Abstract
Leucoptera sinuella is a leaf-miner moth present in several regions in the world, which has been recently introduced into Chile. The larvae feed exclusively on the leaves of poplar and willow trees, and the damage caused by the feeding behavior poses a threat to the wood-producing industry. Besides, L. sinuella larvae invade nearby orchards for pupation, causing rejections in Chilean fresh fruit for export. Here we report the identification of the female-produced sex pheromone of L. sinuella as a first step towards the development of pheromone-based methods for pest management of this species. First, we analyzed hexane extracts of the abdominal glands of virgin females by gas chromatography coupled with mass spectrometry and identified the major compound in these extracts to be 3,7-dimethylpentadecane, while minor compounds in the extracts proved to be 3,7-dimethyltetradecane and 7-methylpentadecane. Structure assignments were carried out by comparison of retention times and mass spectra of the natural products with those of authentic reference samples. Second, we conducted field tests, which showed that traps baited with synthetic 3,7-dimethylpentadecane were significantly attractive to males in a dose-dependent response. Our results also showed that a mixture of 3,7-dimethylpentadecane, 3,7-dimethyltetradecane, and 7-methylpentadecane in proportions similar to those found in gland extracts was the most attractive lure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leucoptera sinuella (Reutti) (Lepidoptera: Lyonetiidae) is a small leaf-mining moth present in Asia, Europe, and northern Africa. In 2015 it was detected for the first time in Chile in the Metropolitan region in the vicinity of Santiago and since then has spread to the adjacent regions of Valparaíso, O’Higgins, Maule, and Biobio (Sandoval et al. 2019). The principal host plants are poplar (Populus spp.) and willow (Salix spp.) trees (Salicaceae), which are important species for the production of wood and derived products. The damage in the host plants is due to the leaf-mining habit of the larvae, which leads to loss of foliage, reducing the photosynthetic capacity of the plants. Additionally, it has been observed that upon completing development, the larvae migrate to nearby fruit tree plantations, seeking protected places to pupate (Fuentes-Contreras 2017, 2020; Sandoval et al. 2019). The presence of L. sinuella pupae in fruit crops causes the rejections of exportations to the USA and México. For example, in the season 2019–2020, a total of 78,570 boxes of apples, oranges, peaches, pears, persimmon fruits, and plum have been rejected for exportation because of the presence of L. sinuella pupae (Fuentes-Contreras 2020).
The adults of L. sinuella are of white color, with a body size of 3–4 mm and a wing span of ca. 1 cm. Females lay eggs in groups on the leaves close to the midvein. Larvae are small (up to 7 mm length), of pale yellowish color, and presenting a flattened body. They are encountered in groups feeding in the mesophyll of the leaves. Pupae are found on the surface of the leaves or in small cracks in the bark, covered by a white silk case (cocoon). When larvae invade orchards, pupae have been found mainly on fruits, leaves, and packing materials (pallets, boxes, etc.). In central Chile, there are at least three generations per year with male flights occurring mainly during spring (late September) and summer (late December and late January-early February) (Fuentes-Contreras 2017, 2020; Sandoval et al. 2019).
Leucoptera sinuella is not of major economic concern in the regions in which it has been established previously (Europe and Asia), and a control program using traditional insecticides or benign alternatives have not been developed yet. Only a very small number of natural enemies are known in Chile (due to the recent introduction), and biological control has not fully achieved its potential (Fuentes-Contreras 2017, 2020; Sandoval et al. 2019). Pheromone-based management tactics have the potential to contribute to integrated pest management programs, and as a first step towards the implementation of more sustainable methods, we report here on the identification, synthesis, and field evaluation of sex pheromone components of L. sinuella.
Methods and Materials
Insects
Diapausing pupae of L. sinuella were collected from silk cocoons on Populus spp. bark and leaves and from corrugated cardboard strips during March 2017 in Coltauco, O’Higgins region, and during March 2018 from campus Talca, Universidad de Talca, Maule region, Chile. Pupae were kept under dark conditions and 5 °C during four months to simulate winter diapause. Later the pupae were transferred to controlled conditions (25 °C and photoperiod of 16:8 [L:D]) to promote adult emergence and to use the females for pheromone gland extraction. During 2019–2020 spring and summer, pupae were collected from cocoons located in leaves and bark of Populus spp. These pupae were maintained under controlled conditions (25 °C and photoperiod of 16:8 [L:D]) to obtain virgin adults females used in extract preparation and field trapping experiments. Pupae were sexed based on examination of terminalia under stereoscopic microscope. Females showed the genital opening on the 8th and extending into the anterior region of the 9th abdominal segment, while males showed the genital opening on the 9th abdominal segment, surrounded by prominent tubercules.
Extraction of Glands
For the preparation of gland extracts, 1–3 d old virgin females were used. The abdominal pheromone glands were removed after gently squeezing the abdomen and extracted for 15 min in hexane (Suprasolv, Merck, Germany; 10 μL per gland). After this time, the solvent was transferred to another vial and stored at −20 °C until analysis. Several glands (between 2 to 12 glands, depending on the availability of adult virgin females) were pooled for the preparation of an extract. Several extracts were obtained following this procedure, which were used for the different analyses.
