Abstract
3-(3′,4′-Dihydroxyphenyl)-l-alanine (l-DOPA), which is synthesized in velvet bean (Mucuna pruriens), inhibits plant growth. The concentration of l-DOPA in soil is reduced by adsorption and transformation reactions, which can result in the reduction of its plant-growth-inhibitory activity. To determine which part of the l-DOPA structure is involved in the adsorption and soil transformation reactions, we compared the kinetics of l-DOPA disappearance in a volcanic ash soil with that of l-phenylalanine (3-phenyl-l-alanine) and l-tyrosine (3-(4′-hydroxyphenyl)-l-alanine), compounds that are similar in structure to l-DOPA but do not have a catechol (o-dihydroxybenzene) moiety. l-Phenylalanine and l-tyrosine were not adsorbed and transformed in the soil at equilibrium pH values between 4 and 7. These results suggest that the adsorption and transformation reactions of l-DOPA in the soil involve the catechol moiety and not the amino and carboxylic acid groups, which are common to all three compounds. Like l-DOPA, (+)-catechin, another allelochemical that contains a catechol moiety, underwent adsorption and soil transformation reactions. Thus, we concluded that the concentrations of allelochemicals bearing a catechol moiety in soils will decrease rapidly owing to adsorption and transformation reactions, and this decrease will be faster in soils with a high pH value or high adsorption ability. Owing to this decrease in concentration, allelopathic phenomena may not occur.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Velvet bean (Mucuna pruriens) inhibits the growth of neighboring plants by releasing the phytotoxic substance 3-(3′,4′-dihydroxyphenyl)-l-alanine (l-DOPA, Fig. 1) (Fujii et al., 1991, 1992; Fujii, 2000). l-DOPA content in fresh leaves and roots of velvet bean ranges from 0.5 to 1.5%, and the yield of fresh leaves and stems ranges from 20 to 30 t ha−1 (Fujii et al., 1991). Therefore, velvet bean could produce 100–450 g ha−1 (0.05–0.23 mol m−2) of l-DOPA. Because plant-growth-inhibitory activity of l-DOPA in the absence of soil has been observed at concentrations of at least 1 × 10−4 to 1 × 10−5 M (Fujii et al., 1991; Nishihara et al., 2004; Furubayashi et al., 2005; Hiradate et al., 2005), l-DOPA released from velvet beans may inhibit the growth of neighboring plants if l-DOPA persists in soil environments.
Shibuya et al. (1994) observed plant-growth-inhibitory activity of l-DOPA in a granitic sandy soil (“Masa” soil) but not in an Andosol, probably because of the high ability of the Andosol to adsorb l-DOPA. Furubayashi et al. (2005) showed that the concentration of l-DOPA in a soil-water mixture decreases over time, owing to (i) adsorption on the soil, (ii) chemical transformation, and (iii) microbial degradation. Hiradate et al. (2005) reported that the adsorption mechanism involves a ligand exchange reaction in an Andosol, and that the soil components catalytically accelerate the transformation of l-DOPA. Hiradate et al. (2005) also demonstrated that the adsorbed and transformed forms of l-DOPA exhibit no plant-growth-inhibitory activity. Furubayashi et al. (2005) determined the amount of l-DOPA adsorbed and the rate of transformation at equilibrium pH values between 4 and 7 in three soil types (volcanic ash, alluvial, and calcareous soils). The amount of adsorbed l-DOPA and the rate of transformation at equilibrium pH 6 in the presence of volcanic ash soil were 0.068 mol kg−1 soil and 0.057 mol kg−1 soil d−1, respectively, and these values indicate that l-DOPA can be expected to disappear rapidly in this type of soil.
