Abstract
The sex pheromones of many aphid species from the subfamily Aphididae comprise a mixture of the iridoids (cyclopentanoids) (1R,4aS,7S,7aR)-nepetalactol and (4aS,7S,7aR)-nepetalactone. In this paper, we investigate whether other chemicals, in addition to nepetalactol and nepetalactone, are released from Dysaphis plantaginea (rosy apple aphid) oviparae as part of their sex pheromone. Four compounds present in an air entrainment sample collected from D. plantaginea oviparae feeding on apple (Malus silvestris c.v. Braburn) elicited electrophysiological responses from male D. plantaginea. Active peaks were tentatively identified by gas chromatography (GC) coupled with mass spectrometry, with identification confirmed by peak enhancement with authentic compounds on GC columns of different polarities. The electroantennography-active chemicals were (1R,4aS,7S,7aR)-nepetalactol, (4aS,7S,7aR)-nepetalactone, (1S,2R,3S)-dolichodial, and phenylacetonitrile. (1S,2R,3S)-Dolichodial elicited a behavioral response from male D. plantaginea and naïve-mated female parasitoids, Aphidius ervi. This is the first report of electrophysiological and behavioral responses from any aphid morph to (1S,2R,3S)-dolichodial. Whether or not (1S,2R,3S)-dolichodial is a third component of the aphid sex pheromone is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mature sexual female aphids (oviparae) release a sex pheromone from scent plaques on their hind tibiae that attracts conspecific males (Pettersson 1970; Marsh 1972, 1975). Dawson et al. (1987) collected two components of the pheromone from excised hind tibiae of Megoura viciae (vetch aphid) oviparae by washing in solvent (pentane). Together with behavioral bioassays, they concluded that the M. viciae sex pheromone was composed of a mixture of two compounds (Fig. 1), (1R,4aS,7S,7aR)-nepetalactol (1) and (4aS,7S,7aR)-nepetalactone (2). To date, with one exception [damson hop aphid, Phorodon humuli (Campbell et al. 1990)], the sex pheromones of many aphid species from the subfamily Aphididae appear to consist of a mixture of these iridoids (cyclopentanoids; Birkett and Pickett 2003), although the enantiomeric composition of the released compounds has not been widely investigated (Goldansaz et al. 2004; Stewart-Jones et al. 2007).
Bioassays and field trials show that males from different species respond to a range of ratios of (1R,4aS,7S,7aR)-nepetalactol (1) and (4aS,7S,7aR)-nepetalactone (2), but the behavioral response is greater with the ratio identified from conspecific oviparae (Dawson et al. 1990; Hardie et al. 1990; Lilley and Hardie 1996; Boo et al. 2000). However, some experiments have suggested that the two iridoids do not always convey species integrity. For example, the ratio of (4aSR,7SR,7aRS)-nepetalactone/(1RS,4aSR,7SR,7aRS)-nepetalactol released from different species of Cryptomyzus oviparae were similar, but Cryptomyzus galeopsidis (European blackcurrant aphid) males could distinguish between the sex pheromone released by conspecific oviparae and the sex pheromone from other species of Cryptomyzus (Guldemond et al. 1992). Male C. galeopsidis also could discriminate between a synthetic pheromone blend 30:1 (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2) and volatiles from conspecific oviparae (Guldemond et al. 1993). A stronger, positive behavioral response to the conspecific oviparae occurred, suggesting that the sex pheromone of some aphids is likely to comprise more than two components.
The holocyclic heteroecious aphid Dysaphis plantaginea (rosy apple aphid) is the second most important pest of apples in Europe and North America after the codling moth (Cydia pomonella) (Graf 1999; Wyss et al. 1999; Blommers et al. 2004). It colonizes apple (Malus domestica) as its primary host and plantain (Plantago spp.) as the secondary host (Blackman and Eastop 2000). In apple, D. plantaginea impairs shoot growth, reduces the formation of flowers over winter, gives rise to leaf curl, and causes malformation and reduction in size of the fruit (Forrest and Dixon 1975; Blommers et al. 2004). Apple yield can be reduced by as much as 45% (De Berardinis et al. 1994; Blommers et al. 2004). Stewart-Jones et al. (2007) determined that D. plantaginea oviparae release (1R,4aS,7S,7aR)-nepetalactol (1) and (4aS,7S,7aR)-nepetalactone (2) in a 4:1 ratio.
In this paper, we investigate whether chemicals in addition to the nepetalactone and nepetalactols are released from oviparae as part of the aphid sex pheromone. The identification of other chemicals that may play a role in species integrity will add a new dimension to our understanding of aphid sex pheromones, and additional chemicals could be exploited as part of integrated pest management systems. To investigate the role of other components, D. plantaginea is used as a model.
Methods and Materials
Insects
Dysaphis plantaginea oviparae were collected from an apple (Malus silvestris c.v. Braburn) orchard (Leckford Fruit Farm, Leckford Estate, Hampshire, UK). Male D. plantaginea were obtained by rearing on apple (M. silvestris c.v. Braburn) in a controlled environment room (12L/12D regime; photophase 16 ± 0.5°C; scotophase 12 ± 0.5°C).
