Abstract
Gilbert (1976) suggested that male-contributed odors of mated females of Heliconius erato could enforce monogamy. We investigated the pheromone system of a relative, Heliconius melpomene, using chemical analysis, behavioral experiments, and feeding experiments with labeled biosynthetic pheromone precursors. The abdominal scent glands of males contained a complex odor bouquet, consisting of the volatile compound (E)-β-ocimene together with some trace components and a less volatile matrix made up predominately of esters of common C16- and C18-fatty acids with the alcohols ethanol, 2-propanol, 1-butanol, isobutanol, 1-hexanol, and (Z)-3-hexenol. This bouquet is formed during the first days after eclosion, and transferred during copulation to the females. Virgin female scent glands do not contain these compounds. The transfer of ocimene and the esters was shown by analysis of butterflies of both sexes before and after copulation. Additional proof was obtained by males fed with labeled D-13C6– glucose. They produced 13C-labeled ocimene and transferred it to females during copulation. Behavioral tests with ocimene applied to unmated females showed its repellency to males. The esters did not show such activity, but they moderated the evaporation rate of ocimene. Our investigation showed that β-ocimene is an antiaphrodisiac pheromone of H. melpomene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male insects may use morphological, behavioral, or physiological adaptations that reduce the probability of female remating (Simmons 2001; Wedell 2005). One of these adaptations consists of male pheromones, transferred during mating, that make mated females repellent to subsequent males (Happ 1969; Gilbert 1976; Kukuk 1985; Andersson et al. 2000). These pheromones, known as antiaphrodisiacs (Happ 1969), confer direct selective advantage for the donor male by reducing sperm competition. However, there are also indirect advantages for other males and the females themselves, as courtship behavior and male harassment may result in reduced longevity and increased predation risk (Thornhill and Alcock 1983; Cook et al. 1994; Clutton-Block and Langley 1997; Bateman et al. 2006). Although antiaphrodisiac pheromones have been found in a wide range of insects, such as mealworm beetles, sweat bees, fruit flies, and butterflies (Happ 1969; Gilbert 1976; Kukuk 1985; Scott 1986), the chemical structures have been identified only for few species (Scott 1986; Andersson et al. 2000, 2003).
In butterflies, antiaphrodisiac pheromones were first found in the genus Heliconius (Lepidoptera: Nymphalidae). Gilbert (1976) suggested that the strong odor of H. erato mated females could inhibit further mating. He concluded that the odor would repel courting males, as males waiting on female pupae rapidly left when exposed to the abdomen of mated females. All species of Heliconius and related genera, when handled, release strong odors that can be detected by humans (Eltringham 1925). These are produced in glands located in the last abdominal segments on both sexes. In females, it is a large yellow gland, which, when extruded, exposes a pair of club-shaped structures that probably serve to distribute the volatiles. Male glands, on the other hand, are located inside the two highly chitinized claspers that grasp the female abdomen during copulation (Eltringham 1925; Emsley 1963; Simonsen 2006).
In addition to their possible role in sexual communication, odors from abdominal glands may serve as warning signals about the toxicity of these butterflies (Eltringham 1925). Fritz Müller described the odors as strong and nauseous, and suggested they are also distasteful to enemies because they are released only after handling the butterflies (Eltringham 1925). The major volatile released from the abdominal scent glands of males and females of the heliconiine Agraulis vanillae is 6-methyl-5-hepten-2-one (Ross et al. 2001). The authors proposed that this ketone has a protective function, as it is a known defensive allomone of ants and cockroaches (Tomalsky et al. 1987; Ross et al. 2001). Their conclusions were based on observations of bird predation, but their experiments did not discriminate between protection due to gland constituents and those resulting from the wing warning coloration and toxicity (Nahrstedt and Davis 1983, 1985; Engler-Chaouat and Gilbert 2007). The idea of a male antiaphrodisiac, already reported in pierid butterflies and Heliconius (Gilbert 1976; Andersson et al. 2000, 2003), was not discussed by Ross et al. (2001).
Although Heliconius butterflies have been extensively studied and their characteristic odors described subjectively more than one century ago, few attempts have been made to elucidate their function and chemical composition (Eltringham 1925; Gilbert 1976; Miyakado et al. 1989; Ross et al. 2001). Here, we report work on the chemical composition of abdominal scent glands of H. melpomene and their role as an antiaphrodisiac pheromone. We show that the chemicals from abdominal scent glands are synthesized by the males only, are transferred to the females during copulation, and repel other courting males from mated females.