Chemical Analyses
Analysis by gas chromatography coupled with mass spectrometry (GC/MS) was carried out using a Shimadzu GCMS-QP2010 Ultra combination equipped with a fused silica Rtx-5MS capillary column (30 m × 0.25 mm id, 0.25 μm film, Restek, Bellefonte, PA, USA) or with a Stabilwax capillary column (30 m × 0.32 mm id, 0.25 μm film, Restek). The oven was programmed from 50 °C (5 min hold) to 270 °C at 8 °C min−1 (Rtx-5MS) or from 50 °C (5 min hold) to 220 °C at 8 °C min−1 (Stabilwax). The GC was operated in split/splitless mode (30 s sampling time) with an injector temperature of 200 °C. Helium was used as the carrier gas at a flow rate of 36 cm min−1 (Rtx-5MS) or 43 cm min−1 (Stabilwax). Electron impact mass spectra were acquired at 70 eV. Resolution of stereoisomers was attempted using a gas chromatograph Shimadzu GC-2010, equipped with fused silica capillary columns coated with permethylated β-cyclodextrin added into 14% cyanopropylphenyl/86% dimethyl polysiloxane (Rt-βDEXm, 30 m × 0.25 mm id, 0.25 μm film, Restek) or 2,3-di-O-methyl-6-O-TBDMS-β-cyclodextrin embedded in an intermediate polarity phase (β-DEX 325, 30 m × 0.25 mm id, 0.25 μm film, Supelco, Bellefonte, PA, USA), using helium as carrier gas at a flow rate of 25 or 30 cm min−1 and various isothermal conditions between 90 and 120 °C.
Synthesis
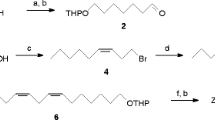
The synthetic route to a stereoisomeric mixture of 3,7-dimethylpentadecane (3,7-dime-C15) was modified from a synthesis for 5,9-dimethylalkanes reported by Liang et al. (2000) and is shown in Fig. 1. Experimental details are described in the Supplementary Information. Briefly, citronellyl tosylate (2), obtained by reaction of citronellol (1) with p-toluenesulfonyl chloride (TsCl), was reduced with lithium aluminum hydride (LAH) to give 2,6-dimethyl-2-octene (3). Allylic oxidation of 3 with selenium dioxide catalyzed by tert-butyl hydroperoxide (Umbreit and Sharpless 1977) yielded a mixture of 2,6-dimethyl-2-octen-1-ol (4) and 2,6-dimethyl-2-octenal (5), plus other byproducts which were not further characterized. After chromatographic separation of the products, pyridinium dichromate (PDC) was used to oxidize alcohol 4 to aldehyde 5, which was employed in a Wittig reaction with the ylide generated by treatment of heptyltriphenylphosphonium bromide with butyl lithium (BuLi). The resulting stereoisomeric mixture of 3,7-dimethyl-6,8-pentadecadiene (6) was hydrogenated to yield a mixture of all stereoisomers of the target compound. The purity of the compound (as determined by GC) was 95%. Main impurities were 3,7-dimethyltetradecane (1.7%) and n-tetradecane (1.3%), plus minor amounts of other hydrocarbons. 1H-NMR (300 MHz, CDCl3): δ = 1.43–1.04 (m, 24H), 0.94–0.83 (m, 12H) - 13C-NMR (75 MHz, CDCl3): δ = 37.5, 37.4, 37.2, 37.1, 37.0, 36.9, 34.4, 32.79, 32.76, 32.0, 30.0, 29.7, 29.6, 29.5, 29.4, 27.1, 24.5, 22.7, 19.8, 19.7, 19.3, 19.2, 14.1, 11.42, 11.40 - MS (70 eV): 41 (36), 43 (62), 55 (25), 57 (100), 71 (90), 85 (65), 97 (15), 99 (14), 113 (6), 126 (5), 127 (12), 140 (10), 141 (7), 155 (2), 183 (2), 211 (3), 240 (<1).
A stereoisomeric mixture of 3,7-dimethyltetradecane (3,7-dime-C14) was synthesized as described above, employing the ylide of hexyltriphenylphosphonium bromide in the Wittig reaction. Again, hydrogenation of the resulting diene yielded all stereoisomers of the target compound, the purity of which was 96%. Main impurities were 3,7-dimethyltridecane (1.3%) and n-dodecane (1.1%), plus minor amounts of other hydrocarbons. 1H-NMR (300 MHz, CDCl3): δ = 1.44–1.02 (m, 22 H), 0.95–0.82 (m, 12H) - 13C-NMR (75 MHz, CDCl3): δ = 37.5, 37.4, 37.2, 37.1, 37.0, 36.9, 34.4, 32.79, 32.77, 32.0, 30.0, 29.6, 29.5, 29.4, 27.1, 24.5, 22.7, 19.8, 19.7, 19.3, 19.2, 14.1, 11.4 - MS (70 eV): 41 (38), 43 (66), 55 (24), 57 (95), 71 (100), 85 (63), 97 (15), 113 (5), 126 (16), 127 (16), 141 (3), 155 (1), 169 (2), 197 (3), 211 (1), 226 (<1).
