Abstract
The sex pheromone of Mnesampela privata, an endemic pest of Eucalyptus plantations in Australia, was previously identified as a single bioactive compound, (3Z,6Z,9Z)-3,6,9-nonadecatriene (C19 triene). Initial field testing of lures containing 1 mg, 5 mg or 10 mg of C19 triene (>98% purity) caught no or very few male M. privata. (3Z,6Z,9Z)-3,6,9-Henicosatriene (C21 triene) was identified as an additional minor pheromone component in abdominal tip extracts of M. privata females from Tasmania. Levels of both compounds extracted from individual females varied greatly, but the ratio was relatively constant at 33:1 C19:C21 trienes. Electroantennograms (EAG) of synthetic C21 triene with male M. privata gave positive but consistently lower responses than elicited by the C19 triene. Field tests showed that the addition of 1–6% C21 triene to 1 mg C19 triene significantly increased trap catch and the detection of M. privata in plantations. Traps baited with a 16:1 ratio caught significantly more moths than those baited with a ratio approximating that of females.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The autumn gum moth, Mnesampela privata Guenée, is a serious pest of eucalypt plantations in southern Australia (Bashford 1993; Steinbauer et al. 2001; Rapley et al. 2007, 2009). A synthetic pheromone lure for monitoring males of this species was developed after identification of the polyene hydrocarbon (3Z,6Z,9Z)-3,6,9-nonadecatriene (C19 triene) as a bioactive component in female abdominal tip extracts (Steinbauer et al. 2004). Östrand et al. (2007) used traps baited with 1 mg of C19 triene to monitor populations of male M. privata in Tasmania and Victoria, and found trap catches were significantly correlated with subsequent oviposition. However, Steinbauer et al. (2004) speculated that M. privata may use other compounds in its sex pheromone, as field catches were low at a site with an outbreak population. Furthermore, in our trapping trials in Eucalyptus plantations in Tasmania, South Australia, Victoria, and Western Australia during 2005 and 2006, using pheromone traps baited with 1 mg of C19 triene, captures of male M. privata were negligible despite the known presence of moths, eggs or larvae (P. Walker unpublished data).
Polyene hydrocarbons and their epoxides constitute a second major class of lepidopteran sex pheromones that have been isolated from four major families: Geometridae, Noctuidae, Arctiidae, and Lymantriidae (Millar 2000; El-Sayed 2008). To date, C19 triene has been identified as an attractant or pheromone component in 34 other species of Geometridae, 24 of which belong to the same subfamily as M. privata (Ennominae) (El-Sayed 2008). In all species that use C19 triene, except M. privata, other bioactive compounds also have been found in pheromone extracts. The use of two or more components in a pheromone is often crucial in maintaining a discrete, species-specific chemical communication channel (Cardé and Baker 1984; Millar et al. 1990). Although M. privata is the only Australian geometrid in which C19 triene has been verified as a sex pheromone component, Steinbauer et al. (2004) gave indirect evidence that this compound is used for communication by other sympatric species as three species of Ennominae (two belonging to the same genus) also were caught during field trials. Therefore, it is likely that additional pheromone components are used by M. privata. Steinbauer et al. (2004) detected two alcohols (1-hexadecanol and 1-octadecanol) in some female M. privata extracts, but neither compound elicited significant antennal responses in males, nor did their addition to lures containing C19 triene enhance trap captures.
The purpose of this study was to search for other pheromone components in female M. privata. The response of male M. privata to a previously unidentified pheromone component was measured in the laboratory by an electroantennogram, and in the field by using pheromone traps in Eucalyptus spp. plantations in Tasmania, Western Australia, Victoria, and South Australia. We also determined the optimal ratio of the two synthetic pheromone components for attraction of male M. privata, compared the trapping efficacy of two types of traps, examined the effect of lure age on trap catch, and, for comparison, analyzed male M. privata abdominal tip extracts.
Methods and Materials
Insects
A colony of M. privata was founded (21°C ± 1°C; 60–70% RH; L12: D12 photoperiod) from collections of eggs and early instar larvae infesting Eucalyptus globulus trees near Cornelian Bay, Hobart, Tasmania, during April and May 2005. Larvae were reared on juvenile E. globulus leaves placed in ventilated plastic boxes lined with paper toweling and, at the prepupal stage, were placed in boxes containing vermiculite for pupation. Pupae were sexed according the position of the genital scar, stored separately, and monitored daily for emergence. Upon emergence, moths were placed in individual 850-ml round plastic containers (Genfac Plastics Ltd, Melbourne, Australia) with a honey/sugar solution (20 g honey, 20 g sugar, and 2 g ascorbic acid in 1 l boiled water) and held until experimentation.
Extracts
Abdomen tips containing the ovipositor and associated pheromone gland were excised from unmated, laboratory-reared, M. privata females, 1 (i.e., newly emerged), 3, 5, 7, and 9-d-old (N = 10, 8, 10, and 5, respectively), and from three mated females of unknown age. Tips were removed during the first 2 h of the scotophase to increase the chance of sampling calling females. The tips from ten female M. privata caught in a light trap 50 km east of Albany, Western Australia, also were analyzed. After freezing females and removing the abdominal tips, each was dissected to determine its mating status. Tips from all females were placed into individual borosilicate vials with 150 μl inserts (Waters Corporation, Australia), soaked in 15 μl of CH2Cl2 containing 100 ng methyl stearate as an internal standard for an hour at room temperature, and then the tips were removed before storing the extract at −20°C. The solutions were allowed to reach room temperature before analysis.