The aphid parasitoid Aphidius ervi was purchased as mummies from Koppert Biological Systems (Product: Ervipar). The mummies were placed into a Petri dish (9-cm diameter, Scientific Laboratory Supplies), and the adult parasitoids emerged into a ventilated polypropylene breeding cage (30 × 30 × 30 cm, Bugdorm 1, Watkins & Doncaster, Kent, UK). Honey solution (1:1 honey/water) on cotton wool was provided as a food source. Emergence cages were kept in a controlled environment room (20°C, 25–40% RH, 16L/8D regime). All parasitoids used in laboratory experiments were naïve-mated females, 1–3 d old.
Isolation of Volatiles
The base of an excised apple (M. silvestris c.v. Braburn) branch, bearing leaves infested with D. plantaginea oviparae (various adult ages), was placed in a glass vessel (500 ml) containing water. The branch was then placed into a glass entrainment vessel (1.5 L). A metal plate containing a hole for the apple branch was clipped to the base of the glass vessel. As a hole was present in the entrainment set-up (an open system), air that had been purified by passage through an activated charcoal filter (BDH, 10–14 mesh, 50 g) was pushed into (700 ml min−1) and pulled out of (600 ml min−1) the vessel. Excised leaves heavily infested with D. plantaginea oviparae (various adult ages) were also placed in a glass vessel (500 ml). Air that had been purified by passage through an activated charcoal filter (BDH, 10–14 mesh, 50 g) was pulled (600 ml min−1) out of the vessels. Volatiles were also collected from uninfested apple leaves and an uninfested apple branch with leaves as controls.
Volatiles were trapped onto Porapak Q 50/80 (50 mg; Supelco, Bellefonte, PA, USA) held in glass tubing (5 mm outer diameter) by two plugs of silanized glass wool. The Porapak Q was conditioned by washing with redistilled diethyl ether (5 ml) and heating at 132°C for 2 hr under a stream of purified N2. After the air entrainment, volatiles were eluted from the Porapak with redistilled diethyl ether (750 µl), and samples were stored in a freezer (−22°C). Because preliminary tests showed that the quantities of pheromone released were low, subsequent entrainment using Porapak Q was carried out over a 4-d period. The procedure was repeated for two additional 4-d periods.
Analysis of Volatiles
Air entrainment samples were analyzed by gas chromatography (GC) on both polar (DB-wax, 30 m × 0.32 mm inner diameter × 0.5 µm film thickness) and non-polar (HP-1, 50 m × 0.32 mm inner diameter × 0.5-µm film thickness) capillary columns with a HP5890 GC (Agilent Technologies, UK) fitted with a cool-on-column injector, a deactivated retention gap (1 m × 0.53 mm inner diameter), and a flame ionization detector (FID). The GC oven temperature was maintained at 30°C for 1 min after sample injection and then raised by 5°C min−1 to 150°C, then 10°C min−1 to 240°C. The carrier gas was hydrogen. Peak enhancement by co-injection with a chemical standard was done to confirm tentative identification of the chemicals present. A multiple-point external standard method was used to quantify the amount of identified chemical components present in the air entrainment samples. Coupled gas chromatography-mass spectrometry (GC-MS) analysis was performed on a Thermofinnigan Instrument MAT95 XP double-focusing magnetic sector mass spectrometer coupled to a TRACE GC fitted with an HP-1 column and integrated data system (Fisons Instruments, Manchester, UK). The GC oven temperature was maintained at 30°C for 5 min and then programmed at 5°C min−1 to 250°C. Ionization was by electron impact at 70 eV, 250°C (source temperature).
Electrophysiology
The air entrainment sample collected from D. plantaginea oviparae was tested by using coupled gas chromatography-electroantennography (GC-EAG) with male D. plantaginea. Two Ag–AgCl glass electrodes were filled with saline solution [composition as in Maddrell (1969), but without glucose]. The head was excised and placed into the indifferent electrode, and the tips of the antennae were severed and inserted into the recording electrode. The signals were passed through a high impedance amplifier (UN-06, Syntech®, The Netherlands) and analyzed by using a customized software package (EAG 2000 and GC-EAG 2000, Syntech®, The Netherlands). Preparations were held in a humidified, charcoal filtered air stream (1 l min−1) coming from a glass tube outlet positioned 0.5 cm from the preparation.
Separation of the volatiles was achieved by GC on a non-polar (HP-1, 50 m × 0.32 mm inner diameter × 0.52-µm film thickness) capillary column using an HP5890 GC (Agilent Technologies, UK) fitted with a cold on-column injector and a FID. The oven temperature was maintained at 30°C for 2 min and then programmed at 5°C min−1 to 100°C and then at 10°C min−1 to 250°C. The carrier gas was hydrogen. The outputs from the EAG amplifier and the FID were monitored simultaneously and analyzed with the software package (EAG 2000, Syntech®, The Netherlands). Chromatograms were compared visually by overlaying traces on a light box and matching corresponding EAG peaks. Six replicates were done.
EAG recordings were also made from male and gynoparous D. plantaginea with three of the identified chemicals (10 µg). The delivery system employed a filter paper in a disposable Pasteur pipette cartridge. The stimulus (2 sec duration, 100 ml min−1) was delivered into a purified air stream (900 ml min−1 during stimulus delivery, 1 l min−1 before and after stimulus delivery) flowing continuously over the preparation. Solutions of synthetic compounds were made in distilled hexane and applied to a filter paper strip. The solvent was allowed to evaporate for 30 sec before the strip was placed in the cartridge. The control stimulus was hexane. Fresh cartridges were prepared immediately prior to each experiment. Responses were compared for significant differences by using Student’s t test. Six replicates were done.