The synthesis of racemic 7-methylpentadecane (7-me-C15) was achieved by Wittig reaction of 2-octanone and the ylide of octyltriphenylphosphonium bromide, followed by hydrogenation of the resulting alkene. Purity: >99% (GC). 1H-NMR (300 MHz, CDCl3): δ = 1.40–1.05 (m, 25 H), 0.95–0.83 (m, 9H) - 13C-NMR (75 MHz, CDCl3): δ = 37.1 (2C), 32.8, 32.0, 31.9, 30.0, 29.7 (2C), 29.4, 27.09, 27.05, 22.7 (2C), 19.7, 14.1 (2C) - MS (70 eV): 41 (34), 43 (66), 55 (20), 57 (100), 71 (86), 85 (39), 99 (10), 112 (21), 113 (7), 140 (13), 141 (6), 211 (1), 226 (<1).
Field Tests
Field tests were carried out at the following locations: 1) Campus Antumapu, College of Agriculture, University of Chile, Metropolitan region (33°34′S, 70°37′W), 2) Coltauco, O’Higgins region (34°17’S, 71°05’O), and 3) Estación Experimental Panguilemo of Universidad de Talca, Maule region (35°21’S, 71°35’W). Traps were placed in infested poplar trees at ca. 2 m height in randomized complete block designs with a distance of ca. 10 m between traps within a block (Bacca et al. 2006) and ca. 50 m between blocks. Red delta traps (Alphascent, West Linn, OR, USA, catalog #AST0091-G) and hot melt pressure adhesive liners (Alphascent, West Linn, OR, USA, catalog #AST0014-G) were used. Lures containing synthetic compounds were prepared by loading white rubber septa (Sigma-Aldrich, St. Louis, MO, USA, catalog #Z553905) with 100 μL of an appropriate solution of pure or mixtures of synthetic compounds in hexane (Merck, Darmstadt, Germany). Septa treated with 100 μL hexane were used as control. Virgin females were placed in stainless steel ball tea strainers inside the traps. To prevent small birds to get into the traps, a plastic screen (mesh 7 × 7 mm) was placed over each end of the delta traps.
Experiment 1
Preliminary experiments had shown that the presence of 3,7-dime-C15 alone is sufficient to attract male L. sinuella. In experiment 1, we examined dose effects of 3,7-dime-C15 on captures of males. Virgin females were included as positive control. Treatments were 1) 1000 μg 3,7-dime-C15, 2) 100 μg 3,7-dime-C15, 3) 10 μg 3,7-dime-C15, 4) hexane control, and 5) two virgin females. The females were 2 d old when placed in the field. This field trial was carried out at the sites in Panguilemo (December 10 to 20, 2019), Antumapu (January 17 to 27, 2020), and Coltauco (January 14 to 24, 2020). Five replications were used for each treatment with the exception of Antumapu, which had three replications per treatment. Every 3–4 days, captured moths were removed from traps, the trap location was rotated within each block, and females were replaced.
Experiment 2
In experiment 2 we examined the effect of the presence of the minor compounds 3,7-dime-C14 and 7-me-C15 in mixtures with the main compound, 3,7-dime-C15, on captures of males, as well as the attractiveness of the minor compounds 3,7-dime-C14 and 7-me-C15 alone. Because of the presence of ca. 2% 3,7-dime-C14 in synthetic 3,7-dime-C15, the treatments containing 3,7-dime-C15 are binary and ternary mixtures. Applying total amounts of 1000 μg per lure, treatments were 1) 980 μg 3,7-dime-C15 + 20 μg 3,7-dime-C14, 2) 880 μg 3,7-dime-C15 + 120 μg 3,7-dime-C14, 3) 950 μg 3,7-dime-C15 + 20 μg 3,7-dime-C14 + 30 μg 7-me-C15, 4) 1000 μg 3,7-dime-C14, 5) 1000 μg 7-me-C15, and 6) hexane control. Because of the presence of 3,7-dime-C14 as an impurity in 3,7-dime-C15 at a level similar to that found found in gland extracts, we incorporated treatment 2, containing a major amount proportion of 3,7-dime-C14. The experiments were carried out at the three sites in Panguilemo (January 28 to February 11, 2020), Antumapu (January 31 to February 17, 2020), and Coltauco (January 17 to 28, 2020). Five replications were used for each treatment, with the exception of Antumapu, which had three replications per treatment, but lacked treatment 5. Captured moths were removed from traps every 3–4 days, and the trap location was rotated within each block on that occasion.