l-DOPA contains a catechol moiety, and catechol is easily oxidized in soils, especially under alkaline conditions (Kumada, 1981). Like catechol, l-DOPA may undergo soil oxidation reactions. Many other allelochemicals also contain a catechol moiety. For example, robinetin, myricetin, and quercetin are produced by Robinia pseudo-acacia L. (Iqbal et al., 2004); caffeic and chlorogenic acids are present in wild perennial buckwheat (Fagopyrum cymosum M.; Tsuzuki and Yamamoto, 1987); digallic and ellagic acids have been detected in soils under the canopy and in the litter of a lowland forest (Lodhi, 1976); (±)-catechins are secreted by spotted knapweed (Centaureamaculosa; Bais et al., 2002, 2003); and (+)-catechin has been isolated from buckwheat (Iqbal et al., 2003). All these compounds show plant-growth-inhibitory activity, but their concentrations in soils may be reduced owing to the presence of the catechol moiety in their structures, which we suspect is involved in their adsorption and soil transformation reactions.
In the present study, to elucidate the role of the catechol moiety in l-DOPA adsorption and soil transformation reactions, we investigated the kinetics of the disappearance of l-phenylalanine and l-tyrosine (Fig. 1), which have chemical structures similar to the structure of l-DOPA but lack the catechol moiety, at various equilibrium pH values in a volcanic ash soil. We also investigated whether (+)-catechin underwent adsorption and transformation reactions in a volcanic ash soil and whether the decrease of (+)-catechin concentration in soil solutions corresponded to the reduction of plant-growth-inhibitory activity. We expect this information to be useful in understanding the chemical behaviors of phytotoxic catechol compounds in soils.
Methods and Materials
Soil Samples
Samples of a volcanic ash soil (Silandic Andosol, FAO, 1998; Melanudand, Soil Survey Staff, 1999) and an alluvial soil (Anthrosol, FAO, 1998; Aquept, Soil Survey Staff, 1999) were collected from the Ap horizons of an upland agricultural field and a paddy field, respectively, in Tsukuba, Japan (Hiradate and Uchida, 2004). A sample of a calcareous soil (Chromic Luvisol, FAO, 1998; Hapludalf, Soil Survey Staff, 1999) was collected from the Ap horizon of an upland agricultural field in Yomitan, Okinawa, Japan. The soil samples were air-dried and then ground and finely sieved (<0.5 mm).
Analysis of Soil Components
Particle size analysis was performed by the pipette method (Day, 1965). Total organic components were determined by a dry combustion method (Sumigraph NC analyzer NC-900, Sumika Chem. Anal. Service, Japan). Determination of soil pH in H2O was performed with a pH meter with a glass electrode. To determine the pH(H2O) values, 10.0 g of air-dried soil were mixed with 25 ml of deionized water. The mixture was allowed to stand over 12 hr with occasional shaking, and the pH was measured in the suspension.
Chemicals
l-Phenylalanine and l-tyrosine were purchased from Wako Pure Chemical Industries (Osaka, Japan). (+)-Catechin was purchased from Spectrum Chemical (Gardena, CA, USA).
Kinetics of l-Phenylalanine, l-Tyrosine, and (+)-Catechin Disappearance in the Presence of the Volcanic Ash Soil
Fifty milligrams of the volcanic ash soil sample (oven-dry basis) were placed in a 12-ml glass centrifuge tube. Deionized water, 1.0 ml of a 1-M NaCl solution (background electrolyte), 50 μl of chloroform (an antibiotic), and a dilute HCl or NaOH solution (for pH adjustment) were added to the tube to obtain a liquid-phase volume of 3.0 ml and equilibrium pH values between 4 and 7. The tube was capped with a screw-on Teflon-sealed cap and shaken overnight to establish equilibrium. Then, 2 ml of a 5-mM l-phenylalanine or (+)-catechin solution were added to the soil suspension (0.2 mol l-phenylalanine or (+)-catechin kg−1 soil). The resulting mixture was shaken at 100 rpm for 1–120 hr at 30°C in the dark. The same procedure was applied to another 50 mg of soil samples, but 0.25 ml of 4 M NaCl and a dilute HCl or NaOH solution were added to the tube to obtain a liquid-phase volume of 1.0 ml and equilibrium pH values between 4 and 7. Then, instead of 2 ml of 5 mM l-phenylalanine or (+)-catechin, 4 ml of 2.5 mM l-tyrosine were added to reach the same final concentration (0.2 mol l-tyrosine kg−1 soil).