Chemical Standards
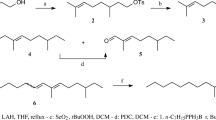
(1R,4aS,7S,7aR)-Nepetalactol (1) and (4aS,7S,7aR)-nepetalactone (2) were synthesized as stated in Dawson et al. (1996). Phenylacetonitrile (benzyl cyanide, 5) was obtained from Sigma-Aldrich, UK (99% purity).
(1S,2R,3S)-Dolichodial (3) (Fig. 1) was extracted from cat thyme, Teucrium marum (Jekka’s Herb Farm, Bristol, UK). The aerial parts of T. marum (102.58 g) were extracted with chloroform (2 × 800 ml) for 24 hr at ambient temperature. The solvent was removed under reduced pressure to yield a golden-brown gum (2.97 g). The extract was subjected to liquid chromatography over Florisil (100–200 mesh, Sigma-Aldrich) with hexane/diethyl ether (1:1) to yield a pale oil (912 mg). Bulb-to-bulb distillation using a Kugelrohr apparatus (90°C, 2 mmHg) yielded four fractions, one of which was shown by comparison of MS, 1H and 13C NMR data with literature values (Pagnoni et al. 1976) to contain (1S,2R,3S)-dolichodial (3) and (1S,2S,3S)-dolichodial (4) (Fig. 1) in a 9:1 ratio (Bellesia et al. 1983a).
Four-Way Olfactometer
Aphid behavioral assays were done by using a Perspex four-way olfactometer [modified from Pettersson (1970), 120 mm diameter]. Air was removed from the center of the olfactometer by a vacuum pump, buffered by a 2-l jar and adjusted with a flow meter to 400 ml min−1. Air was thus pulled through each of the four side arms at 100 ml min−1, and again verified with airflow meters. Teflon tubing was used to attach a glass vessel (25 ml) and a flow meter to each of the four side arms. Polytetrafluoroethylene tape was used to ensure airtight seals between the olfactometer and the Teflon tubing. All five holes were covered with a layer of muslin to prevent access by the aphid during bioassays. To remove any visual stimuli, the olfactometer was placed in the center of a black-walled box with an observation opening at the front and lit from above with diffuse uniform lighting.
Two experiments were conducted. First, the response of male D. plantaginea to different amounts of (1S,2R,3S)-dolichodial (3) (10, 1, 0.1, and 0.01 µg) and hexane (control) was tested. Second, two ratios, 4:1:0 (40:10:0 µg) and a 4:1:0.05 (40:10:0.5 µg) of (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)/(1S,2R,3S)-dolichodial (3) and hexane (control) were tested. An aliquot of the test solution was applied with a micropipette (Drummond “microcaps,” Drummond Scientific Co., USA) to a filter paper strip (solvent allowed to evaporate for 30 sec). The filter paper was placed into one of the glass vessels (25 ml). The three control vessels were similarly treated with the same volume of solvent on the filter paper. Sixteen replicates were done. All treatments were tested in a randomized block experimental design.
The four-way olfactometer arena was split into five areas (four areas by each arm and a central area). A single aphid was introduced into the central area. The time spent and number of entries into each area was recorded by using specialist software (OLFA, Udine, Italy) over a 16-min period. The apparatus was rotated 90° every 4 min to eliminate bias. The proportion of time spent in each of the four side areas was logit-transformed with a correction factor in order to avoid extreme values (Rawlings et al. 1998). The proportion of entries into each of the four areas out of the total number of entries was also calculated. The data were compared by analysis of variance (ANOVA) with randomized blocking (Montgomery 1997), as implemented in Genstat 8.0 (Payne et al. 2005). The analysis was used to look at two parameters: (1) the difference between the treated and control arms and (2) the difference between the three control arms.
Four-Choice Olfactometer
A four-choice olfactometer (Vamvatsikos 2006) [modified from Douloumpaka and van Emden (2003)] was used to test the behavioral response of naïve female A. ervi to (1S,2R,3S)-dolichodial (3). The olfactometer was made from four cylindrical Perspex tubes (test-odor chambers, 9.5 × 2.5 cm internal diameter) each connected to a flow meter by Teflon tubing and a central arena by four smaller pieces of Perspex tubing (4 × 0.5 cm internal diameter). These small tubes protruded 2 cm into the cylindrical Perspex tubes but were flush with the internal surface of the central arena. The central arena comprised a Perspex tube (4.5 cm internal diameter × 2.5 cm height) and a Perspex lid covered the pot firmly, forming an air-tight central arena. The lid was fitted with a small tube connecter (0.5 cm internal diameter) by Teflon tubing to a flow meter and a vacuum pump, buffered by a 2-l jar.
Two experiments were conducted. First, the response of A. ervi to different amounts of (1S,2R,3S)-dolichodial (3) (10, 1, 0.1, and 0.01 µg), a control (hexane), and a positive control [1:1 (10:10 µg) (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)] was tested. Second, two ratios 1:1:0 (10:10:0 µg) and a 1:1:0.05 (10:10:0.5 µg) of (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)/(1S,2R,3S)-dolichodial (3), a control (hexane), and a positive control [1:1 (10:10 µg) (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)] were tested. Glinwood (1998) showed that, in a four-way olfactometer, mated naïve female A. ervi spent significantly more time in the test arm containing 1:1 (10:10 µg) (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2) compared to the control arms (10 µl of hexane), hence the use of this ratio.