Abdomen tips (the genitalia, claspers, and surrounding tissue) were excised from laboratory-reared male M. privata moths 1, 3, and 5-d-old (N = 3 per age category), and soaked in CH2Cl2 as described above. All males were unmated, and had no access to females.
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
GC-MS analyses were carried out on a Varian 3800 GC coupled to a Varian 1200 triple quadrupole MS using 70 eV electron ionization in single quadrupole mode. The column was a Varian ‘Factor Four’ VF-5 ms (30 m × 0.25 mm internal diam and 0.25 micron film). Injections of 1 μl of abdominal tip extracts were made either manually or with a Varian CP-8400 autosampler in conjunction with a Varian 1177 split/splitless injector at 250°C in the splitless mode. The ion source was held at 220°C, and the transfer line at 290°C. The carrier gas was helium at 1.22 ml/min using a constant flow mode. The GC oven was held at 60°C for 1 min then ramped to 150°C at 30°/min, then to 280°C at 8°/min.
Identities of major compounds were assigned based on the previous report from Steinbauer et al. (2004) on this species, mass spectra, and Kovats retention indices relative to those reported in the NIST Chemistry WebBook (http://webbook.nist.gov/chemistry/). Lower levels of trienes were detected in moth extracts by selected ion monitoring (SIM) using characteristic ions at m/z 79 and 108, which were used for comparison of C19 and C21 triene levels. Levels of trienes were estimated in each moth from the ratio of the response in full scan mode of the triene to the methyl stearate internal standard.
Solutions of C19 and C21 trienes for EAG and field testing were prepared in various C19/C21 proportions ranging from 3:2 to 67:1. These ratios were independently verified by GC-MS analysis of an aliquot of each solution.
Synthesis
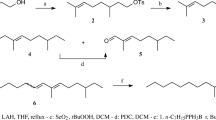
(3Z,6Z,9Z)-3,6,9-Nonadecatriene (>98% purity) was prepared by JAS and PPM, at the University of Tasmania, as described by Davies et al. (2007). Synthesis of (3Z,6Z,9Z)-3,6,9-henicosatriene (>99.7% purity) was conducted by AN, FA and EH, at Mid Sweden University, as shown in Scheme 1. Unless otherwise stated, starting materials and solvents were used as received from commercial suppliers. Dry THF (benzophenone and potassium) and Et2O (LiAlH4) were distilled from the indicated drying agents and either used immediately or stored under argon. NMR spectra were recorded on a Varian 500 instrument. GC analyses were carried out using a 30 m × 0.25 mm id capillary column coated with EC-1, df = 0.25 μm, carrier gas: N2 (15 psi) and a split ratio of 1:20. Mass spectra were recorded on a Varian Saturn 2000 GC-MS instrument. Silica gel 60 (230–400 mesh; Merck, Sigma-Aldrich, Scandinavia) was used in preparative liquid chromatography. Thin layer chromatography (TLC) was performed on silica gel plates (Merck 60, pre-coated aluminium foil) eluted with EtOAc in cyclohexane (40%).
(9Z,12Z,15Z)-9,12,15-Octadecatrien-1-ol (2)
Methyl linolenate (1) (1.08 g, 3.69 mmol) dissolved in Et2O (5 ml) was added to a suspension of LiAlH4 (295 mg, 7.39 mmol) in Et2O (20 ml) at 0°C, under an argon atmosphere. After 1.75 h, the reaction was quenched with 2 M HCl (aqueous, 15 ml) at 0°C. The organic layer was separated, followed by extraction with Et2O (3 × 30 ml), washing of the combined extracts with brine (30 ml), and drying (MgSO4). Evaporation of solvent under reduced pressure resulted in a clear bright yellow oil that was purified by flash chromatography (silica, EtOAc/cyclohexane). Compound 2 was isolated as a clear bright yellow oil, 919 mg (3.48 mmol, 94% yield), with 100% purity according to GC. 1H and 13C NMR spectra were identical with those reported in literature (Wang and Zhang 2007).
(3Z,6Z,9Z)-Henicosa-3,6,9-triene (C21 triene)
4-Methylbenzene-1-sulfonyl chloride (1.29 g, 6.78 mmol) was added to a solution of alcohol 2 (897 mg, 3.39 mmol) and pyridine (0.80 ml, 10.2 mmol) in CH2Cl2 (30 ml) at 0°C, under an argon atmosphere. The reaction mixture was allowed to remain at room temperature for 4 d, after which Et2O (50 ml) and H2O (30 ml) were added to the mixture. The organic layer was separated, followed by extraction with Et2O (3 × 30 ml), washing of the combined extracts with 2 M HCl (aqueous, 2 × 30 ml) and brine (2 × 30 ml), and drying (MgSO4). Evaporation of solvent under reduced pressure resulted in a clear bright yellow oil that was purified by flash chromatography. Tosylate 3 was isolated as a clear, colorless oil, (1.56 g) containing large amounts of 4-methylbenzene-1-sulfonyl chloride. The excess of sulfonyl chloride was removed by reaction of the product mixture with 1,4-butandiol (0.70 ml) in pyridine (1.0 ml) at 5°C overnight. Et2O (25 ml) and H2O (25 ml) were added, and the aqueous phase was acidified with 2 M HCl. The organic layer was separated, followed by extraction with Et2O (4 × 30 ml), washing of the combined extracts with brine (50 ml), and drying (MgSO4). Evaporation of solvent under reduced pressure resulted in a yellow turbid oil that was purified by flash chromatography. The tosylate 3 was isolated as a clear colorless oil, 1.07 g (2.56 mmol, 76% yield, pure according to TLC), and used in the next step without further characterization.