After filtration of the soil suspension through a cellulose acetate filter membrane (pore size, 0.2 μm; Advantec Toyo Kaisha, Tokyo, Japan), the equilibrium pH was determined with a pH meter with a glass electrode, and the concentration of the test chemical was determined with a high-performance liquid chromatograph (HPLC: pump, L-6200; UV–vis detector, L-4200H; autosampler, AS-2000; column oven, L-5025; chromatogram integrator, D-2500; Hitachi, Tokyo, Japan) equipped with a reversed-phase analytical column (Inertsil ODS-3, 5 μm, 4.6-mm i.d., 250-mm length, GL Sciences, Tokyo, Japan). l-Phenylalanine and l-tyrosine were eluted with a solution of 0.1 M phosphate buffer (pH 2) and methanol (l-phenylalanine, 8:2 v:v; l-tyrosine, 9:1 v:v) at a flow rate of 1 ml min−1 at a column temperature of 40°C; the detection wavelength was 200 nm. (+)-Catechin was eluted with a mixed solution of deionized water and methanol (7:3 v:v) at a flow rate of 1 ml min−1 at a column temperature of 40°C; the detection wavelength was 200 nm. l-Phenylalanine, l-tyrosine, and (+)-catechin eluted at 6.2, 6.0, and 5.4 min, respectively.
Calculation of l-Phenylalanine, l-Tyrosine, and (+)-Catechin Disappearance at Constant Equilibrium pH Value in the Presence of the Volcanic Ash Soil
To adjust accurately the equilibrium pH at the end of the reaction time was difficult because the equilibrium pH of the soil mixture changed gradually over the reaction period, owing to the buffering action of the soil and the reaction of the test chemicals. Therefore, we calculated the amounts of chemicals at equilibrium pH values of 4, 5, 6, and 7 by plotting the relationship between the amounts of the test chemicals and equilibrium pH values of the solutions after filtration through the cellulose acetate membrane. This calculation method is similar to that used for l-DOPA in our previous work (Furubayashi et al., 2005).
Kinetics of l-Phenylalanine and (+)-Catechin Disappearance in the Absence of Soil
To clarify the effects of solution pH on the stability of l-phenylalanine and (+)-catechin in the absence of soil, we prepared a 5-ml solution of 2 mM l-phenylalanine or (+)-catechin in a 12-ml glass centrifuge tube in the presence of 0.1 M phosphoric acid–sodium phosphate solution buffered at pH values between 4 and 7. After the tube was shaken at 100 rpm for 1–120 hr at 30°C in the dark, the test solution was passed through a cellulose acetate filter membrane (pore size, 0.2 μm; Advantec Toyo Kaisha). Then, the equilibrium pH and the concentrations of the test chemicals in the filtrate were measured by using a pH meter and an HPLC system, as described above.
Kinetics of l-DOPA Disappearance in the Presence and Absence of Soil
We measured the kinetics of the disappearance of l-DOPA in the presence and absence of the volcanic ash soil in a previous study (Furubayashi et al., 2005) under experimental conditions identical to those used in the present study. The kinetic data from the previous study were used for comparison with the data obtained in this study.
Measurement of the Plant-Growth-Inhibitory Activity of (+)-Catechin in the Presence of Three Types of Soil
A 500-mg portion (oven-dry basis) of the soil (volcanic ash soil, calcareous soil, or alluvial soil) was placed in each well (15.6-mm i.d., 17-mm high, 1.9-cm2 growing area) of a 24-well plate (Nunclon, NalgeNunc, Denmark). Then, 1.0 ml of a solution containing 10−6–10−2 M (+)-catechin in the presence of 0.75% agar (gelling temperature, 30 to 31°C; Nacalai Tesque, Kyoto, Japan) at 40°C was added to each soil-containing well. After the soil suspension was stirred with a stick and incubated at 30°C for 24 hr in the dark, four lettuce seeds (Lactuca sativa cv. Great Lakes 366) were placed on the gelled agar–soil mixture. The lettuce seeds were grown for 72 hr at 20°C in the dark, and the inhibitory activity was determined by measuring root elongation and comparing it with that of the control (without (+)-catechin, but in the presence of the corresponding soil). This experiment was replicated three times.