Statistical Analysis
Field data (expressed as cumulative catches) were analyzed with generalized linear mixed models (GLMM) using the package lme4 (Bates et al. 2015) in R (R Core Team 2020). A negative binomial distribution with a loge link function was used by controlling over-dispersion of the male catch. The means obtained were obtained by the inverse of the link function on the linear predictor and the standard errors were calculated using the Delta method (Agresti 2013). To verify if there were significant differences between the treatments, the Wald Chi-Squared test was used. In the case of finding differences, Fisher’s LSD (least significance difference) test for multiple comparisons (P < 0.05) was used.
Results
Chemical Analyses of Gland Extracts
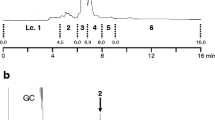
GC/MS analyses of gland extracts consistently revealed the presence of one major compound (A) and two minor compounds (B and C) (Fig. 2), with an approximate relative abundance (±SD) of 96 ± 1.1, 1 ± 0.4, and 3 ± 0.9 (n = 8). The retention indices were 1605 (Rtx-5MS) and 1600 (Stabilwax) for compound A, 1503 (Rtx-5MS) and 1502 (Stabilwax) for B, and 1541 (both stationary phases) for C. The almost identical retention indices on the apolar and polar stationary phases pointed to compounds without functional groups, and concomitantly, the mass spectra of all three compounds showed fragmentation patterns characteristic of branched, saturated hydrocarbons (Pomonis et al. 1980). The mass spectrum of A (Fig. 3) showed a molecular ion at m/z 240, indicating a molecular formula of C17H36. The diagnostic fragment at m/z 211 (M-29) implied the loss of an ethyl group, which is consistent with a methyl group in position 3 of the main chain. Two additional diagnostic fragments at m/z 127 and 140 pointed to a second methyl branching in either position 7 or 8. Considering known biosynthetic pathways for methyl-branched alkanes (Juárez et al. 1992), the 3,7-branching pattern appeared to be more plausible than a 3,8-branching, so we synthesized 3,7-dime-C15. The mass spectrum and retention times of the synthetic compound were an exact match of those of compound A, thus confirming its identity. The amount of 3,7-dime-C15 in the pheromone gland extracts was determined to be 0.42 ± 0.38 ng/gland (n = 6, ranging from 0.12 to 0.85 ng). Unfortunately, the stereoisomers of 3,7-dime-C15 were not resolved on any of the columns and conditions used for analysis.
The retention indices of compound B were roughly 100 units less than those of A, suggesting it to be a nor-analogue of A. The mass spectrum showed a molecular ion at m/z 226, indicating a molecular formula of C16H34. Similar as for compound A, the diagnostic fragments m/z 197 (M-29) and 126 indicated methyl branches at carbons 3 and 7, respectively, suggesting 3,7-dime-C14 as the structure of this compound. The identity of B was confirmed by matching mass spectra and retention times with those of an authentic standard.
Compound C eluted almost halfway between compounds A and B, indicating a different methyl-branching pattern. The mass spectrum exhibited a molecular ion at m/z 226 (molecular formula C16H34) and diagnostic fragments at m/z 112 and 140, which suggested 7-me-C15 as its likely structure. Again, comparison of the analytical data of the natural compound with those of a synthetic sample confirmed the structure proposal.
Field Tests
In experiment 1, a total of 1200 moths were captured at the three test sites (279 in Panguilemo, 220 in Antumapu, and 701 in Coltauco). At all three locations, the 1000 μg dose of 3,7-dime-C15 captured significantly more males than any of the other treatments (Fig. 4). Traps containing lures loaded with 100 μg of 3,7-dime-C15 captured significantly more males than control traps at all sites. The results from all three sites also showed that the 100 μg dose was equally attractive as the virgin females, which in turn captured more males than the 10 μg dose and the control traps in Coltauco and more than the control traps in Panguilemo (Fig. 4).
Cumulative captures of male Leucoptera sinuella in traps (n = 3–5) baited with synthetic compounds and virgin females at different locations (A: Coltauco, B: Antumapu, C: Panguilemo). Treatments: T1 = 1000 μg 3,7-dimethylpentadecane (3,7-dime-C15), T2 = 100 μg 3,7-dime-C15, T3 = 10 μg 3,7-dime-C15, T4 = blank, T5 = virgin female. Columns with different letters are significantly different according to Fisher’s LSD test (P < 0.05)
A total of 3288 males were captured in experiment 2 (987 in Panguilemo, 476 in Antumapu, and 1825 in Coltauco). All treatments containing synthetic compounds were more attractive than the control traps at all locations (Fig. 5). Throughout all experimental sites, the highest numbers of males were captured in traps baited with the ternary blend T3 (3,7-dime-C15 + 3,7-dime-C14 + 7-me-C15, 95:2:3), and this treatment was significantly more attractive than the other treatments with the exception of T1 (3,7-dime-C15 + 3,7-dime-C14, 98:2) in Panguilemo and T2 (3,7-dime-C15 + 3,7-dime-C14, 88:12) in Antumapu. With two exceptions (T1 in Coltauco and T2 in Panguilemo), all treatments containing the main compound 3,7-dime-C15 were more attractive than the minor compounds 3,7-dime-C14 and 7-me-C15 alone.