Propyl magnesium bromide was prepared by addition of propylbromide (0.47 ml, 5.2 mmol) in THF (5 ml) to magnesium chips (256 mg, 10.5 mmol) under an argon atmosphere. The reaction started spontaneously. After 15 min, the Grignard reagent was transferred by syringe to a flask loaded with CuI (488 mg, 2.56 mmol) at −30°C under an argon atmosphere. After another 15 min, tosylate from above (1.07 g, 2.56 mmol) was added to the grey suspension. The mixture was allowed to reach room temperature slowly and turned black. After 4 d, the reaction was quenched with aqueous saturated NH4Cl (25 ml) followed by addition of H2O (25 ml), extraction with Et2O (3 × 30 ml), washing of the combined extracts with brine (30 ml), and drying (MgSO4). Evaporation of solvent under reduced pressure resulted in a yellow oil that was purified by flash chromatography. (3Z,6Z,9Z)-Henicosa-3,6,9-triene was isolated as a clear colorless oil, 544 mg (1.87 mmol, 73% yield), with 99.7% purity according to GC. 1H and 13C NMR spectra were identical with those reported in literature (Wang and Zhang 2007). MS (EI) m/z (relative intensity): 290 (13 %, M+), 261 (6), 247 (4), 234 (23), 219 (3), 205 (5), 191 (4), 177 (6), 163 (11), 149 (16), 135 (30), 121 (34), 108 (100), 95 (83), 81 (90), 79 (83), 67 (60), 57 (20), 55 (19), 41 (32), 39 (29).
Electrophysiology
Electroantennogram (EAG) recordings were prepared by placing an antenna excised from a male between two silver wire electrodes, covered by thin glass capillaries filled with Ringers solution. Antennal responses were recorded via an Intelligent Data Acquisition Controller (IDAC-02, Syntech, Hilversum, the Netherlands) connected to a PC. Antennal preparations were exposed continuously to charcoal-filtered and moistened air with a velocity of 0.5 m/s. Test compounds (5 μl of hexane on filter paper in a Pasteur pipette) were delivered via a 1 s puff of air (5 ml/s) into an opening in the glass tube 150 mm upstream from the antenna, with the outlet 10 mm from the antenna. Stimuli were administered by that device. The solvent was allowed to evaporate from the filter paper prior to testing. The EAG responses of males to the C19 and C21 trienes, hexadecyl acetate, and 1-octadecanol (all >99% purity) were tested on the antennae of four to 12 males. EAG recordings were analyzed using ‘GcEad’ software, version 4.1 (Syntech, the Netherlands 2005). For data analysis, the mean solvent signal was subtracted from each mean stimulus signal. All amplitudes were normalized to the amplitude recorded in response to the reference stimulus.
GC-Electroantennogram (EAD) analysis that used combined gland extracts from three virgin females was conducted on antennae of males prepared as described above. Extracts (1 μl) were injected into a Varian Vista 6000 GC in splitless mode with an injection temperature of 220°C, and the oven was held at 60°C for 1 min and then ramped to 240°C at 20°/min, with a 5 min hold. The GC was equipped with an HP-5, (5% phenyl)-methylpolysiloxane column, 12 m × 0.2 mm id, phase thickness 0.33 ìm. Nitrogen was used as carrier gas. A GC effluent splitter (split ratio 1:1) was used. One stream was directed to a flame ionization detector (FID), and the second was added through a heated transfer line to the air stream and directed over the excised antenna of a male moth, as described above. EAD signals and FID responses were recorded simultaneously by the IDAC2 connected to a PC via Syntech GcEad software.
Field Trials
Field trials were carried out in commercial plantations of Eucalyptus nitens and E. globulus, 2–5-y-old, in Tasmania, Western Australia, Victoria, and South Australia in 2007 and 2008. Lures were prepared by dissolving compounds in hexane, with ca 1% of the antioxidant butylated hydroxytoluene, and applying 100 μl of solution into rubber septa (Sigma-Aldrich, Australia), then allowing the solvent to evaporate in a fume hood before storing at −20°C. Control septa were loaded with 100 μl of hexane. Unless otherwise stated, green Unitraps (PheroBank, the Netherlands) were used in trapping studies. The base of each trap was sprayed with a persistent surface insecticide to kill trapped moths and to prevent moths from being removed by ants and wasps. Traps were suspended within tree rows from metal poles at ca 1.5 m height.
Experiment 1.