Results and Discussion
In the presence of volcanic ash soil, l-DOPA disappeared over time at equilibrium pH values between 4 and 7, and in the absence of soil, l-DOPA disappeared only at pH values higher than 7 (Fig. 2). Hiradate et al. (2005) previously reported that l-DOPA disappeared in soils because of adsorption and transformation reactions. In contrast, the amounts of l-phenylalanine and l-tyrosine in the presence and absence of soil remained stable at equilibrium pH values between 4 and 7 during the experimental period (1–120 hr; Fig. 2), which indicates that l-phenylalanine and l-tyrosine were neither absorbed nor transformed by the soil.
In our previous work (Furubayashi et al., 2005), we found that when l-DOPA is incubated with soils, it disappears by means of two chemical processes (Fig. 3). The rapid and sudden reduction of l-DOPA concentration observed during the first 4-hr period was attributed to adsorption. The second process was characterized by a constant rate of disappearance caused by catalytic transformation reactions. The adsorption process ends within 8 hr, and after that time, only the catalytic transformation reactions contribute to the disappearance of l-DOPA (Fig. 3; Furubayashi et al., 2005). The amount of l-DOPA disappearing after 8 hr can be expressed by the following linear equation:
Determination of the transformation rate and the amount of adsorption of l-DOPA (left) and (+)-catechin (right) using the first-order regression curve Y = aX + b, where Y is the amount of l-DOPA or (+)-catechin lost (mol kg−1 soil), a is the rate of l-DOPA or (+)-catechin disappearance by transformation (mol kg−1 hr−1), X is the reaction time (hr), and b is the amount of l-DOPA or (+)-catechin lost by adsorption (mol kg−1). For the regression analysis, data between 8 and 120 hr of reaction time were used for each series. The reaction experiments were conducted at an equilibrium pH value of 6.0 in the absence (○) and presence (●) of a volcanic ash soil
where Y is the amount of l-DOPA disappeared (mol kg−1 soil), a is the rate of l-DOPA disappearance by the transformation reactions (mol kg−1 soil hr−1), X is the reaction time (hr), and b is the amount of l-DOPA disappeared by adsorption (mol kg−1 soil). The rate of transformation (a) and the amount of l-DOPA adsorbed (b) were calculated by using Eq. 1, and the values obtained were plotted against the equilibrium pH values (Figs. 4 and 5).
The presence of volcanic ash soil increases adsorption of l-DOPA (Fig. 4). The amounts of l-DOPA adsorbed by the soil range from 0.020 to 0.068 mol kg−1 soil at equilibrium pH values between 4 and 7, and the amounts increased with increasing equilibrium pH. In contrast, the amounts of adsorbed l-phenylalanine and l-tyrosine were negligible. The differences in adsorption among the three compounds cannot be attributed to the amino and carboxylic acid groups, which are common to all three structures, and are instead attributable to the number and positions of the hydroxyl group on the benzene rings: l-DOPA has 3′ and 4′ hydroxyl groups, l-tyrosine has a 4′ hydroxyl group, and l-phenylalanine has no hydroxyl groups (Fig. 1). Therefore, we concluded that the 3′,4′-dihydroxyphenyl moiety (that is, the catechol moiety) is responsible for the adsorption.