An aliquot of the test solution was applied with a micropipette (Drummond “microcaps,” Drummond Scientific Co., USA) to a filter paper strip (solvent allowed to evaporate for 30 sec). The filter paper was placed into two of the cylindrical, Perspex test-odor chambers. The two control test-odor chambers were similarly treated with the same volume of solvent on the filter paper. Sixteen replicates were done. All treatments were tested in a randomized block experimental design.
Twelve mated naïve female A. ervi were drawn into the central arena of the olfactometer by using a pooter. The insects were left to acclimatize for 15 min before the experiment commenced. To remove any visual stimuli, the olfactometer was placed in the center of a black-walled box. Air was removed from the center of the olfactometer at a flow rate of 1.6 l min−1. Air was thus pulled through each of the four side arms at 400 ml min−1. After 30 min, parasitoids that had entered the odor chambers were counted. The whole system (central arena and side arms) was rotated 90° clockwise after each replicate to cancel out any directional bias in the apparatus.
Chi-square analysis was performed on the results from the four-choice olfactometer. The choice chi (which tests the significance of variation between choices) and the heterogeneity chi (which tests the significance of variation between the replicates) were taken into consideration (Gomez 1984).
Results
Electrophysiology
Coupled GC-EAG analysis with male D. plantaginea revealed four EAG-active compounds in the air entrainment sample collected from D. plantaginea oviparae (Fig. 2). Gas chromatography-mass spectrometry and peak enhancement by co-injection using non-polar (HP-1) and polar (DB-Wax) columns confirmed that the peaks were phenylacetonitrile (5), (1SR,2RS,3SR)-dolichodial (3), (1R,4aS,7S,7aR)-nepetalactol (1), and (4aS,7S,7aR)-nepetalactone (2). (1R,4aS,7S,7aR)-Nepetalactol (1), (4aS,7S,7aR)-nepetalactone (2), and (1SR,2RS,3SR)-dolichodial (3) were present in a 4:1:0.05 ratio. These compounds were not detected in the air entrainment samples of apple leaves without D. plantaginea.
Example of a coupled GC-EAG trace of male Dysaphis plantaginea responses to an air entrainment sample from conspecific oviparae. Top trace corresponds to the FID detector on the GC, and the bottom trace corresponds to the antennal response of the insect preparation. Numbers refer to chemicals 1 (1R,4aS,7S,7aR)-nepetalactol (1), 2 (4aS,7S,7aR)-nepetalactone (2), 3 (1S,2R,3S)-dolichodial (3) and 5 phenylacetonitrile (5)
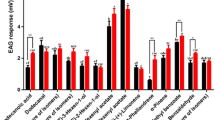
Male and gynoparous D. plantaginea showed a significantly greater EAG response to (1R,4aS,7S,7aR)-nepetalactol (1), (4aS,7S,7aR)-nepetalactone (2), and (1S,2R,3S)-dolichodial (3) compared with the control (hexane) (Fig. 3).
Electrophysiological response (mean ± SE) of male and gynoparous Dysaphis plantaginea to (1R,4aS,7S,7aR)-nepetalactol (1), (4aS,7S,7aR)-nepetalactone (2), and (1S,2R,3S)-dolichodial (3) standards. Asterisks indicate significant differences from the control (solvent) determined using Student’s t test (see text) (**P < 0.01, ***P < 0.001, N = 6)
Behavioral Response of Male Dysaphis plantaginea
Male D. plantaginea spent more time in (P = 0.025) and made a higher proportion of entries (P = 0.012) into the arm containing 1 µg (1S,2R,3S)-dolichodial (3) compared to the control arms (Fig. 4). Male D. plantaginea also spent more time (P = 0.016) in the arm containing 0.1 µg (1S,2R,3S)-dolichodial (3) compared to the control arms. The time male D. plantaginea spent in and the proportion of entries into the arms containing 10 µg and 0.01 µg of (1S,2R,3S)-dolichodial (3) were not significantly different compared to the control arms. A significant difference was not seen between any of the control arms.
The response, a back-transformed mean proportion of time spent and b mean proportion of entries ± SE, by male Dysaphis plantaginea to different amounts of (1S,2R,3S)-dolichodial (3) in the four-way olfactometer. Asterisks indicate significant differences from the control (solvent) determined using ANOVA (see text) (*P < 0.05, **P < 0.01, ***P < 0.001, N = 16)
Male D. plantaginea spent a greater proportion of time in (P = 0.014) and made a higher proportion of entries (P < 0.001) into the arm of the olfactometer where the three-component mixture [4:1:0.05 (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)/(1S,2R,3S)-dolichodial (3)] was present compared to the control arms (Fig. 5). The proportion of time spent in and the proportion of entries into the arms containing the two-component mixture [4:1 (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)] compared to the control arms were not significantly different. A significant difference was not observed between any of the control arms.