Field Bioactivity of C21 triene: Starting in March 2007, captures of male M. privata in Unitraps baited with 1 mg, 5 mg, or 10 mg C19 triene and 1 mg C19 triene + 25 μg C21 triene (40:1 ratio) were compared in commercial Eucalyptus plantations at various locations in southern Australia. In Tasmania, three replicates of each lure type were placed in 2–5-yr-old E. nitens plantations at the Surrey Hills estate, near Hampshire (NW Tasmania), three in Gould’s Country (NE Tasmania), three in the Geeveston area and one near Pittwater and one near Nugent (Southern Tasmania). Three replicates of each lure type (except the 5 mg C19 triene dose) were placed in 2–5-yr-old E. globulus plantations near Albany, Western Australia, and four replicates were placed in the Green Triangle area on the Victoria/South Australian border. Single lines of Unitraps containing each lure type were placed 10–30 m apart at a height of 1.5 m in random order, spacing replicate rows at least 100 m apart. The position of each lure type within a replicate row was assigned randomly. Traps were checked on a weekly or fortnightly basis, and trap positions within rows were rotated in order to reduce any positional bias in moth capture. Captured moths were identified, counted, and sexed. In Victoria/South Australia and Western Australia, trapping was terminated in May and June 2007, respectively. In Tasmania, trapping extended to February 2008, and lures were changed in December 2007 as detailed in Experiment 2.
Experiment 2.
Optimal Ratio of C19 and C21 Trienes for Trapping Male M. privata: Lures loaded with blends of C19:C21 trienes at 3:2, 16:1, 33:1, 40:1, and 67:1 ratios were made by adding the required amount of C21 triene to 1 mg C19 triene. Lures loaded with 1 mg C19 triene only, 25 μg of C21 triene only (equivalent to the amount of C21 triene added to 40:1 ratio C19:C21 triene baits), and hexane only (controls) also were formulated. Single rows of randomly assigned Unitraps each containing one of the test lures were spaced 10 m apart at 1.5 m height in 2–5-yr-old E. nitens plantations at five locations in the Surrey Hills estate. Replicate rows of traps were spaced at least 100 m apart. The number of male M. privata caught was monitored 19 December 2007 to 18 March 2008, checking traps and rotating their positions about every 2 wk. Baits were not replaced over the trapping period.
Experiment 3.
Comparison of Trap Design: The trapping efficiency of the green Unitraps was compared with white Delta traps in a 4-yr-old E. nitens plantation near Buckland, southern Tasmania. Five pairs of each trap design were placed 10 m apart, with a distance of 5 m between pairs and a trap height of 1.5 m. All traps were baited with lures containing 1 mg C19 triene + 60 μg C21 triene (16:1 ratio). The order of trap design within each pair was assigned randomly. Trap catch was checked once after a 4 d trapping period between 4 and 8 April, 2008.
Experiment 4.
Effect of Lure Age on Trap Catch: The number of male M. privata caught in five Unitraps baited with fresh lures loaded with 1 mg C19 triene + 60 μg C21 triene (16:1 ratio) was compared with the catch for five traps baited with comparable lures that had been aged in the field for 3 mo from experiment 2. Deployment of traps was as for Experiment 3. The order of trap design within each pair was assigned randomly. Trap catch was checked twice over a 24 d trapping period between 8 April and 2 May, 2008, in the E. nitens plantation near Buckland, southern Tasmania.
Statistical Analysis
SPSS version 16.0 for Windows (SPSS Inc. 2007) was used to analyze EAG and field trapping data. EAG responses to test compounds (mV) were normalized to the mean response to hexane as the reference stimulus (= 100%). Normalized antennal responses and pheromone trapping data from Experiments 1 and 2 were analyzed using ANOVA, while data from Experiments 3 and 4 were compared using t-tests. Data was checked for variance homogeneity by using Levene’s homogeneity-of-variance test and transformed if necessary. If the ANOVA F-value was significant, differences between means were then tested for significance by the least significant difference test (LSD) at the 5% probability level.
Results
GC-MS Analysis of Solvent Extracts
Twenty-one compounds were identified tentatively from gland extracts of 41 individual M. privata females from Tasmania by GC-MS (Table 1). Detailed examination of the mass spectra of all major and minor compounds in an individual female with relatively high levels of C19 triene revealed one compound (peak 8 in Fig. 1) that had very similar MS characteristics of a polyunsaturated compound (i.e., ions at m/z 67, 79, and 108). Also, the diagnostic ion at m/z 206 observed in the C19 triene (M- 56) was seen at m/z 234, consistent with an extra C2H4 unit (Fig. 2). Given that (3Z,6Z,9Z)-3,6,9-henicosatriene already had been recorded as a pheromone component in some Geometridae species (Millar 2000; El-Sayed 2008), this compound was synthesized. The synthetic C21 triene retention characteristics and mass spectrum were indistinguishable from that of the moths’ compound, and the standard co-eluted with female moth component 8. Other components of interest found in female extracts were hexadecyl acetate along with the previously reported n-hexadecanol and n-octadecanol (Steinbauer et al. 2004). These three compounds were found in only a few females in variable quantities. Levels of trienes also were extremely low in some moths, and it was not possible (even by SIM-MS) to determine the C19/C21 triene ratio in all individuals. The C21 triene was below the detection limit in 23 out of 41 extracts, while the C19 triene was below detection limit in one female extract. Consequently, the calculated C19:C21 ratio is based on 15 individual females of known age and three of unknown age in which both compounds could be measured. Mean estimated titers of trienes per female (calculated as internal standard equivalents) were 30.74 ng (± 6.1 SE) (range = 604 pg to 143 ng) for C19 triene, and 1.44 ng (± 0.3 SE) (range = 368 pg to 3.8 ng) for C21 triene. Estimated titers of C19 and C21 trienes were highly correlated (Pearson's correlation coefficient = 0.956, P < 0.001, N = 15) (Fig. 3a). Mean titers of both compounds peaked in 7-d-old females and decreased markedly in 9-d-old females (Fig. 3b), but was again highly variable within age categories. The mean ratio of C19:C21 trienes in female M. privata from Tasmania was 33:1 (± 2.3 SE, range 8:1 to 54:1), compared to females from Western Australia which exhibited a mean C19:C21 trienes ratio of 5.7:1 (± 0.6 SE, range 3.5:1 to 9.7:1), which was significantly different from the ratio found for moths from Tasmania (t-test, t = 7.614, df = 26, P < 0.001). Dissections revealed that all of these females had mated once.