Methods and Materials
Chemicals
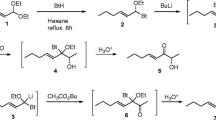
Ocimene was prepared according to the method of Matsushita and Negishi (1982), resulting in a 7:3 mixture of (Z)- and (E)-ocimene. The amount of the (E)-isomer was increased to a 4:6 Z/E ratio by argentation-chromatography with AgNO3-treated silica (Williams and Mander 2001). We were unable to obtain a higher ratio, as no convenient and reproducible synthetic route to pure (E)-ocimene currently exists.
The ester mixture was prepared in a combinatorial way by using a mixture of fatty acids (ratios, see Table 1). The acids were converted into their acid chlorides by reaction with oxalyl chloride (Drutu et al. 2001). The acid chloride mixture was treated with an equimolar amount of an alcohol mixture (ratios, see Table 1) according to standard methods (Franklin et al. 2003). This ensured that every acid present in the mixture was esterified with each alcohol, resulting in a mixture of 25 esters. The analysis of the purified reaction product revealed that this ester mixture (hereafter called esters) was similar in composition and relative abundance of each component to the ester bouquet produced by male H. melpomene.
Analytical Procedures
Chemical analyses were carried out on individual butterflies from a colony of H. melpomene rosina held in a greenhouse at Freiburg (Germany). The culture, originating from butterflies collected in Corcovado, Osa Peninsula, Costa Rica, was reared for about five generations with Passiflora caerulea as host plant and Lantana camara as source of pollen. The butterflies also had access to sucrose solution supplemented with pollen. Additional samples were obtained by a colony of H. melpomene rosina held at the University of Texas at Austin, originating from individuals brought from Costa Rica. They were reared in greenhouses at about 32°C and high humidity (>80%). Butterflies had access to P. oerstedi (larval host plant), sugar, and honey water, and sources of pollen from Psiguria sp., Psychotria poeppigiana and L. camara flowers.
Butterflies were analyzed individually. The extracts were prepared either immediately after emergence from pupae or 5 d later. Some 5-d-old males were allowed to mate, and their glands were analyzed either directly after copulation or 3 d later.
Claspers of males and the last abdominal segment of females were dissected from bodies of freshly killed butterflies and placed individually in vials with approximately 100 μl of pentane. The lower tips of the abdomens were also cut and stored in vials with pentane. Analyses of the latter served to identify compounds found in tissues surrounding scent glands. Samples were kept at −70°C until analyzed.
Gas chromatography and mass spectrometry (GC-MS) of pentane extracts were performed with a Hewlett-Packard model 5973 mass selective detector connected to a Hewlett-Packard model 6890 gas chromatograph with a BPX5-fused silica capillary column (SGE, 30 m × 0.25 mm, 0.25-μm film thickness). Injection was performed in splitless mode (250°C injector temperature) with helium as the carrier gas (constant flow of 1 ml/min). The temperature program started at 50°C, was held for 1 min, and then rose to 320°C with a heating rate of 5°C/min. All compounds were identified by comparison of the mass spectra and retention times with those of authentic reference samples in the different compound classes as well as by analysis of mass spectral fragmentation patterns.
The quantification of individual components was difficult because of many overlapping peaks, therefore peak areas of several compound classes were combined, and the sum of this area determined against an internal standard (hecadecanenitril). Results in Table 3 show individual variation of the scent gland composition. These data and results of the analysis of additional individuals were combined to examine the proportion of compounds transferred during copulation (Fig. 3).
Odors released by mated females or females of treatment 3 (see below) were analyzed by using headspace Solid Phase Microextraction (SPME) with a 65-μm polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber (Supelco) for 5 min. Analysis was performed with a Finnigan Q-GC gas chromatograph with a DB5-MS-fused silica capillary column (13 m × 0.32 mm, 0.25-μm film thickness) coupled to a GCQ Polaris mass spectrometer. Splitless injection at 250°C was used with a temperature program rising from 60°C to 220°C, with heating rate of 10°C/min. Carrier gas was helium at a pressure of 4 psi.