The response (back-transformed mean proportion of time-spent) by male Dysaphis plantaginea to different ratios of (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)/(1S,2R,3S)-dolichodial (3) in a four-way olfactometer. Asterisks indicate statistically significant differences from the control (solvent) determined using ANOVA (see text) (***P < 0.001, N = 16)
Behavioral Response of Aphidius ervi
More naïve-mated female A. ervi were counted in the arms containing the positive control (P < 0.001), 10 µg (P < 0.001), and 1 µg (P < 0.01) of (1S,2R,3S)-dolichodial (3) compared to the control arms (Fig. 6). The number of naïve-mated female A. ervi counted in the arms containing 0.1 and 0.01 µg (1S,2R,3S)-dolichodial (3) was not significantly different compared to the control arms.
The response (mean number ± SE) of naïve-mated female Aphidius ervi to different amounts of (1S,2R,3S)-dolichodial (3) in a four-choice olfactometer. Asterisks indicate statistically significant differences from the control (solvent) determined using χ 2-test (see text) (**P < 0.01, ***P < 0.001, N = 16)
The number of naïve-mated female A. ervi counted in arms containing 1:1:0.05 (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)/(1S,2R,3S)-dolichodial (3) was not significantly different compared to the arms containing 1:1 (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2) (Fig. 7).
The response (mean number ± SE) of naïve-mated female Aphidius ervi to different ratios of (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)/(1S,2R,3S)-dolichodial (3) in a four-choice olfactometer. Asterisks indicate statistically significant differences between treatments 1 and 2 determined using a χ 2-test (see text) (***P < 0.001, N = 16)
Discussion
Electrophysiological responses by male D. plantaginea to four chemicals present in volatiles collected from D. plantaginea oviparae were recorded. The EAG-active chemicals were identified as (1R,4aS,7S,7aR)-nepetalactol (1), (4aS,7S,7aR)-nepetalactone (2), (1S,2R,3S)-dolichodial (3), and phenylacetonitrile (5). These four compounds were not detected in the air entrainment sample collected from the host plant, suggesting that they are either released by oviparae or by the plant in response to oviparae feeding. As previously discussed, (1R,4aS,7S,7aR)-nepetalactol (1) and (4aS,7S,7aR)-nepetalactone (2) may be components of D. plantaginea sex pheromone (Stewart-Jones et al. 2007).
Past research has identified phenylacetonitrile (5) as an insect and a plant volatile, involved in insect–plant (Leal et al. 1994; Bartlet et al. 1997) and insect–insect (Norris and Pener 1965; Obeng-Ofori et al. 1993; Torto et al. 1994; Loughrin et al. 1995) interactions. With regard to plant volatiles, phenylacetonitrile (5) has been identified from leaf tissue (Macleod et al. 1981; Loughrin et al. 1995) and flowers (Tatsuka et al. 1990; Knudsen et al. 1993; Leal et al. 1994) but, relevant to this study, occurs as a major volatile from apple fruit (Boeve et al. 1996). This may suggest that phenylacetonitrile (5) present in the air entrainment sample of D. plantaginea oviparae originated from the aphid/plant complex. Thus, phenylacetonitrile (5) is most likely not a component of the aphid sex pheromone, but male D. plantaginea may utilize it synergistically with the sex pheromone components to locate conspecific oviparae (Powell and Hardie 2001). As phenylacetonitrile (5) is thought not to be a component of the aphid sex pheromone, no behavioral studies were conducted.
The fourth compound, (1S,2R,3S)-dolichodial (3), is released by insects. Dolichoderus and Iridomyrmex species of ants release (1S,2R,3S)-dolichodial (3), and it may play a defense or trail role (Cavill and Hinterberger 1960; Cavill and Houghton 1974; Cavill et al. 1982). The diastereoisomer anisomorphal [(1S,2S,3S)-dolichodial (4) (Pagnoni et al. 1976)] is a major component of the defense secretion of the Southern walking stick insect, Anisomorpha buprestoides (Meinwald et al. 1962). In addition, (1S,2R,3S)-dolichodial (3) is structurally related to (4aS,7S,7aR)-nepetalactone (2), a known component of the aphid sex pheromone. Both are methylcyclopentanoid terpenes thought to originate biosynthetically from citronellol. Dawson et al. (1996) suggested that citronellol may be a precursor for the cyclopentanoids biosynthesized in aphids, as during studies on the composition of the sex pheromone of several species of aphid, including D. plantaginea, citronellol accompanied the cylcopentanoid sex pheromone components but was electrophysiologically and behaviorally inactive. Although the biosynthetic pathways from citronellol to (4aS,7S,7aR)-nepetalactone (2) in Nepeta cataria (Lamiaceae = Labiatae) (Bellesia et al. 1984) and (1S,2R,3S)-dolichodial (3) in cat thyme, T. marum (Bellesia et al. 1983b), are thought to be different, when [10–3H](1S,2R,3S)-dolichodial was fed to cut stalks of N. cataria, partial incorporation into (4aS,7S,7aR)-nepetalactone (2) was observed (Bellesia et al. 1984). This suggests that the (1SR,2RS,3SR)-dolichodial (3) present in the air entrainment sample of D. plantaginea oviparae may originate from the oviparae and may be a third component of the sex pheromone.
In behavioral assays, (1S,2R,3S)-dolichodial (3) elicited a response by male D. plantaginea. This suggests that (1S,2R,3S)-dolichodial (3) may be an attractant or an arrestant. This is the first time that electrophysiological and behavioral responses by any aphid morph to (1S,2R,3S)-dolichodial (3) have been reported. Behavioral responses were not recorded when the two-component mixture [4:1 (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)] was present in the bioassay. However, when (1S,2R,3S)-dolichodial (3) was present in a three component mixture [4:1:0.05 (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2)/(1S,2R,3S)-dolichodial (3)] with a ratio equivalent to the ratio in the air entrainment sample, a behavioral response by male D. plantaginea was recorded.