Representative total ion current chromatogram of extract from a female Mnesampela privata abdominal tip. Number designations for peaks are as listed in Table 1. Insert shows magnified portion of chromatogram to indicate where compounds 7 (n-octadecanal) and 8 ((3Z,6Z,9Z)-3,6,9-henicosatriene), eluted
a Correlation between estimated titers of C19 and C21 trienes in individual, unmated, female Mnesampela privata abdominal tip extracts, originating from Tasmania (N = 15), calculated as methyl stearate equivalents (see text). Levels were positively correlated (Pearson's correlation coefficient = 0.956, P < 0.001). b Mean (± SE) estimated titers of C19 and C21 trienes (nanograms) in abdominal tip extracts of unmated female M. privata, originating from Tasmania 1, 3, 5, 7 and 9-d-old and for all moths analysed (C19 triene: N = 10, 7, 10, 5, 5 and 37 females, respectively; C21 triene: N = 2, 4, 5, 3, 1, and 15 females, respectively). Letters above C19 triene means indicate that they were not significantly different by ANOVA (F = 1.4, df = 36, P = 0.256). C21 triene means were not statistically compared due to the small sample size in some age categories
SIM analysis of solvent extracts from the abdominal tips of nine male M. privata from Tasmania, detected C21 triene in six individuals, and (3Z,6Z,9Z)-3,6,9-tricosatriene (C23 triene) was tentatively identified in all individuals. No C19 triene was found in any of the male extracts. Levels of both trienes varied widely between males as did the ratio of C23:C21 triene. Mean estimated of C21 triene titers in extracts of males was 1.56 ng (± 0.3 SE, range = 415 pg to 2.27 ng), which was not significantly different from that found in female extracts (t-test, t = 0.24, df = 19, P > 0.05). Mean estimated titers of C23 triene was 7.33 ng (± 1.7 SE, range 961 pg to 16.71 ng), while the mean ratio of C23:C21 triene was 5.2:1 (± 1.1 SE, range 2.3:1 to 10.6:1).
EAG and GC-EAD
EAG responses of male M. privata antennae to C19 and C21 trienes differed (Fig. 4). Antennal responses to 5 μg of C21 triene were consistently higher than the blank stimulus, but lower than that to 5 μg C19 triene (P < 0.05, LSD). EAG response to C21 triene was greater than that of 1-octadecanol and hexadecyl acetate (P < 0.05, LSD), which elicited an antennal response similar to the blank stimulus. When C21 triene was added to 5 μg C19 triene at a range of ratios, including similar to that found in female extracts, there was a slightly but not significantly enhanced antennal response (P > 0.05, LSD) (Fig. 4).
Mean (± SE) EAG response of male Mnesampela privata antennae to 5 μg of C19 triene, C21 triene, 1-octadecanol (C18 alcohol), hexadecyl acetate (C16 acetate) and various blends of C19:C21 trienes dissolved in hexane, expressed as a percentage of the control stimulus (dashed line). Means with different letters atop bars are significantly different by one-way ANOVA (F = 12.18, df = 75, P < 0.001) followed by the LSD test (P = 0.05). Number of antennae tested: C19 and C21 trienes alone N = 12, C18 alcohol and C16 acetate N = 4 and triene blends N = 8
GC-EAD runs that used the combined abdominal extracts from three female M. privata, which had relatively high levels of C19 and C21 trienes as previously determined by GC-MS, elicited a weak responses (<2 mV) in antennae of males to the C19 triene. No antennal response or FID peak was detected in the region where the C21 triene was expected to elute.
Field Trials
Experiment 1.
Field Bioactivity of C19 and C21 Trienes: Significantly more M. privata males were caught in traps baited with a 40:1 ratio of C19:C21 trienes than in traps baited with C19 triene alone, but there was no significant difference in the number of males caught in traps baited with 1 mg, 5 mg or 10 mg C19 triene (ANOVA, log10 + 1 transformed counts, F = 4.9, df = 62, P < 0.05, followed by LSD test at P = 0.05) (Table 2). The majority of males were caught at Surrey Hills, NW Tasmania, where a high M. privata population developed in late summer 2007. No M. privata were caught in traps placed in plantations in Victoria/South Australia, and trap captures were also low in Western Australia where trials were conducted late in the flight season. Across all plantations (N = 18 locations), M. privata males were caught at 61.1% of locations when traps were baited with a 40:1 blend of C19:C21 trienes compared to 16.7% when traps were baited with 1 mg C19 triene only.