Feeding Experiments
Feeding experiments were carried out at the University of Texas at Austin. Freshly eclosed males were fed daily with isotopically labeled compounds of any of three potential biosynthetic precursors of ocimene: 25 mg of 99% D-13C6-glucose, 5 mg of 99% 13C2-sodium acetate, and 10 mg of 4,4,6,6,6-D5-mevalolactone (Dickschat et al. 2005), all in 5 ml of honey water. Each group of males was enclosed in a 2 × 2 × 2 m insectary with access to sugar water and one of the feeding mixtures added to an artificial flower. Males were also fed by gently holding the butterfly and placing drops of solution directly onto the proboscis with a syringe until satiation (ca. 25 μl). This was done daily during 10 d or more, until they mated to a virgin female. After mating, terminal abdominal segments of both sexes containing putative pheromone glands were dissected and analyzed by GC-MS as described in the analytical procedures.
Behavioral Bioassays
The experiment were also conducted in greenhouses at the University of Texas at Austin. Abdomens of 1- to 5-d-old virgin females were painted with 2 μl of one of three chemical mixtures: (1) analytical grade hexane used as control; (2) a 1:1 solution of the synthetic ester mixture (Table 1) and hexane (1 mg/individual of ester mixture); and (3) a 0.1:1:1 solution of 6:4 (E/Z)-β-ocimene, ester mixture, and hexane (100 μg/individual of β-ocimene).
Males, randomly chosen from a population held in a separate greenhouse and never exposed to females, were tested individually for their behavior in the presence of treated virgin females. Only males more than 3 d old were used in the experiments. Each test consisted of two phases. The male was first exposed to a virgin female for 10 min, during which time the number and duration of courtship bouts were recorded. Once the male grasped the female abdomen with his claspers, the couple was immediately separated, and the male was allowed to rest for 10 min. The immediate separation after males initiated copulation assured that there was no transfer of spermatophore or scents from males. The aim of this phase was to verify the sexual receptivity of both butterflies; therefore, only males that succeeded on grasping female abdomens were used in the second phase of the test. In the second phase, the same female was transferred back to the male insectary after applying on the top of her abdomen 2 μl of one of the test solutions. Here, number and duration of courtship bouts were measured for 10 min or until the male grasped the female’s abdomen again. A total of 30 test were carried out, 10 for each treatment of virgin females. Females were used once and 4 out of 26 males were used in more than one test. Whenever males were reused, tests were done at least 2 d apart, and different chemical mixtures were used in the experimental phase.
We tested whether the proportion of matings in experiments done with females painted with test mixtures that contained possible repellents was lower than those with control females by using Fisher’s exact test (Sokal and Rohlf 1969). Mean courtship events and courtship times between the two phases of tests were compared with a nonparametric Wilcoxon Matched Pairs Test. We also compared these variables between experiments with females painted either control or test mixtures using nonparametric Mann–Whitney U Tests (STATISTICA for Windows 1999).
Results
Chemical Analyses
The GC-MS analyses of abdominal gland extracts of males showed a bouquet made up of more than 100 components belonging to different chemical classes (Figs. 1 and 2, Table 2). The extracts of mated females showed a similar chemical composition, albeit in lower concentrations (Fig. 1, Table 2), whereas those of unmated female glands exhibited a markedly reduced composition (Fig. 1, Table 2). It is clear from the total ion chromatogram (TIC, Fig. 1) that the male blend consisted of early and late eluting parts. The early eluting compounds are made up of several volatile components clustered in the TIC mostly around the major constituent, (E)-β-ocimene (1). Minor amounts of the terpenes (Z)-β-ocimene, allo-ocimene, β-cyclocitral, (E,E)-α-farnesene, and traces of the alcohols hexanol and (Z)-3-hexenol were detected. Additional trace compounds include three pyrazines (e.g., 2), as well as guaiacol (3).