These behavioral data add weight to the possibility that (1S,2R,3S)-dolichodial (3) is a component of the aphid sex pheromone. In addition, mass spectrometric analysis on air entrainment samples collected at Rothamsted Research from Rhopalosiphum padi (bird-cherry-oat aphid), Aphis fabae (black bean aphid), Cryptomyzus maudamanti, and Cryptomyzus ribis (redcurrent blister aphid) oviparae all contain a chemical with the same mass spectra as (1S,2R,3S)-dolichodial (3) (Pickett, Wadhams and Woodcock, unpublished data). As discussed, biological evidence suggests that the sex pheromone of the Cryptomyzus species is likely to comprise more than just the (4aSR,7SR,7aRS)-nepetalactone and (1RS,4aSR,7SR,7aRS)-nepetalactol. As (1S,2R,3S)-dolichodial (3) elicits a behavioral response in male D. plantaginea, this chemical may also be a component of the sex pheromone of Cryptomyzus species and play a role in species integrity.
(1S,2R,3S)-Dolichodial (3) not only elicits a behavioral response by male D. plantaginea but also an electrophysiological response by gynoparous D. plantaginea. Electrophysiological and behavioral responses by gynoparae to (1R,4aS,7S,7aR)-nepetalactol (1) and (4aS,7S,7aR)-nepetalactone (2) have been reported previously (Hardie et al. 1994; Lösel et al. 1996; Park et al. 2000; Zhu et al. 2006). It was suggested that the aphid sex pheromone may act as an aggregation pheromone for gynoparae in order to locate conspecific oviparae on suitable host plants (Lilley and Hardie 1996; Powell and Hardie 2001; Zhu et al. 2006). Therefore, if (1S,2R,3S)-dolichodial (3) is part of the aphid sex pheromone, an EAG response in gynoparae would be expected. Behavioral assays are required to determine whether this compound elicits a behavioral response by gynoparae.
Aphid pheromones provide an ideal method for parasitoids to locate hosts, as they are specific to aphids. If (1S,2R,3S)-dolichodial (3) is part of the sex pheromone of certain aphid species, it may be perceived by A. ervi as a pheromone component and, therefore, should elicit a behavioral response. In this paper, a behavioral response by naïve-mated female A. ervi to (1S,2R,3S)-dolichodial (3) was indeed recorded. However, when a choice was available between the two-component mixture and the three-component mixture, no significant differences were recorded in a four-choice olfactometer. A behavioral response of naïve-mated female A. ervi toward a 1:1 (1R,4aS,7S,7aR)-nepetalactol (1)/(4aS,7S,7aR)-nepetalactone (2) mixture and to (4aS,7S,7aR)-nepetalactone (2) alone has already been reported (Glinwood 1998). This suggests that the presence of (4aS,7S,7aR)-nepetalactone (2) may be needed only to elicit a strong behavioral response by A. ervi.
Air entrainments with oviparae on artificial diets and radio-labeling studies could be conducted to assess whether (1SR,2RS,3SR)-dolichodial (3) is released from the oviparae or from the host/aphid complex. In addition, enantiomeric studies need to be conducted to determine whether (1S,2R,3S)-dolichodial (3) or its enantiomer (1R,2S,3R)-dolichodial is present in the air entrainment sample. The absolute stereochemical configuration is presumed to be (1R,2S,3R)-dolichodial (3), as this enantiomer is behaviorally active and is structurally related to (4aS,7S,7aR)-nepetalactone (2). If (1SR,2RS,3SR)-dolichodial (3) is a third component of the aphid sex pheromone, it will add a new dimension to the ratios of sex pheromone components and may play an important role in species integrity.
References
Bartlet, E., Blight, M. M., Lane, P., and Williams, I. H. 1997. The responses of the cabbage seed weevil Ceutorhynchus assimilis to volatile compounds from oilseed rape in a linear track olfactometer. Entomol. Exp. Appl. 85:257–262.
Bellesia, F., Pagnoni, U. M., Pinetti, A., and Trave, R. 1983a. Teucrein, a new iridolactol from Teucrium marum, and its biosynthetic relationship with dolichodial. J. Chem. Res. Synop. 12:328–329.
Bellesia, F., Pagnoni, U. M., Pinetti, A., and Trave, R. 1983b. The biosynthesis of dolichodial in Teucrium marum. Phytochemistry 22:2197–2201.
Bellesia, F., Grandi, R., Pagnoni, U. M., Pinetti, A., and Trave, R. 1984. Biosynthesis of nepetalactone in Nepeta cataria. Phytochemistry 23:83–87.
Birkett, M. A., and Pickett, J. A. 2003. Aphid sex pheromones: from discovery to commercial production. Phytochemistry 62:651–656.
Blackman, R. L., and Eastop, V. F. 2000. Aphids on the Worlds Crops. 2nd edn. p. 414. Wiley, Chichester.
Blommers, L. H. M., Helsen, H. H. M., and Vaal, F. W. N. M. 2004. Life history data of the rosy apple aphid Dysaphis plantaginea (Pass.) (Homoptera: Aphididae) on plantain and as migrant to apple. J. Pest Sci. 77:155–163.