A large number of Androchela smithi McQuillan (Ennominae) also were caught in traps at Surrey Hills, north western Tasmania, during the M. privata flight period; 194 in 1 mg C19 triene only baited traps, and 60 in traps baited with a 40:1 ratio of C19:C21 triene. At this location, three moths of an unidentified geometrid species were also caught in C19 triene traps in March 2008. In traps placed in southern Tasmania, 12 Mnesampela heliochrysa (Lower) were caught in C19 triene only baited traps, but none were caught in 40:1 ratio of C19:C21 triene baited traps
Experiment 2.
Optimal Ratio of C19 and C21 Trienes for Trapping M. privata: A total of 868 male M. privata were caught in five replicate traps of eight test baits between 19 December 2007 and 18 March 2008, at Surrey Hills, NW Tasmania. Lures containing C19 and C21 trienes in a ratio of 16:1, 33:1, and 40:1 caught significantly more M. privata males than those with a 3:2 and 67:1 ratio or C19 triene only (Table 3). Traps baited with C21 triene only (loaded at an amount equivalent to that in the 40:1 ratio C19:C21 triene baits) or hexane only did not catch any male M. privata. When trap catch was expressed as the proportion of male M. privata caught in each bait type at each replicate site, captures in 16:1 ratio of C19:C21 trienes were significantly higher than other blends (Fig. 5).
A total of 73 A. smithi were caught in traps baited with C19 triene only (mean = 14.6 moths/trap) compared to 2, 5, 2, 3, and 2 A. smithi caught in 3:2, 16:1, 33:1, 40:1, and 67:1 ratio of C19:C21 triene, respectively (overall mean for blends = 0.6 moths/trap). While no M. privata were caught in traps baited with C21 triene only, 1 A. smithi (mean = 0.6 moths/trap) and 1 unidentified geometrid were caught. One unidentified species of geometrid also was caught in a trap baited with a 3:2 ratio of C19:C21 triene. No moths were caught in the control traps.
Mean proportion (± SE) of Mnesampela privata males caught at each site (N = 5) in Unitraps baited with 1 mg C19 triene alone, 0.25 μg C21 triene alone, hexane only or 1 mg C19 triene with varying amounts of C21 triene to give indicated ratios. Means with different letters atop bars are significantly different by ANOVA (F = 82.54, df = 39, P < 0.001) followed by the LSD test (P = 0.05). Trapping was conducted between 19 December 2007 and 18 March 2008 in E. nitens plantations at the Surrey Hills estate, NW Tasmania
Experiment 3.
Comparison of Trap Design: The mean number of male M. privata caught in Delta traps (33.0 ± 1.4 SE, range 29–36 moths per trap) was higher than in Unitraps (11.6 ± 2.5 SE, range 7–19 moths per trap) (t-test, t = 7.31, df = 8, P < 0.001).
Experiment 4.
Effect of Lure Age on Trap Catch: There was no significant difference in the number of male M. privata caught in traps baited with new lures of 16:1 C19:C21 trienes (mean = 19.6 ± 1.2 SE) vs. lures aged in the field for 90 days (mean = 23.4 ± 2.1 SE) (t-test, t = 1.12, df = 8, P > 0.05).
Discussion
We identified (3Z,6Z,9Z)-3,6,9- henicosatriene as a second bioactive compound in the sex pheromone of M. privata, in addition to the major component (3Z,6Z,9Z)-3,6,9-nonadecatriene. Field trials in Tasmania demonstrated that the addition of 1–6% of C21 triene to 1 mg C19 triene significantly increased the number of male M. privata caught in traps. Addition of C21 triene to C19 triene also increased the sensitivity of pheromone traps to detect low populations of M. privata in Eucalyptus plantations, and significantly reduced cross-attraction of other geometrid species. Essential minor pheromone components have been documented in several other geometrid species that utilize polyene hydrocarbons. For example, Szöcs et al. (2004) found that captures of male Operophtera fagata Scharf. increased significantly when as little as 0.1% of (1,3Z,6Z,9Z)-nonadecatetraene was added to the main component, (6Z,9Z)-nonadecadiene.
C19 and C21 trienes are components of the female sex pheromones in many species of Geometridae, Noctuidae, Arctiidae, and Lymantriidae (El-Sayed 2008). To our knowledge, identification of both C19 and C21 trienes in the same sex pheromone has been verified in only one other species, Colotois pennaria L. (Szöcs et al. 1993; El-Sayed 2008), which belongs to the same subfamily as M. privata. Millar et al. (1992) identified both C19 and C21 trienes in pheromone gland extracts of Epirrhoe sperryi (H.). However, only C19 triene was attractive in the field, despite EAG activity of C21 triene in the laboratory. Wong et al. (1985) and Millar et al. (1991) reported that males of several other species of Geometridae and Noctuidae responded to both C19 and C21 trienes during electroantennogram testing, but the actual presence of these compounds in the sex pheromone was not determined, nor was the attraction to both trienes tested in the field. Several species of moths utilize blends of polyene hydrocarbons with mixed chain lengths, but usually the blend consists of compounds differing by only one carbon (Millar 2000). Therefore, M. privata and C. pennaria appear to be unique in that they use a blend of 3Z,6Z,9Z trienes that differ by two carbons.