Cumulative captures of male Leucoptera sinuella in traps (n = 3–5) baited with synthetic compounds at different locations (A: Coltauco, B: Antumapu, C: Panguilemo). Treatments: T1 = 980 μg 3,7-dime-C15 + 20 μg 3,7-dimethyltetradecane (3,7-dime-C14), T2 = 880 μg 3,7-dime-C15 + 120 μg 3,7-dime-C14, T3 = 950 μg 3,7-dime-C15 + 20 μg 3,7-dime-C14 + 30 μg 7-methylpentadecane (7-me-C15), T4 = 1000 μg 3,7-dime-C14, T5 = 1000 μg 7-me-C15, T6 = blank. The field trial conducted in Antumapu lacked treatment T5. Columns with different letters are significantly different according to Fisher’s LSD test (P < 0.05)
Discussion
We have identified three methyl-branched hydrocarbons as sex pheromone components of Leucoptera sinuella, including 3,7-dimethylpentadecane as the major component. To our knowledge, this compound has not been described as an insect pheromone component before. It is, however, consistent with structures of pheromones identified from other species of the genus Leucoptera, as the main components of the sex pheromones of L. coffeella and L. malifoliella (= scitella) are 5,9-dimethylpentadecane (Francke et al. 1988) and 5,9-dimethylheptadecane (Francke et al. 1987), respectively. Additionally, both species share 5,9-dimethylhexadecane as a minor compound (Francke et al. 1988), and L. malifoliella produces also 5,9-dimethyloctadecane as a minor component (Riba et al. 1990). Furthermore, the pheromones of two other related species from the family Lyonetiidae are also methyl-branched hydrocarbons. Females of Lyonetia prunifoliella produce 10,14-dimethyl-1-octadecene as the main component, plus 5,9-dimethylheptadecane and 5,9-dimethyloctadecane as minor components (Gries et al. 1997). The pheromone of Lyonetia clarkella has been identified as 14-methyl-1-octadecene (Sugie et al. 1984). Another lyonetiid pheromone was reported to contain unsaturated, unbranched nitrate esters (Hall et al. 1992). Outside the Lyonetiidae, a few other methylated hydrocarbon pheromones have been identified from Lepidoptera (Roelofs and Cardé 1971; Gries et al. 1991, 1993a, 1993b, 1994), including a male pheromone component from Galleria mellonella (Svensson et al. 2014).
Our first field trial evaluated the dose effect of the main compound 3,7-dime-C15 on captures of males and included virgin females as positive control. At all three experimental sites, traps baited with 1000 μg of 3,7-dime-C15 were more attractive to males than traps containing 100 μg, 10 μg, virgin females, and hexane. We also observed that the 100 μg doses captured significantly more males than the 10 μg doses at two locations and that this treatment was more attractive than control traps at all sites. Finally, the 10 μg doses were more attractive than the controls in two experiments. These results show that there is a dose-dependent response of male L. sinuella to synthetic 3,7-dime-C15. Traps containing virgin females captured more males than control traps, confirming that females were actually releasing pheromone during the experiment. Captures in these traps were comparable to the 100 μg and 10 μg doses and much inferior to the 1000 μg dose, suggesting that this latter dose is a more powerful attractant than virgin females. The results using virgin females are quite consistent between the experimental sites, however, it should be kept in mind that the confinement of the females in the traps exposes them to conditions which may alter their natural behavior, including the release of pheromone.
The second field trial was designed to explore the biological activity of the minor compounds 3,7-dime-C14 and 7-me-C15, either in mixtures with the main compound or alone. The interpretation of the results of these experiments must consider that our synthetic 3,7-dime-C15 contained ca. 1.7% of 3,7-dime-C14, which is just about the proportion we have found to be present in female glands. Consequently, it becomes clear that this small percentage of 3,7-dime-C14 in 3,7-dime-C15 is biologically relevant. The first treatment (T1), a 98:2 binary blend of 3,7-dime-C15 and 3,7-dime-C14, is a binary blend in proportions similar to those found in gland extracts. In order to explore if greater amounts of 3,7-dime-C14 have an effect on the attractivene ss of the lure, we included a 88:12 blend of these two compounds (T2). The third treatment (T3) is a 95:2:3 ternary mixture of 3,7-dime-C15, 3,7-dime-C14, and 7-me-C15, which is very close to the natural composition of these compounds. The results of the field tests at the three locations show various similarities. First, the ternary blend (T3) is the most attractive treatment at all three test sites. In two of the trials, there was one treatment each (T2 in Antumapu and T1 in Panguilemo, respectively) which was not statistically different from T3, but this latter consistently attracted the largest number of males. This suggests that at least 7-me-C15 has a synergistic effect when present together with 3,7-dime-C15. We cannot draw conclusions about the possible effect of the other minor compound 3,7-dime-C14, because the responses to treatments T1 and T2 containing different proportions of 3,7-dime-C15 and 3,7-dime-C14 are not consistent between the three sites. Second, all treatments containing synthetic compounds are more attractive than control traps, and third, with the exception of T1 in Coltauco and T1 and T2 in Panguilemo, the mixtures containing the main compound 3,7-dime-C15 are more attractive than the minor compounds alone. These two observations show that males are able to perceive all compounds tested and suggest that these are attractive rather than neutral or inhibitory, with the highest response to the compound with the “correct” structure. The difference between the structures of the main and the two minor compounds is just a methyl group, and the results of the field tests suggest that this difference is sufficient to allow discrimination between the compounds.