In both the presence and the absence of soil, the rate of l-DOPA transformation is faster at higher pH values, whereas the transformation rates of l-phenylalanine and l-tyrosine were negligible at equilibrium pH values between 4 and 7 (Fig. 5). In the absence of soil, the rate of l-DOPA transformation is almost zero at equilibrium pH values lower than 6.0, but the rates increase to 0.007 and 0.029 mol kg−1 soil d−1 at equilibrium pH 6.5, and 7.0, respectively. Hiradate et al. (2005) reported that l-DOPA is automatically transformed into polymerized forms in the absence of soils at equilibrium pH values higher than 6.5 and that this transformation is accompanied by the consumption of OH−. In the presence of the soil, the l-DOPA transformation rates increase to 0.003 and 0.028 mol kg−1 soil d−1 at equilibrium pH 4 and 7, respectively. Hiradate et al. (2005) also reported that an Andosol and its components catalytically accelerate the transformation of l-DOPA into heterogeneous components, such as humic substances, as indicated by their spectrum in the proton nuclear magnetic resonance. In contrast, l-phenylalanine and l-tyrosine, which lack the catechol moiety, remained stable both in the absence and in the presence of the soil, which indicates that the catechol structure, rather than the amino group or the carboxylic acid group, is responsible for l-DOPA adsorption and soil transformation reactions.
l-DOPA content in fresh leaves and roots of velvet bean has been reported by Fujii et al. (1991). If the estimated total production of l-DOPA from velvet bean were added to a soil of 10-cm depth, the maximum amount of l-DOPA in the soil would range from 0.0005 to 0.0023 mol kg−1 soil. If a certain amount, close to the above estimated value of 0.0023 mol kg−1 soil of l-DOPA, is added to a volcanic ash soil, the concentration of l-DOPA is markedly reduced after 24 hr of reaction time (Fig. 2). Furubayashi et al. (2005) likewise reported that at a concentration of 10−3 M (0.002 mol kg−1 soil), the plant-growth-inhibitory activity of l-DOPA in the presence of the three soils (volcanic ash, calcareous, and alluvial) is less than 25%. In the presence of these soils, therefore, allelopathic phenomena caused by l-DOPA released from velvet bean may not be observed.
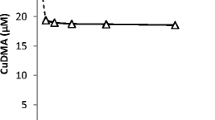
To clarify the influence of the catechol moiety on the adsorption and soil transformation reactions of these compounds, we examined the adsorption and transformation reactions of another catechol-containing allelochemical, (+)-catechin (Fig. 1), both in the presence and in the absence of the volcanic ash soil. When (+)-catechin was incubated with the soil, it disappeared rapidly during the first 4 hr, and then the rate of disappearance slowed and become constant, just as is the case for l-DOPA (Fig. 2). We attributed the disappearance of (+)-catechin in the presence of the soil to adsorption and transformation reactions, as for l-DOPA (Fig. 3). The amount of (+)-catechin adsorbed and the rate of (+)-catechin transformation were calculated by using Eq. 1. (+)-Catechin adsorption on the soil increased with increasing equilibrium pH: 0.013 and 0.054 mol kg−1 soil were adsorbed at equilibrium pH 4 and 7, respectively (Fig. 4). The rate of (+)-catechin transformation in the presence of the soil increased with increasing equilibrium pH, to 0.005 and 0.027 mol kg−1 soil d−1 at equilibrium pH 4 and 7, respectively (Fig. 5). In the absence of soil, the rate of (+)-catechin transformation was negligible at equilibrium pH values lower than 5, but the transformation rate was clearly detected at equilibrium pH values between 5.5 and 7.0 (0.005 and 0.018 mol kg−1 soil d−1, respectively). The kinetics of the disappearance of (+)-catechin in the absence and presence of the soil were similar to those of l-DOPA, which indicates again that the presence of the catechol moiety, common to l-DOPA and (+)-catechin, was involved in the adsorption and soil transformation reactions. Oszmianski and Lee (1990) reported that the enzymatic oxidation reaction products of catechin and chlorogenic acid are mainly polymers, and that the production of these is associated with browning. In our study, the gradual change of the (+)-catechin solutions from colorless to yellowish brown likely indicates that (+)-catechin was transformed by oxidative polymerization. Shindo and Huang (1984) found that dihydroxybenzene compounds (hydroquinone, resorcinol, and catechol) are converted into humic acid through semiquinones during the catalytic oxidative reduction of Mn(IV) and Fe(III) oxides. The catechol moieties of l-DOPA and (+)-catechin probably underwent similar reactions in our study.