While levels of the C19 and C21 trienes in individual females varied widely, the ratio of these two compounds was more constant and highly correlated (Fig. 3a). In Tasmanian M. privata, the mean ratio of C19:C21 trienes changed linearly from a 29:1 in 1-d-old females to 43:1 in 7-d-old females. Although this difference was not significant here, Allison and Cardé (2006) found that the ratio of pheromone components in a pyralid moth (Cadra cautella (Walker)) changed with female age, and suggested that males could use this to assess the reproductive value of females, which declined markedly with age. If this is the case in M. privata, it may explain why significantly more males were caught in traps baited with a lower ratio of C19:C21 triene (16:1) than in traps baited with a ratio closer to the average found in females (33:1) (see also: Szöcs et al. 1993; Strong et al. 2008).
GC-MS analysis of gland extracts from females caught in a light trap near Albany, Western Australia, found significant differences in the ratio of the two trienes compared to populations from Tasmania. Preliminary field trials in Western Australia suggested that increased trap sensitivity could be gained from the addition of more C21 triene to C19 triene but further research is needed to confirm the optimal ratio of these compounds to use for monitoring male M. privata in this and other geographical areas.
In their trapping study that used C19 triene baits, Steinbauer et al. (2004) reported catching three closely related species of geometrid: Dolabrossa amblopa Guest, Mnesampela heliochrysa, and M. arida McQuillan. In our study, we caught large numbers of A. smithi and M. heliochrysa in Tasmania during the flight period of M. privata. No M. heliochrysa were caught in traps baited with a blend of C19 and C21 trienes thus suggesting that the latter compound may act as a behavioral antagonist to this species. Little is known about the biology of A. smithi, which was described relatively recently and has been previously collected only from the Tasmanian highlands (McQuillan 1996). Captures of A. smithi occurred in traps baited with a range of blends of C19 and C21 trienes, but were significantly lower than in traps baited with C19 triene alone. Similarly, in field trials in Canada, Millar et al. (1992) reported the indiscriminate cross attraction of E. sperryi to lures containing C19 triene regardless of whatever other components were added, suggesting that other physiological or behavioral mechanisms (e.g., closely synchronized female calling and male response) minimize male attraction to pheromone blends produced by female moths of sympatric species.
Our discovery that male M. privata also produce the C21 triene found in females is similar to the finding of Heath et al. (1988). They also found that abdominal tip extracts from males of the noctuid Anticarsa gemmatalis (Hübner) contained C21 triene, which is a major component of the female sex pheromone of this species, and suggested that it is a hairpencil compound used during courtship.
In summary, we recommend that traps used to monitor M. privata populations in Tasmania be baited with lures that contain both C19 and C21 trienes, at a ratio of 16:1. However, further research is needed to verify whether this is an optimal ratio of trienes to use for monitoring M. privata in other geographical areas. We also recommend the Delta trap design for monitoring M. privata in preference to the Unitrap design due to lower costs and more efficient capture rates. In the light of the recommendation to include C21 triene in the pheromone bait, the relationship between trap catch and subsequent M. privata egg and larval infestations needs to be re-examined as Östrand’s et al. (2007) trapping studies were conducted with baits containing C19 triene only. The use of synthetic pheromone for the control of M. privata, through mating disruption or mass trapping, is not economically feasible at present given the high costs associated with the synthesis of the C19 and C21 trienes and the sporadic nature of this pest.
References
ALLISON J. D. and CARDÉ, R.T. 2006. Heritable variation in the sex pheromone of the almond moth, Cadra cautella. J. Chem. Ecol. 32:621–641.
Bashford, R. 1993. Insect pest problems of eucalypt plantations in Australia. 4. Tasmania. Aust. For. 56:375–377.
CARDÉ, R.T. and BAKER, T.C. 1984. Sexual communication with pheromones, pp. 355–383, in W.J. Bell and R.T. Cardé (eds.). Chemical ecology of insects. Chapman and Hall, London.
DAVIES, N.W., MEREDITH, G., MOLESWORTH, P.P., and SMITH, J.A. 2007. Use of anti-oxidant BHT in situ for the synthesis of readily oxidised compounds: Application to the synthesis of the moth pheromone (Z,Z,Z)-nonadecanonadeca-3,6,9-triene. Aust. J. Chem. 60: 848–849.
El-Sayed, A.M. 2008. The pherobase: database of insect pheromones and semiochemicals. <http://www.pherobase.com>. ©2003–2008 The pherobase—extensive database of insect pheromones and semiochemicals.
Heath, R.R., Landolt, P.J., Leppla, N.C., and Dueben, B.D. 1988. Identification of a male-produced pheromone of Anticarsia gemmatalis (Hübner) (Lepidoptera: Noctuidae) attractive to conspecific males. J. Chem. Ecol. 14:1121–1130.
Mcquillan, P. B. 1996. The Tasmanian geometrid moths associated with the genus Amelora auctorum (Lepidoptera: Geometridae: Ennominae). Invertebr. Taxon. 10:433–456.
Millar, J.G. 2000. Polyene hydrocarbons and epoxides: A second major class of lepidopteran sex attractant pheromones. Annu. Rev. Entomol. 45:575–604.