In other lyonetiid species it was observed that minor components do exert a synergistic effect. While no information is available about a possible effect of the minor compound on the attraction of male L. coffeella to the main pheromone compound, Riba et al. (1990) reported that in high-density populations of L. malifoliella (captures ranging from ca. 8–16 males per trap per day), the addition of 0.1 to 5% of the minor component 5,9-dimethyloctadecane to the main component 5,9-dimethylheptadecane significantly enhanced the attraction of males to traps when compared to traps baited with the main component alone. In lower-density populations (captures ca. 5–6 males per trap per day), a synergistic effect was observed only in lures containing 5% of the minor component. In Lyonetia prunifoliella, the presence of the main compound and at least one of the minor compounds is necessary to attract males, and a ternary blend is more attractive than the binary blends (Gries et al. 1997).
Because of the presence of two chiral carbon centers in 3,7-dime-C15 and 3,7-dime-C14, both of these hydrocarbons exist as four possible stereoisomers. Similarly, 7-me-C15 possesses one chiral carbon, and, consequently, two enantiomers are possible. The stereoisomers of these compounds were not resolved under any of the conditions used in our analysis, and the absolute configuration of the female-produced compounds could not be determined. However, the stereoisomeric mixtures of each compound used in field tests were attractive to males, indicating that the mixture is appropriate for use in pheromone-based monitoring of the pest. Similar results were obtained for L. coffeella (Zarbin et al. 2004) and L. malifoliella (Francke et al. 1987), where a stereoisomeric mixture of the respective main pheromone component was attractive to males. The absolute configuration of the female-produced compound has not been determined in neither L. coffeella nor in L. malifoliella, but the biological activity of single isomers of the pheromones of these two species has been examined in field tests. In a study carried out in Hungary, it was shown that males of L. malifoliella were attracted only to (5S,9S)-5,9-dimethylheptadecane, while no males were caught in traps baited with any of the other isomers. Addition of any of the isomers, or of a mixture of the three remaining isomers, to the pure (5S,9S)-isomer did not alter trap catches (Toth et al. 1989). In the case of L. coffeella, a study performed in Mexico showed that traps baited with (5S,9R)-5,9-dimethylpentadecane caught significantly more males than traps baited with the (5R,9S)- or (5R,9R)-isomer, while the (5S,9S)-isomer had intermediate catches (Malo et al. 2009). The biological activity of the stereoisomers of the main pheromone component of Lyonetia prunifoliella have been evaluated in field tests in Korea and Japan, showing that males in both countries were strongly attracted to (10S,14S)-10,14-dimethyl-1-octadecene, while the other isomers were either only weakly or not attractive and did not show a synergistic or inhibitory effect (Park et al. 2002; Taguri et al. 2014). The pheromone of Lyonetia clerkella, 14-methyl-1-octadecene, has only one chiral center, and field tests with pure synthetic enantiomers showed that males were strongly attracted to the (S)-isomer, while the (R)-isomer was only weakly attractive (Sato et al. 1985).
The compounds identified here and from other lyonetiid species, together with other methyl-branched structures have recently been proposed to be classified as Type III lepidopteran pheromones (Löfstedt et al. 2016), in addition to the Type I and Type II classification established earlier (Ando et al. 2004). The biosynthesis of Type III structures is probably similar to the de novo synthesis of fatty acids (and ultimately hydrocarbons), and likely occurs outside the pheromone gland, presumably in oenocyte cells (Löfstedt et al. 2016). Repetitive chain elongation steps using acetate or malonate units assembles the chain, and the incorporation of propionate or methylmalonate accounts for the methyl branching in the final molecules (Juárez et al. 1992). This pathway leads to structures in which the methyl branching points in the main chain are separated by an odd number of methylene groups, which is the case in the known Leucoptera pheromones.
In summary, by means of chemical analysis, synthesis, and field tests we have identified the main sex pheromone component of L. sinuella as 3,7-dimethylpentadecane. Minor compounds produced by females are 3,7-dimethyltetradecane and 7-methylpentadecane. Our results suggest that at least the latter has a synergistic effect on the attraction of males. Future studies will be directed at evaluating the biological activity of pure stereoisomers and the potential for the use of the pheromone in pest management programs. Reports on the use of sex pheromones in monitoring and mating disruption have been published for a few lyonetiids affecting agricultural crops such as coffee (Bacca et al. 2006) and peach (Nakano et al. 2014). The development of management tools based on the pheromone of L. sinuella could contribute to the reduction of Populus spp. defoliation that affects timber production and to the reduction of export fruit rejections without the need to eliminate poplar and willow trees from windbreak barriers of fruit orchards in Chile, thus, providing more sustainable alternatives for the management of this pest.