We investigated the plant-growth-inhibitory activity of (+)-catechin in three soil types (volcanic ash, calcareous, or alluvial). Under natural pH conditions (without pH adjustment, Table 1), (+)-catechin activity became markedly weaker in the presence of soils, whereas in the absence of soils, growth inhibition of at least 50% was observed at initial concentrations higher than 10−3 M (Fig. 6). At initial concentrations lower than 3 × 10−3 M (0.006 mol kg−1 soil), the inhibitory activity was negligible in the presence of the soils. When 0.006 mol (+)-catechin kg−1 soil was incubated with volcanic ash soil at pH 5.8 (no pH adjustment), the (+)-catechin concentration was markedly reduced after 24 hr (Fig. 2). As a result of the disappearance of (+)-catechin, almost no plant-growth-inhibitory activity was observed. In the environment, allelochemicals are released from living plants at a fairly constant rate during the growth season, and, therefore, the amounts of these chemicals deposited in the soils remain fairly constant. However, adsorption and transformation reactions would prevent the accumulation of the highly toxic allelochemicals l-DOPA and (+)-catechin.
Inhibitory activity of (+)-catechin, measured in terms of root elongation of lettuce (Lactuca sativa cv. Great Lakes 366) grown in agar media in the absence (♦) and presence of soils (●; volcanic ash soil, ▲; calcareous soil, ■; alluvial soil), at various initial concentrations. Inhibition of root growth was detected by measuring root elongation and comparing it with that of the control (with no (+)-catechin, but in the presence of the corresponding soil) after incubation for 72 hr at 20°C in the dark. Bars indicate standard deviation (n = 12)
Dalton et al. (1983, 1989) extensively studied the role of clays and organic matter in similar reactions of ferulic acid in soils. These workers found that organic matter was the most active soil component involved in the irreversible retention of ferulic acid in sterile soil. In our study, the clay contents of the soils decreased in the order calcareous soil > volcanic ash soil > alluvial soil, and the organic matter contents decreased in the order volcanic ash soil > calcareous soil > alluvial soil (Table 1). However, we could not confirm the influence of variations in the clay and organic matter contents of the soil on adsorption and transformation reactions of catechin, because the plant-growth-inhibitory activity of (+)-catechin was extremely low in the presence of the soils. In a previous study (Furubayashi et al., 2005), we observed the largest amount of l-DOPA adsorption in the presence of the volcanic ash soil, which has the highest organic matter content of the three soil types, but the influence of variations in clay content on the adsorption and transformation reactions of l-DOPA was not clear. However, we suspect that the influence may be small because l-DOPA is more polar than ferulic acid and dissolves readily in water. Hiradate et al. (2005) and Furubayashi et al. (2005) have reported that volcanic ash soil, which has high P-retention ability, a high pH (NaF) value, and large amounts of Fe and Al (hydr)oxides, absorbs large amounts of l-DOPA, which suggests that the mechanism of the adsorption of l-DOPA involves a ligand exchange reaction. The mechanisms of the adsorption and transformation reactions of both l-DOPA and (+)-catechin in soils and the effect of variations in clay and organic matter soil contents on their disappearance require further study.
Blum et al. (1987) reported that the retention of ferulic acid (i.e., by adsorption, polymerization, etc.) by clay and organic matter in nonsterile soil is secondary to microbial utilization and uptake by cucumber roots. Likewise, Weidenhamer and Romeo (2004) reported that certain allelochemicals (gallic acid, hydroquinone, arbutin, and benzoquinone) from Polygonella myriophylla are degraded rapidly by soil microbes. In our previous study of the behavior of l-DOPA in soil sterilized with chloroform and in nonsterile volcanic ash soil (Furubayashi et al., 2005), we found that l-DOPA disappears after 72 hr, owing to degradation caused by the soil microbes. Therefore, biodegradation by soil microbes, in addition to adsorption and transformation reactions, is a significant factor in the disappearance of l-DOPA and (+)-catechin in soils. In volcanic ash soil environments, the plant-growth-inhibitory activity of l-DOPA and (+)-catechin can be expected to be reduced quickly mainly because of adsorption and transformation reactions that occur rapidly (within 24 hr). The disappearance might be even faster in high-microbial-activity soil than in low-microbial-activity soil because all three processes will occur. The importance of adsorption and transformation reactions relative to that of microbial activity in reducing the concentrations of these compounds in soils also requires further study.
