3: Z11–16:Ac, 4: Z9,E12–14:OH, 5: Z9–14:OH). Bars with the same letter within the same trial are not significantly different (ANOVA, LSD test, P < 0.05)
In 2008, formulation F7 (1+5+3 in a 56:37:7 ratio) was the most attractive in the two fields (489 ± 8 males/trap caught in field A and 620 ± 95 in field B), being significantly more efficient than F2, F4, and F6 (Fig. 8). F7 was also superior to the two commercial lures C1 and C2 in field B and to C1 in field A. By contrast, F6 was the least active formulation (Fig. 8).
Mean number (± SE) of Spodoptera exigua males caught in traps baited with various pheromone formulations in two different fields (A,B) in 2008: C1, C2 (commercial baits), F2: 1+5+3 (87:2.5:10.5), F4: 1+5 (60:40), F5: 1+5 (80:20), F6: 1+5+2 (35:23:42), F7: 1+5+3 (56:37:7) (1: Z9, E12–14:Ac, 2: Z9–14:Ac, 3: Z11–16:Ac, 4: Z9,E12–14:OH, 5: Z9–14:OH). Bars with the same letter within the same trial are not significantly different (ANOVA, LSD test, P < 0.05)
Discussion
The sex pheromone of the Spanish population of the BAW, determined from female gland extracts, has been identified as a mixture of compounds 1–6 in a 26:11:1:22:31:9 ratio, although volatiles emitted by virgin females lack the presence of alcohols Z9,E12–14:OH (4) and Z11–16:OH (6). This is not surprising because it is known that the composition of the pheromone gland and volatiles released by females may differ remarkably (Cross et al. 1976; Hill et al. 1975). The composition of female volatiles was similar to that found by Tumlinson et al. (1990) with the exception of Z9,Z12–14:Ac, which was absent in our extracts. The relative differences in both strains could be due to the different origin of the strains or the different extraction methods used: volatile collections followed by entrainment on charcoal by the American team and SPME analysis of female effluvia in this work. The diene alcohol 4 was moderately EAG-active and had a neutral effect on male behavior. However, in the Japanese population, this chemical stimulated the receptor cells tuned to alcohol 5 (Mochizuki et al. 1993). Moreover, combining alcohol 4 with the corresponding acetate 1 resulted in an efficient formulation in the field (Rogers and Underhill 1981; Mochizuki et al. 1994). Alcohol 6, in turn, was practically inactive by EAG, but inhibited the responses of males when mixed with the highly active formulation, 1+5 (60:40). Its presence in gland extracts suggests that it is likely a biosynthetic precursor of the corresponding Z11–16:Ac (3) (Bjostad et al. 1987). However, to our knowledge, no biosynthetic studies of the pheromone have been undertaken to prove this assumption.
In wind tunnel studies, the combination 1+5 in a 60:40 ratio (blend A) was the most active among all the binary and ternary mixtures tested, but this proportion is not critical because similar levels of activity were achieved by blends 80:20 to 40:60. This result is consistent with the relative amounts of both compounds found in gland extracts and female volatiles. Both chemicals have been found to be tuned to two different receptor neurons in male-specific sensilla trichodea (Dickens et al. 1993; Mochizuki et al. 1993), and, in the field, this formulation in a 10:1 ratio was reported to be active in Florida (Mitchell et al. 1983), Taiwan (Cheng et al. 1985), and in a 70:30 ratio in Japan (Wakamura 1987).
In field tests, initially we compared the most effective formulations found by Mitchell et al. (1983) (F1) and Tumlinson et al. (1990) (F2) with one commercially available (C1), along with a new formulation (F3) of similar composition to the pheromone gland extract lacking compounds 3 and 6. In the first trial, all formulations were significantly more attractive than the C1, although without a significant difference among them. In the second trial, F2 was the most efficient formulation. Based on these results, a new set of experiments was designed in 2008 to further improve formulation F2. Thus, addition of compound 3 (7%) to formulation F4 (blend A above) resulted in a more efficient blend (F7), not only in comparison to its parent F4 but also to F2. In fact, F7 was the most attractive formulation found for BAW males. Our results show that in the Spanish population the presence of the minor component 3 appears to be important for the development of new attractant formulations to catch BAW males.
Compound 2, the major component of the pheromone released by females, deserves some comments. First, this compound was only moderately active on male antennae, activity similar to that of diene alcohol 4, a component absent in female volatiles. Second, in a wind tunnel, compound 2 decreased the attractant activity of the major component 1, and that of the highly active blend A as well. Third, in the field, addition of 2 to formulation F4 resulted in a less efficient lure, the resulting formulation F6 being the least efficient of all tested. These data are in agreement with those of Tumlinson et al. (1990) in which compound 2, also the major component released by American BAW females, also reduced the number of catches when it was incorporated into the lure. It is surprising that this compound emitted by females as a major component reduces the attraction of conspecific males in the laboratory and in the field. Does the female use this compound to modulate the activity of the key components 1 and 5 under some specific but yet unknown conditions? It was also unexpected that occasionally compound 2 elicited in males spikes from neurons sensitive to the major compound 1 (Dickens et al. 1993), with the threshold of the cells responding to acetate 2 being ca. 1000 times higher than that of the cells responding to acetate 1 (Mochizuki et al. 1993). These data do not correspond to the relatively high emission of compound 2 by females relative to compounds 1 and 5, the two components from which receptor cells have so far been identified (Mochizuki and Shibuya 1991; Mochizuki et al. 1993) (see above). These data indicate that more research is needed to clarify the role of compound 2 in the chemical communication system of the BAW.