The later eluting (and thus higher boiling) fraction was dominated by a complex ester mixture (Table 2) of common C16- and C18-fatty acids with the alcohols tabulated in Table 1 (6–11). A range of macrolides derived from a bishomolog series of C12- to C20-acids, oxidized at the ω or ω-1 position, were also present in trace amounts (4 and 5). Along with esters, this fraction also contained typical cuticular components of insects as unbranched and methyl-branched alkanes, together with small amounts of 2,5-dialkyltetrahydrofurans (Schulz et al. 1998). The fatty acid content was variable (ranging from major to minor constituents of the bouquet). However, analyses of abdominal samples without the scent glands showed that fatty acids, alkanes, cholesterol, squalene, and dialkyltetrahydrofurans are not specific to the scent glands, so their presence probably reflects more the quality of the sample preparation than the physiological state of an individual. Consequently, we excluded these compounds from the bioassays. The composition of the male scent glands was remarkably similar among individual butterflies raised at the different locations and on different host plants, showing only slight variations in the trace components and the proportions of esters and alkanes.
An obvious difference between the two parts of the blend is volatility. The calculated vapor pressure of 1, the most prominent volatile component, is 1.56 Torr, whereas a major late eluting compound, hexyl octadecenoate, has a value of 4.94 × 10-8 Torr. One of the earliest eluting, and thus more volatile esters, ethyl hexadecanoate, has a value of 7.63 × 10−5 Torr (Scifinder database, ACS 2007). We, therefore, designate the ester part of the mixture as a matrix. Furthermore, because of their vapor pressures, macrolides, alkanes, tetrahydrofuranes, ketones, fatty acids, and cholesterol were all considered to belong to the matrix.
Abdominal glands from individuals of different age were also analyzed (Table 3). Freshly emerged males and females lacked volatile compounds, except for small amounts of guaiacol (3) in their scent glands. The only other compounds identified were the common C16- and C18-fatty acids together with hydrocarbons, cholesterol, and squalene, which are not gland-specific compounds. Five days after eclosion, males had developed the whole bouquet of compounds with 1 reaching up to 75 μg/individual. In males, bouquets before and after mating did not change significantly. Unmated females only produced 3, but had a bouquet similar to that of males after mating. Even 3 d after mating, bouquet components were detectable. A quantitative analysis of a limited number of individual butterflies is found in Table 3. Occurrence of 1 and the esters in individuals of different physiological state is shown in Fig. 3.
Feeding Experiments with Labeled Percursors
Deuterated mevalolactone, often incorporated by plants and bacteria into terpenes, was incorporated into 1 in males, but at a low rate (0.5%). 13C-labeled acetate also gave low incorporation into 1, but proved to be toxic, and surviving males did not mate. The best results were obtained by using uniformly labeled U-13C-glucose. Glucose can be converted by butterflies into doubly labeled acetate via the citric acid cycle. This acetate can enter the mevalonate pathway of terpene synthesis. Together with two additional unlabeled acetate units, present in excess in experimental butterflies, it is transformed into isopentenyl pyrophosphate building blocks that contain one or two 13C-atoms. This labeling pattern is expected because one carbon from the three acetate units is lost during this process, being either a 12C- or 13C-atom (Cane 1999). Indeed, 1 labeled with one or two 13C-atoms was observed in the experiments. The possibility of glucose entering into terpene biosynthesis by the novel desoxyxylulose pathway (Eisenreich et al. 2004) was tested, but there was no evidence that this occurred.
The incorporation rate was determined by the relative abundance of the ions m/z 136/137/138 and m/z 93/94/95 in the mass spectrum of 1, taking into account the natural abundance of 13C (Fig. 4). The obtained values of 6 to 10% showed that males indeed biosynthesized 1 de novo. Females contained labeled 1 after mating with U-13C-glucose fed males. Labeled esters, hydrocarbons, and tetrahydrofurans were also found in those females. These findings confirm that not only 1, but also the esters and hydrocarbons, are transferred from males to females during mating.
Laboratory Bioassays
In preliminary tests, β-ocimene showed a repellent effect on males, but its effect vanished quickly. We, therefore, choose to test this compound in combination with an artificial mixture of esters, similar in proportion to those found in the abdominal scent glands. Our assumption was that the esters might serve as a matrix, reducing the rate of evaporation of β-ocimene, so that the signal would stay longer on the females. We used the synthetic ester mixture because the esters are the most important compound class of the low boiling constituents of the secretion. The other compounds were present only in low concentration or were part of the cuticule, as stated above. The odor emitted from females painted with the β-ocimene/ester solution was similar to the odor sampled from recently mated females when compared by GC-MS.