References
Agresti A (2013) Categorical data analysis, 3rd edn. John Wiley & Sons, Inc., Hoboken, New Jersey, USA
Ando T, Inomata S, Yamamoto M (2004) Lepidopteran sex pheromones. Top Curr Chem 239:51–96. https://doi.org/10.1007/b95449
Bacca T, Lima ER, Picanco MC, Guedes RNC, Viana JHM (2006) Optimum spacing of pheromone traps for monitoring the coffee leaf miner Leucoptera coffeella. Entomol Exp Appl 119:39–45. https://doi.org/10.1111/j.1570-7458.2006.00389.x
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Francke W, Franke S, Toth M, Szӧcs G, Guerin P, Arn H (1987) Identification of 5,9-dimethylheptadecane as a sex pheromone of the moth Leucoptera scitella. Naturwissenschaften 74:143–144. https://doi.org/10.1007/BF00366529
Francke W, Toth M, Szöcs G, Krieg W, Ernst H, Buschmann E (1988) Identifizierung und Synthese von Dimethylalkanen als Sexuallockstoffe weiblicher Miniermotten (Lyonetiidae) / identification and synthesis of dimethylalkanes as sex attractants of female leaf miner moths (Lyonetiidae). Z Naturforsch C 43:787–789. https://doi.org/10.1515/znc-1988-9-1025
Fuentes-Contreras E (2017) Situación actual y manejo de plagas en manzano. Bol Técnico Pomáceas 17:2–4
Fuentes-Contreras E (2020) Polilla del Álamo (Leucoptera sinuella) y otras plagas emergentes en fruticultura. Bol Técnico Pomáceas 20:2–4
Gries G, Gries R, Borden JH, Li J, Slessor KN, King GGS, Bowers WW, West RJ, Underhill EW (1991) 5,11-Dimethylheptadecane and 2,5-dimethyl-heptadecane: sex pheromone components of the geometrid moth, Lambdina fiscellaria fiscellaria. Naturwissenschaften 78:315–317. https://doi.org/10.1007/BF01221418
Gries G, Gries R, Krannitz SH et al (1993a) Sex pheromone of the western hemlock looper, Lambdina fiscellaria lugubrosa (Hulst) (Lepidoptera: Geometridae). J Chem Ecol 19:1009–1019. https://doi.org/10.1007/BF00992534
Gries G, King GGS, Gries R, Wimalaratne PDC, Gray TG, Shepherd RF, Li J, Slessor KN, Khaskin G (1993b) 3,13-Dimethylheptadecane: major sex pheromone component of the western false hemlock looper, Nepytia freemani Munroe (Lepidoptera: Geometridae). J Chem Ecol 19:1501–1510. https://doi.org/10.1007/BF00984893
Gries R, Gries G, Li J, Maier CT, Lemmon CR, Slessor KN (1994) Sex pheromone components of the spring hemlock looper, Lambdina athasaria (Walker) (Lepidoptera: Geometridae). J Chem Ecol 20:2501–2511. https://doi.org/10.1007/BF02036187
Gries R, Gries G, King GGS, Maier CT (1997) Sex pheromone components of the apple leafminer, Lyonetia prunifoliella. J Chem Ecol 23:1119–1130. https://doi.org/10.1023/B:JOEC.0000006390.43868.3b
Hall DR, Beevor PS, Campion DG, Chamberlain DJ, Cork A, White RD, Almestar A, Henneberry TJ (1992) Nitrate esters: novel sex pheromone components of the cotton leafperforator, Bucculatrix thurberiella Busck. (Lepidoptera: Lyonetiidae). Tetrahedron Lett 33:4811–4814
Juárez P, Chase J, Blomquist GJ (1992) A microsomal fatty acid synthetase from the integument of Blattella germanica synthesizes methyl-branched fatty acids, precursors to hydrocarbon and contact sex pheromone. Arch Biochem Biophys 293:333–341. https://doi.org/10.1016/0003-9861(92)90403-J
Liang T, Kuwahara S, Hasegawa M, Kodama O (2000) Simple synthesis of 5,9-dimethylated long-chain alkanes, the sex pheromones of leaf miner moths. Biosci Biotechnol Biochem 64:2474–2477. https://doi.org/10.1271/bbb.64.2474
Löfstedt C, Wahlberg N, Millar JG (2016) Evolutionary patterns of pheromone diversity in Lepidoptera. In: Allison J, Cardé RT (eds) . University of California Press, Pheromone communication in moths, pp 43–78
Malo EA, Rojas JC, Lopez-Guillen G, Barrera JF (2009) Chemical analysis of female volatiles and field response of the coffee leafminer moth (Lepidoptera: Lyonetiidae) to stereoisomers of its major sex pheromone component. Florida Entomol 92:548–553. https://doi.org/10.1653/024.092.0403
Nakano R, Ihara F, Mishiro K, Toyama M, Toda S (2014) Confuser® MM can be used as an attractant to enable monitoring of the peach leafminer moth, Lyonetia clerkella (Lepidoptera: Lyonetiidae), in peach orchards treated with a mating disrupter. Appl Entomol Zool 49:505–510. https://doi.org/10.1007/s13355-014-0277-8