Bais et al. (2003) used methanol to extract natural rhizosphere soil of spotted knapweed in Montana, and estimated that the concentration of (−)-catechin in the soil is about 1.1 mg g−1 soil (0.004 mol kg−1 soil), and that (−)-catechin inhibits the growth and germination of native species in field soils at natural concentrations. However, they did not report the soil pH, and the extraction method they used was different from our method, making comparison with our results difficult. Under the soil conditions used for the present study, we estimate that the rates of transformation of (+)-catechin were 0.013 and 0.054 mol kg−1 soil d−1 at equilibrium pH 4 and 7, respectively, and these values indicate that the occurrence of allelopathic phenomena of spotted knapweed in the field would require the release of catechin at the rate of 0.013 mol kg−1 soil d−1 or more. Blair et al. (2005) demonstrated a rapid decline of catechin concentration within 24 hr after its addition to wet North Carolina soil (Loamy sand/sand, pH 5.2), and those results support ours. We expected, therefore, that the occurrence of allelopathic phenomena caused by catechin and l-DOPA would be low in a soil such as volcanic ash soil, calcareous soil, or alluvial soil at pH values between 4 and 7, because in this range, the rate of transformation of catechin and l-DOPA would be high. Many other phytotoxic allelochemicals contain a catechol moiety (e.g., caffeic acid, digallic acid, ellagic acid, robinetin, myricetin, quercetin, and (−)-catechin). These compounds may disappear rapidly by means of mechanisms similar to those responsible for the disappearance of l-DOPA and (+)-catechin. We conclude that the phytotoxicity of all catechol-bearing compounds released from plants into soils is likely to be substantially reduced by adsorption and transformation reactions, and that the soil concentrations of these compounds can be expected to be reduced more quickly in soils with high pH values or high adsorption ability. Therefore, allelopathic phenomena may not be observed under such conditions.
References
Bais, H. P., Walker, T. S., Stermitz, F. R., Hufbauer, R. A., and Vivanco, J. M. 2002. Enantiomeric-dependent phytotoxic and antimicrobial activity of (±)-catechin. A rhizosecreted racemic mixture from spotted knapweed. Plant Physiol. 128:1173–1179.
Bais, H. P., Vepachedu, R., Gilroy, S., Callaway, R. M., and Vivanco, J. M. 2003. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 301:1377–1380.
Blair, A. C., Hanson, B. D., Brunk, G. R., Marrs, R. A., Westra, P., Nissen, S. J., and Hufbauer, R. A. 2005. New techniques and findings in the study of a candidate allelochemical implicated in invasion success. Ecol. Lett. 8:1039–1047.
Blum, U., Weed, S. B., and Dalton, B. R. 1987. Influence of various soil factors on the effects of ferulic acid on leaf expansion of cucumber seedlings. Plant Soil 98:111–130.
Dalton, B. R., Blum, U., and Weed, S. B. 1983. Allelopathic substances in ecosystems: Effectiveness of sterile soil components in altering recovery of ferulic acid. J. Chem. Ecol. 9:1185–1201.
Dalton, B. R., Blum, U., and Weed, S. B. 1989. Plant phenolic acids in soils: Sorption of ferulic acid by soil and soil components sterilized by different techniques. Soil Biol. Biochem. 21:1011–1018.
Day, P. R. 1965. Particle fractionation and particle-size analysis, pp. 545–567, in C. A. Black (ed.). Methods of soil analysis. American Society of Agronomy, Madison.