Males did not significantly change their behaviors toward females painted with hexane (control) as neither the mean number of courtship bouts (Z = 1.069, P = 0.285, Wilcoxon matched pair test, N = 10) nor the mean courtship duration (Z = 0.815, P = 0.451, Wilcoxon matched pair test, N = 10) differed in the two experimental phases. Moreover, in 8 out of 10 tests males initiated copulation by grasping female abdomens after hexane was added to their abdomens.
Similarly, the ester mixture alone did not make females less attractive to courting males, as courtships were as long as those directed to control females (Mann–Whitney Test, P = 0.705, N = 10, Fig. 5). Furthermore, 70% of the couples engaged in copulation after painting females with esters, a percentage comparable to the one observed in control experiments (Fisher’s exact test, P = 1, N = 10). Male behavior toward control or ester-painted females was similar to that observed in the first experimental phase. In all cases, males quickly approached and courted females, and in more than 90% of these tests, they grasped female abdomens within the first 2 min of the experiment.
In contrast to esters, β-ocimene strongly reduced female attractiveness. Although males exposed to females with β-ocimene plus esters quickly and repeatedly approached them, they courted them only for a few seconds and then left. The courtship duration was in average significantly shorter than in control experiments (Mann–Whitney Test, P = 0.002, N = 10, Fig. 5). In addition, only one of the 10 couples engaged in copulation in the second experimental phase, a lower proportion compared to that in control tests (Fisher’s exact test, P = 0.006, N = 10).
Discussion
Our results show that male H. melpomene produce a complex odor profile that consists of volatile components as 1, and a matrix of low-boiling-point components dominated by esters and hydrocarbons. This mixture is transferred to females during copulation, and the volatile component β-ocimene (1) makes females unattractive to courting males. As both ocimene isomers occur naturally and our synthetic sample also contained both, we cannot determine their relative strengths as repellents at this time. However, the prevalence of the (E)-isomer suggests this isomer to be active. The semivolatile ester matrix does not repel males, but in our experiments it reduced the evaporation rate of the ocimene, thus suggesting that this could be, at least in part, its function in female glands. Because the abdominal scent glands in this butterfly produce a bouquet that contains many different compounds, the possibility that other constituents of the scent gland also have antiaphrodisiac activity cannot be excluded. Such components might also provide other kinds of information to conspecifics, as in insects, even trace compounds of extracts can be important cues (e.g., Danci et al. 2006).
Interestingly, (E)-β-ocimene is one of the most prominent plant semiochemicals. It is commonly released by leaves in response to insect feeding or by flowers to attract pollinators (Pare and Tumlinson 1999; Andersson et al. 2002). Although the presence of this volatile has not been investigated in natural host plants or preferred pollen sources of H. melpomene (Boggs et al. 1981; Estrada and Jiggins 2002), 1 has been found in larval and adult host plants that are common in disturbed areas or that are frequently used to keep these butterflies in captivity, e.g. L. camara and P. caerulea (Piel et al. 1998; Andersson et al. 2002; Andersson and Dobson 2003b). Therefore, even if our experiments with labeled precursors show that H. melpomene has the capacity to synthesize 1 de novo, the additional contribution of 1 obtained from flowers or larval host plants cannot be ruled out.
Andersson and Dobson (2003a) showed that male and female H. melpomene antennae detect 1 and, in combination with other flower volatiles and color cues, was attractive to both sexes of butterflies. Taking into account the acute vision of Heliconius (Swihart 1972; Stavenga 2002; Zaccardi et al. 2006), the attractive or repellent activity of 1 might be explained by context specificity. In combination with other flower volatiles and visual flower cues, ocimene might be attractive, whereas with other conspecific gland constituents and visual signals, 1 could be repellent. This model points to further functions of the other male bouquet components besides the matrix effect discussed above. It also supports the observation that the antiaphrodisiac acts only over a short range.