Boeve, J. L., Lengwiler, U. B., Tollsten, L., Dorn, S., and Turlings, T. C. J. 1996. Volatiles emitted by apple fruitlets infested by larvae of the European apple sawfly. Phytochemistry 42:373–381.
Boo, K. S., Choi, M. Y., Chung, I. B., Eastop, V. F., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 2000. Sex pheromone of the peach aphid, Tuberocephalus momonis and optimal blends for trapping males and females in the field. J. Chem. Ecol. 26:601–609.
Campbell, C. A. M., Dawson, G. W., Griffiths, D. C., Pettersson, J., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1990. Sex attractant pheromone of damson-hop aphid Phorodon humuli (Homoptera: Aphididae). J. Chem. Ecol. 16:3455–3465.
Cavill, G. W. K., and Hinterberger, H. 1960. The chemistry of ants. IV. Terpenoid constituents of some Dolichoderus and Iridomyrmex species. Aust. J. Chem. 13:514–519.
Cavill, G. W. K., and Houghton, E. 1974. Volatile constituents of argentine ant, Iridomyrmex humilis. J. Insect Physiol. 20:2049–2059.
Cavill, G. W. K., Robertson, P. L., Brophy, J. J., Clark, D. V., Duke, R., Orton, C. J., and Plant, W. D. 1982. Defensive and other secretions of the Australian cocktail ant, Iridomyrmex nitidiceps. Tetrahedron 38:1931–1938.
Dawson, G. W., Griffiths, D. C., Janes, N. F., Mudd, A., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1987. Identification of an aphid sex-pheromone. Nature 325:614–616.
Dawson, G. W., Griffiths, D. C., Merritt, L. A., Mudd, A., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1990. Aphid semiochemicals: a review, and recent advances on the sex-pheromone. J. Chem. Ecol. 16:3019–3030.
Dawson, G. W., Pickett, J. A., and Smiley, D. W. M. 1996. The aphid sex pheromone cyclopentanoids: synthesis in the elucidation of structure and biosynthetic pathways. Bioorg. Med. Chem. 4:351–361.
De Berardinis, E., Baronio, P., and Baumgartner, J. 1994. The effect of aphid (Dysaphis plantaginea Pass., Homoptera: Aphididae) feeding on apple fruit-growth. Ecol. Model. 72:115–127.
Douloumpaka, S., and Van Emden, H. F. 2003. A maternal influence on the conditioning to plant cues of Aphidius colemani Viereck, parasitizing the aphid Myzus persicae Sulzer. Physiol. Entomol. 28:108–113.
Forrest, J. M. S., and Dixon, A. F. G. 1975. The induction of leaf-roll galls by the apple aphids Dysaphis devecta and D. plantaginea. Ann. Appl. Biol. 81:281–288.
Glinwood, R. T. 1998. Response of aphid parasitoids to aphid sex pheromones: laboratory and field studies, Ph.D. Thesis. The University of Nottingham.
Goldansaz, S. H., Dewhirst, S. Y., Birkett, M. A., Hooper, A. M., Smiley, D. W. M., Pickett, J. A., Wadhams, L. J., and McNeil, J. N. 2004. Identification of two sex pheromone components of the potato aphid, Macrosiphum euphorbiae (Thomas). J. Chem. Ecol. 30:819–834.
Gomez, K. A. 1984. Statistical Procedures for Agricultural Research. p. 680. Wiley, New York.
Graf, B. 1999. Optimising the control of rosy apple aphid Dysaphis plantaginea (Pass.) (Homoptera: Aphididae). IOBC/WPRS Bull. 22:71–76.
Guldemond, J. A., Dixon, A. F. G., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1992. The role of host plant odour and sex pheromones in mate recognition in the aphid Cryptomyzus. Proceedings of the 8th International Symposium of Insect-Plant Relationships, 119–121.
Guldemond, J. A., Dixon, A. F. G., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1993. Specificity of sex-pheromones, the role of host-plant odor in the olfactory attraction of males, and mate recognition in the aphid Cryptomyzus. Physiol. Entomol. 18:137–143.
Hardie, J., Holyoak, M., Nicholas, J., Nottingham, S. F., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1990. Aphid sex pheromone components: age-dependent release by females and species-specific male response. Chemoecology 1:63–68.
Hardie, J., Visser, J. H., and Piron, P. G. M. 1994. Perception of volatiles associated with sex and food by different adult forms of the black bean aphid, Aphis fabae. Physiol. Entomol. 19:278–284.
Knudsen, J. T., Tollsten, L., and Bergstrom, L. G. 1993. Floral scents—a checklist of volatile compounds isolated by headspace techniques. Phytochemistry 33:253–280.
Leal, W. S., Ono, M., Hasegawa, M., and Sawada, M. 1994. Kairomone from dandelion, Taraxacum officinale, attractant for scarab beetle Anomala octiescostata. J. Chem. Ecol. 20:1697–1704.
Lilley, R., and Hardie, J. 1996. Cereal aphid responses to sex pheromones and host-plant odours in the laboratory. Physiol. Entomol. 21:304–308.
Lösel, P. M., Lindemann, M., Scherkenbeck, J., Maier, J., Engelhard, B., Campbell, C. A. M., Hardie, J., Pickett, J. A., Wadhams, L. J., Elbert, A., and Thielking, G. 1996. The potential of semiochemicals for control of Phorodon humuli (Homoptera: Aphididae). J. Pest Sci. 48:293–303.