Park JH, Han KS, Mori K, Boo KS (2002) Right stereoisomers for sex pheromone components of the apple leafminer, Lyonetia prunifoliella, in Korea. J Chem Ecol 28:2515–2525. https://doi.org/10.1023/A:1021488103491
Pomonis JG, Nelson DR, Fatland CL (1980) Insect hydrocarbons - 2. Mass spectra of dimethylalkanes and the effect of the number of methylene units between methyl groups on fragmentation J Chem Ecol 6:965–972. https://doi.org/10.1007/BF00994653
R Core Team (2020). R: a language and environment for statistical computing. R Foundation for Computing, Vienna, Austria. http://www.r-project.org/
Riba M, Rosell JA, Eizaguirre M, Canela R, Guerrero A (1990) Identification of a minor component of the sex pheromone of Leucoptera malifoliella (Lepidoptera, Lyonetiidae). J Chem Ecol 16:1471–1483. https://doi.org/10.1007/BF01014082
Roelofs WL, Cardé RT (1971) Hydrocarbon sex pheromone in tiger moths (Arctiidae). Science 171:684–686. https://doi.org/10.1126/science.171.3972.684
Sandoval A, Ide S, Rothmann S et al (2019) Detección de Leucoptera sinuella (Reutti) (Lepidoptera: Lyonetiidae) en Chile, con la identificación de algunos parasitoides asociados. Rev Chil Entomol 45:65–77
Sato R, Abe N, Sonnet P et al (1985) Biological activity of (R)- and (S)-14-methyl-1-octadecene, as the chiral component of the sex pheromone of the peach leafminer moth, Lyonetia clerkella Linne (Lepidoptera : Lyonetiidae). Appl Entomol Zool 20:411–415. https://doi.org/10.1303/aez.20.411
Sugie H, Tamaki Y, Sato R, Kumakura M (1984) Sex pheromone of the peach leafminer moth, Lyonetia clerkella Linne: isolation and identification. Appl Entomol Zool 19:323–330. https://doi.org/10.1303/aez.19.323
Svensson GP, Gündüz EA, Sjöberg N, Hedenström E, Lassance JM, Wang HL, Löfstedt C, Anderbrant O (2014) Identification, synthesis, and behavioral activity of 5,11-dimethylpentacosane, a novel sex pheromone component of the greater wax moth, Galleria mellonella (L.). J Chem Ecol 40:387–395. https://doi.org/10.1007/s10886-014-0410-8
Taguri T, Yaginuma K, Yamamoto M, Fujii T, Ando T (2014) Enantiospecific synthesis and filed evaluation of four stereoisomers of 10,14-dimethyloctadec-1-ene, a sex pheromone component secreted by female moths of the apple leafminer. Biosci Biotechnol Biochem 78:761–765. https://doi.org/10.1080/09168451.2014.905187
Toth M, Helmchen G, Leikauf U et al (1989) Behavioral activity of optical isomers of 5,9-dimethylheptadecane, the sex pheromone of Leucoptera scitella L. (Lepidoptera: Lyonetidae). J Chem Ecol 15:1535–1543. https://doi.org/10.1007/BF01012381
Umbreit MA, Sharpless KB (1977) Allylic oxidation of olefins by catalytic and stoichiometric selenium dioxide with tert-butyl hydroperoxide. J Am Chem Soc 99:5526–5528. https://doi.org/10.1021/ja00458a072
Zarbin PHG, Princival JL, de Lima ER, dos Santos AA, Ambrogio BG, de Oliveira ARM (2004) Unsymmetrical double Wittig olefination on the syntheses of insect pheromones. Part 1: synthesis of 5,9-dimethylpentadecane, the sexual pheromone of Leucoptera coffeella. Tetrahedron Lett 45:239–241. https://doi.org/10.1016/J.TETLET.2003.10.183
Acknowledgments
Technical assistance and collection of insects in the field from, Cristian Gálvez, Javiera Fernández, Elías González, Matías Olivera, Francisca Santibañez, Alexis Muñoz, Patricio Nuñez, Jorge Jaque and Luciano Veliz is acknowledged. Financial support from Vicerrectoría de Investigación y Estudios Avanzados (via grants VRIEA-PUCV 039.399/2017 and 039.498/2018 to JB), Fondo de Equipamiento Cientifico y Tecnologico (Fondequip-Conicyt) (grant EQM130154), and Fondo de Innovación para la Competitividad de la Región de O’Higgins (FIC-O’Higgins grant IDI 40008896-0) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 24.0 kb)
Rights and permissions
About this article
Cite this article
Barros-Parada, W., Bergmann, J., Curkovic, T. et al. 3,7-Dimethylpentadecane: a Novel Sex Pheromone Component from Leucoptera sinuella (Lepidoptera: Lyonetiidae). J Chem Ecol 46, 820–829 (2020). https://doi.org/10.1007/s10886-020-01208-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01208-z