In summary, we have identified the pheromone composition of the Spanish population of the BAW, and found that extracts of female glands exhibit a remarkably different composition relative to volatiles emitted by females. Electrophysiological and behavioral activity of the pheromone components, and mixtures thereof, have resulted in a new highly active formulation (F7) for the Spanish BAW population, which should be useful in future IPM strategies.
References
Bjostad, L. B., Wolf, W. A., and Roelofs, W. L. 1987. Pheromone biosynthesis in Lepidopteran desaturation and chain shortening, pp 77–120, in G. D. Prestwich, G. J. Blomquist (eds), Pheromone Biochemistry. Academic Press. Orlando, Florida.
Brady, U. E., and Ganyard, M. C. 1972. Identification of a sex pheromone of the female beet armyworm Spodoptera exigua. Ann. Entomol. Soc. Am. 65:898–899.
Buser, H. R., Arn, H., Guerin, P., and Rauscher, S. 1983. Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal. Chem. 55:818–822.
Cheng, E. Y., Lin, D. F., Kao, C. H., Chen, S. H., Wang, S. S., and Lee, H. C. 1985. Studies on the synthetic pheromone of the beet armyworm, Spodoptera exigua (Hübner). Evaluation of Mitchell’s formula in Taiwan. J. Agric. Res. China 34:315–322.
Cross, J. H., Byler, R. C., Cassidy, R. F., Silverstein, R. M., Greenblatt, R. E., Burkholder, W. E., Levinson, A. R., and Levinson, H. Z. 1976. Porapak-Q collection of pheromone components and isolation of (Z)—and (E)-14-methyl-8-hexadecenal, sex pheromone components, from the females of four species of Trogoderma (Coleoptera: Dermestidae). J. Chem. Ecol. 2:457–468.
Dickens, J. C., Visser, J. H., and Van der Pers, J. N. C. 1993. Detection and deactivation of pheromone and plant odor components by the beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). J. Insect Physiol. 39:503–516.
Dong, S., and Du, J. 2002. Chemical identification and field tests of sex pheromone of beet armyworm Spodoptera exigua. Acta Phytophyl. Sin. 29:19–24.
Hill, A. S., Cardé, R. T., Kido, H., and Roelofs, W. L. 1975. Sex pheromone of the orange tortrix moth Argyrotaenia citrana (Lepidoptera: Tortricidae). J. Chem. Ecol. 1:215–224.
Matich, A. J., Rowan, D. D., and Banks, N. H. 1996. Solid phase microextraction for quantitative headspace sampling of apple volatiles. Anal. Chem. 68:4114–4118.
Mitchell, E. R., Sugie, H., and Tumlinson, J. H. 1983. Spodoptera exigua: capture of feral males in traps baited with blends of pheromone components. J. Chem. Ecol. 9:95–104.
Mochizuki, F., and Shibuya, T. 1991. Antennal single sensillum responses to sex pheromone in male beet armyworm, Spodoptera exigua, Hübner (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 26:409–411.
Mochizuki, F., Shibuya, T., Ihara, T., and Wakamura, S. 1993. Electrophysiological responses of the male antenna to compounds found in the female sex pheromone gland of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 28:489–496.
Mochi z uki, F., Takai, M., Shibuya, T., and Wakamura, S. 1994. Field trap responses of male Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) to (Z,E)-9,12-tetradecadien-1-ol and (Z)-9-tetradecenyl acetate. Appl. Entomol. Zool. 29:436–438.
Persoons, C. J., Van Der Kraan, C., Nooijen, W. J., Ritter, F. J., Voerman, S., and Baker, T. C. 1981. Sex pheromone components of the beet armyworm, Spodoptera exigua: Isolation, identification and preliminary field evaluation. Ent. Exp. Appl. 30:98–99.
Quero, C., Camps, F., and Guerrero, A. 1995. Behavior of processionary males (Thaumetopoea pityocampa) induced by sex pheromone and analogs in a wind tunnel. J. Chem. Ecol. 21:1957–1969.
Rogers, C. E., and Underhill, E. W. 1981. A sex attractant for monitoring beet armyworm populations. Southwest. Entomol. 6:211–214.
Tumlinson, J. H., Mitchell, E. R., and Sonnet, P. E. 1981. Sex pheromone components of the beet armyworm, Spodoptera exigua (Hubner). J. Environ. Sci. Health A 16:189–200.
Tumlinson, J. H., Mitchell, E. R., and Yu, H. S. 1990. Analysis and field evaluation of volatile blend emitted by calling virgin females of beet armyworm moth, Spodoptera exigua (Hubner). J. Chem. Ecol. 16:3411–3423.
Wakamura, S. 1987. Sex pheromone of the beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Field attraction of male moths in Japan to (Z,E)-9,12-tetradecadienyl acetate and (Z)-9-tetradecen-1-ol. Appl. Entomol. Zool. 22:348–351.
Acknowledgements
We gratefully acknowledge T. Cabello (University of Almería) for providing us with BAW larvae for rearing and useful advice, P. Fuchs (Kenogard, S.A.) for conducting the field tests, and SEDQ S.A. for providing us with compounds 3–6. We are also indebted to MICINN for an I3P fellowship to P. Acín and a Ramón y Cajal contract to C.Q. Financial support was provided by CICYT (project AGL 2006-13489-C02-01/AGR).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Acín, P., Rosell, G., Guerrero, A. et al. Sex Pheromone of the Spanish Population of the Beet Armyworm Spodoptera exigua . J Chem Ecol 36, 778–786 (2010). https://doi.org/10.1007/s10886-010-9817-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9817-z