Loughrin, J. H., Potter, D. A., and Hamilton-Kemp, T. R. 1995. Volative compounds induced by herbivory act as aggregation kairomones for the Japanese beetle (Popillia japonica Newman). J. Chem. Ecol. 21:1457–1467.
MacLeod, A. J., Pieris, N. M., and Gil, V. 1981. Volatile components of sugar beet leaves. Phytochemistry 20:2292–2295.
Maddrell, S. H. 1969. Secretion by malpighian tubules of Rhodnius: movements of ions and water. J. Exp. Biol. 51:71–97.
Marsh, D. 1972. Sex pheromone in the aphid Megoura viciae. Nature 238:31–32.
Marsh, D. 1975. Responses of male aphids to female sex pheromone in Megoura viciae Buckton. J. Entomol. Ser. A. Physiol. Behav. 50:43–64.
Meinwald, J., Chadha, M. S., Hurst, J. J., and Eisner, T. 1962. Defense mechanisms of arthropods. 9. Anisomorphal, the secretion of a phasmid insect. Tetrahedron 1:29–33.
Montgomery, D. C. 1997. Design and Analysis of Experiments. 4th edn. p. 704. Wiley, New York.
Norris, M. J., and Pener, M. P. 1965. A inhibitory effect of allatectomized males and females on the sexual maturation of young male adults of Schistocerca gregaria (Forskal) (Orthoptera: Acrididae). Nature 208:1122.
Obeng-Ofori, D., Torto, B., and Hassanali, A. 1993. Evidence for mediation of two releaser pheromones in the aggregation behavior of the gregarious desert locust, Schistocerca gregaria (Forskal) (Orthoptera, Acrididae). J. Chem. Ecol. 19:1665–1676.
Pagnoni, U. M., Pinetti, A., Trave, R., and Garanti, L. 1976. Monoterpenes of Teucrium marum. Aust. J. Chem. 29:1375–1381.
Park, K. C., Elias, D., Donato, B., and Hardie, J. 2000. Electroantennogram and behavioural responses of different forms of the bird cherry-oat aphid, Rhopalosiphum padi, to sex pheromone and a plant volatile. J. Insect Physiol. 46:597–604.
Payne, R. W., Murray, D. A., Harding, S. A., Baird, D. B., and Soutar, D. M. 2005. GenStat® for Windows®—Introduction. 8th edn. p. 359. VSN International, Hemel Hempstead.
Pettersson, J. 1970. An aphid sex attractant I. Biological studies. Entomol. Scan. 1:63–73.
Powell, G., and Hardie, J. 2001. The chemical ecology of aphid host alternation: how do return migrants find the primary host plant? Appl. Entomol. Zoolog. 36:259–267.
Rawlings, J. O., Pantula, S. G., and Dickey, D. A. 1998. Applied Regression Analysis: A Research Tool. p. 657. Springer, New York.
Stewart-Jones, A., Dewhirst, S. Y., Durrant, L., Fitzgerald, J. D., Hardie, J., Hooper, A. M., Pickett, J. A., and Poppy, G. M. 2007. Structure, ratios and patterns of release in the sex pheromone of an aphid, Dysaphis plantaginea. J. Exp. Biol. 210:4335–4344.
Tatsuka, K., Suekane, S., Sakai, Y., and Sumitani, H. 1990. Volatile constituents of kiwi fruit flowers: simultaneous distillation and extraction versus headspace sampling. J. Agric. Food Chem. 38:2176–2180.
Torto, B., Obeng-Ofori, D., Njagi, P. G. N., Hassanali, A., and Amiani, H. 1994. Aggregation pheromone system of adult gregarious desert locust Schistocerca gregaria (Forskal). J. Chem. Ecol. 20:1749–1762.
Vamvatsikos, P. G. 2006. Olfactory behaviours associated with host-aphid location in a generalist parasitoid wasp, PhD. University of London.
Wyss, E., Villiger, M., Hemptinne, J. L., and Scharer, H. M. 1999. Effects of augumentative release of eggs and larvae of the ladybird beetle, Adalia bipuncata, on the abundance of the rosy apple aphid, Dysaphis plantaginea, in organic apple orchards. Entomol. Exp. Appl. 90:167–173.
Zhu, J. W., Zhang, A. J., Park, K. C., Baker, T. C., Lang, B., Jurenka, R., Obrycki, J. J., Graves, W. R., Pickett, J. A., Smiley, D., Chauhan, K. R., and Klun, J. A. 2006. Sex pheromone of the soybean aphid, Aphis glycines Matsumura, and its potential use in semiochemical-based control. Environ. Entomol. 35:249–257.
Acknowledgments
Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) of the UK. This work was supported by funds from the UK Department for Environment, Food and Rural Affairs. Rothamsted Research receives grant-aided support from the UK. Many thanks to Dr Salvador Gezan who provided statistical guidance and Tom Palmer, manager of the Leckford Estate Fruit Farm, for permission to use the apple orchards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dewhirst, S.Y., Birkett, M.A., Fitzgerald, J.D. et al. Dolichodial: A New Aphid Sex Pheromone Component?. J Chem Ecol 34, 1575–1583 (2008). https://doi.org/10.1007/s10886-008-9561-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9561-9