MILLAR, J.G., GIBLIN, M., BARTON, D., and UNDERHILL, E.W. 1990. 3Z,6Z,9Z-nonadecatriene and enantiomers of 3Z,9Z-cis-6,7-epoxy-nonadecadiene as sex attractants for two geometrid and one noctuid moth species. J. Chem. Ecol. 16:2153–2166.
Millar, J.G., Giblin, M., Barton, D., Wong, J.W., and Underhill, E.W. 1991. Sex attractants and sex pheromone components of noctuid moths Euclidea cuspidea, Caenurgina distincta, and geometrid moth Eupithecia annulata. J. Chem. Ecol. 17:2095–2111.
Millar, J.G., Giblin, M., Barton, D., and Underhill, E.W. 1992. Sex pheromone components of the geometrid moths Lobophora nivigerata and Epirrhoe sperryi. J. Chem. Ecol. 18:1057–1068.
Östrand, F., Elek, J.A., and Steinbauer, M.J. 2007. Monitoring autumn gum moth (Mnesampela privata): relationships between pheromone and light trap catches and oviposition in eucalypt plantations. Aust. For. 70:185–191.
Rapley, L.P., Allen, G.R., Potts, B.M., and Davies, N.W. 2007. Constitutive or induced defences—how does Eucalyptus globulus defend itself from larval feeding? Chemoecology. 17:235–243.
RAPLEY, L.P., POTTS, B. M., BATTAGLIA, M. B., PATEL V.S., and ALLEN, G. R. 2009. Long-term realised and projected growth impacts caused by autumn gum moth defoliation of 2-year-old Eucalyptus nitens plantation trees in Tasmania, Australia. For. Ecol & Manag. 258:1896–1903.
Szöcs, G., Tóth, M., Francke, W., Schmidt, F., Philipp, P., König, W.A., Mori, K., Hansson, B.S., and Löfstedt, C. 1993. Species discrimination in five species of winter-flying geometrids (Lepidoptera) based on chirality of semiochemicals and flight season. J. Chem.Ecol. 19:2721–2735.
Szöcs, G., Tóth, M., Karpati, Z., Zhu, J.W., Löfstedt, C., Plass, E., and Francke, W. 2004. Identification of polyenic hydrocarbons from the northern winter moth, Operophtera fagata, and development of a species specific lure for pheromone traps. Chemoecology. 14:53–58.
Steinbauer, M.J., McQuillan, P.B., and Young, C.J. 2001. Life history and behavioural traits of Mnesampela privata that exacerbate population responses to eucalypt plantations: comparisons with Australian and outbreak species of forest geometrid from the northern-hemisphere. Austr. Ecol. 26:525–534.
Steinbauer, M.J., Östrand, F., Bellas, T.E., Nilsson, A., Andersson, F., Hedenström, E., Lacey, M.J., and Schiestl, F.P. 2004. Identification, synthesis and activity of sex pheromone gland components of the autumn gum moth (Lepidoptera: Geometridae), a defoliator of Eucalyptus. Chemoecology 14:217–223.
STRONG, W.B., MILLAR, J. G., GRANT, G.G., MOREIRA, J.A., CHONG, J.M., and RUDOLPH, C. 2008. Optimization of pheromone lure and trap design for monitoring the fir coneworm, Dioryctria abietivorella. Entomol. Exp. Appl. 126: 67–77.
Wang, S. and Zhang, A. 2007. Facile and efficient syntheses of (3Z,6Z,9Z)-3,6,9-nonadecatriene and homologues: Pheromone and attractant components of Lepidoptera. J. Agric. Food Chem. 55:6929–6932.
Wong, J.W., Underhill, E.W., MacKenzie, S.L., and Chisholm, M.D. 1985. Sex attractants for geometrid and noctuid moths. Field trapping and electroantennographic responses to their hydrocarbons and monoepoxydiene derivatives. J. Chem. Ecol. 11:727–756.
Acknowledgements
This research was funded by the Australian Research Council Linkage Projects grant LP0455303. We thank Gunns Ltd, Forestry Tasmania, Timbercorp, WAPRES, and FFIC for funding and assistance. PPM is thankful to the University of Tasmania and the Thomas Crawford Foundation for a postgraduate scholarship. Thanks also to: Dr. Peter McQuillan (University of Tasmania) and Dr. Cathy Young (Tasmanian Museum and Art Gallery) for identifying geometrids, Dr. Mamoru Matsuki (IPMG, Albany) for conducting trapping trials in Western Australia and supplying moths for analysis, Mr. Ben Bradshaw (Timbercorp, Penola) for conducting trapping trials in Victoria and South Australia, and Dr. Martin Steinbauer for help with comments on the manuscript and the formulation of the original grant. We are also grateful for the financial support (for AN, FA, and EH) from the European Regional Development Fund and Länsstyrelsen i Västernorrlands län.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walker, P.W., Allen, G.R., Davies, N.W. et al. Identification, Synthesis and Field Testing of (3Z,6Z,9Z)-3,6,9-Henicosatriene, a Second Bioactive Component of the Sex Pheromone of the Autumn Gum Moth, Mnesampela privata . J Chem Ecol 35, 1411–1422 (2009). https://doi.org/10.1007/s10886-009-9717-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9717-2