FAO. 1998. World Reference Base for Soil Resources. World Soil Resources Reports 84, FAO, Rome.
Fujii, Y. 2000. Allelopathy in Vicia, Mucuna and related leguminous species, pp. 191–204, In Proceedings of the 7th MAFF International Workshop on Genetic Resources. Part 1. Wild Legumes. AFFRC and NIAR, Japan.
Fujii, Y., Shibuya, T., and Yasuda, T. 1991. l-3,4-Dihydroxyphenylalanine as an allelochemical candidate from Mucuna pruriens (L.) DC var. utilis. Agric. Biol. Chem. 55:617–618.
Fujii, Y., Shibuya, T., and Yasuda, T. 1992. Allelopathy of velvetbean; its determination and identification of l-DOPA as a candidate of allelopathic substances. JARQ 25:238–247.
Furubayashi, A., Hiradate, S., and Fujii, Y. 2005. Adsorption and transformation reaction of l-DOPA in soils. Soil Sci. Plant Nutr. 51:819–825.
Hiradate, S. and Uchida, N. 2004. Effects of soil organic matter on pH-dependent phosphate sorption by soils. Soil Sci. Plant Nutr. 50:665–675.
Hiradate, S., Furubayashi, A., and Fujii, Y. 2005. Changes in chemical structure and biological activity of l-DOPA as influenced by an Andosol and its components. Soil Sci. Plant Nutr. 51:477–484.
Iqbal, Z., Hiradate, S., Noda, A., and Fujii, Y. 2003. Allelopathic activity of buckwheat: isolation and characterization of phenolics. Weed Sci. 51:657–662.
Iqbal, Z., Nasir, H., Hiradate, S., and Fujii, Y. 2004. Role of allelopathy in invasion of an exotic plant Robinia pseudo-acacia L. J. Weed Sci. Tech. 49:98–99.
Kumada, K. 1981. Chemistry of Soil Organic Matter. 2nd edn. Japan Sci. Soc. Press, Tokyo.
Lodhi, M. A. K. 1976. Role of allelopathy as expressed by dominating trees in a lowland forest in controlling the productivity and pattern of herbaceous growth. Am. J. Bot. 63:1–8.
Nishihara, E., Parvez, M. M., Araya, H., and Fujii, Y. 2004. Germination growth response of different plant species to the allelochemical l-3,4-dihydroxyphenylalanine (l-DOPA). Plant Growth Regul. 42:181–189.
Oszmianski, J. and Lee, C. Y. 1990. Enzymatic oxidative reaction of catechin and chlorogenic acid in a model system. J. Agric. Food Chem. 38:1202–1204.
Shibuya, T., Fujii, Y., and Asakawa, Y. 1994. Effects of soil factors on manifestation of allelopathy in Cytisus scoparius. Weed Res. Japan 39:222–228 (in Japanese with English summary).
Shindo, H. and Huang, P. M. 1984. Catalytic effects of manganese(IV), iron(III), aluminum, and silicon oxides on the formation of phenolic polymers. Soil Sci. Soc. Am. J. 48:927–934.
Soil Survey Staff. 1999. Soil Taxonomy, 2nd edn. U.S. Dept. Agric., Agriculture Handbook no. 436. U.S. Gov. Printing Off., Washington, DC.
Tsuzuki, E. and Yamamoto, Y. 1987. Isolation and identification of phenolic substances from wild perennial buckwheat (F. cymosum M.). Miyazaki Daigaku Nogakubu Kenkyu Hokoku 34:289–295.
Weidenhamer, J. D. and Romeo, J. T. 2004. Allelochemicals of Polygonella myriophylla: Chemistry and soil degradation. J. Chem. Ecol. 30:1067–1082.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Furubayashi, A., Hiradate, S. & Fujii, Y. Role of Catechol Structure in the Adsorption and Transformation Reactions of l-Dopa in Soils. J Chem Ecol 33, 239–250 (2007). https://doi.org/10.1007/s10886-006-9218-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9218-5