Male behavior of H. melpomene toward mated females was similar to that described for pierid butterflies (Andersson et al. 2000) and Drosophila melanogaster (Scott 1986). Males approach mated females as often as unmated ones, clearly attracted by visual cues (Jiggins et al. 2004). When males come into close range to the mated females, the latter take mate-refusal posture by exposing their scent glands and release previously transferred compounds. Males immediately leave, so they spend less time courting mated than virgin females. In Heliconius, wing color patterns of females and males are identical, and males are visually attracted to both sexes. Males also release β-ocimene from their genitalia (claspers, unpublished data), which might serve for recognition of males and terminate male–male encounters. Therefore, it does appear that the antiaphrodisiac(s) of H. melpomene make mated females unattractive because they smell like males, as suggested earlier in Heliconius (Gilbert 1976) and found in Pieris brassicae and D. melanogaster (Scott 1986; Andersson et al. 2003).
Although antiaphrodisiac pheromones have been found in other groups of butterflies (Andersson et al. 2003), only the females of the Heliconiinae expose abdominal scent glands when they are courted or disturbed (Emsley 1963). This suggests that these odor mixtures might serve both as antiaphrodisiac and protection. In fact, defense mechanism and sexual communication are tightly linked in unpalatable butterflies and moths (Boppré 1978, 1984; Weller et al. 1999; Conner et al. 2000; Schulz et al. 2004). The Lepidopteran scent glands studied so far show that the bouquets contain many different compounds (Miyakado et al. 1989; Ross et al. 2001; Schulz et al. 2007). Hence, it is possible that such odors provide conspecifics and predators with information. For example, the odor bouquet of H. melpomene contains three pyrazines, e.g., 3-isopropyl-2-methoxypyrazine (2), which are general warning odors of chemically defended insects and deter rats and birds (Kaye et al. 1989; Moore et al. 1990; Lindström et al. 2001). Unpalatable insects often use combinations of warning signals that target different sensory modalities (Lindström et al. 2001; Jetz et al. 2001). There is no evidence, other than the response to rough handling, that Heliconius females expose glands during attacks by predators. However, components in Heliconiinae odors, together with visual signals from the yellow glands, could alert, remind, and discourage predators.
Our findings that semiochemicals with evidence of antiaphrodisiac function are transferred from male to female H. melpomene support the observations published earlier by Gilbert (1976) for H. erato. Furthermore, we have similar results for other Heliconius and two other Heliconiinae in the genus Argynnis (Schulz, Yildizhan, Boppré, Estrada, Gilbert, unpublished; Schulz et al. 2007). Although the composition of these bouquets varies widely, male secretions of most Heliconiinae consists of more volatile compounds and a complex matrix of semivolatile components. This suggests a similar function of the scent glands in other Heliconiinae.
References
Andersson, S. and Dobson, H. E. M. 2003a. Antennal responses to floral scents in the butterfly Heliconius melpomene. J. Chem. Ecol. 29:2319–2330.
Andersson, S. and Dobson, H. E. M. 2003b. Behavioral foraging responses by the butterfly Heliconius melpomene to Lantana camara floral scent. J. Chem. Ecol. 29:2303–2318.
Andersson, J., Borg-Karlson, A. K., and Wiklund, C. 2000. Sexual cooperation and conflict in butterflies: A male-transferred anti-aphrodisiac reduces harassment of recently mated females. Proc. R. Soc. Lond. B 267:1271–1275.
Andersson, S., Nilsson, L. A., Groth, I., and Bergström, G. 2002. Floral scents in butterfly-pollinated plants: possible convergence in chemical composition. Botan. J. Linn. Soc. 140:129–153.
Andersson, J., Borg-Karlson, A. K., and Wiklund, C. 2003. Antiaphrodisiacs in pierid butterflies: A theme with variation! J. Chem. Ecol. 29:1489–1499.
Bateman, P. W., Ferguson, J. W. H., and Yetman, C. A. 2006. Courtship and copulation, but not ejaculates, reduce the longevity of female field crickets (Gryllus bimaculatus). J. Zool. 268:341–346.
Boggs, C. L., Smiley, J. T., and Gilbert, L. E. 1981. Patterns of pollen exploitation by Heliconius butterflies. Oecologia 48:284–289.
Boppré, M. 1978. Chemical communication, plant relationships, and mimicry in the evolution of danaid butterflies. Entomol. Exp. Appl. 24:264–277.
Boppré, M. 1984. Chemically mediated interactions between butterflies, pp. 259–275, in R. I. Vane-Wright and P. R. Ackery (eds.). The Biology of Butterflies. GB-London: Academic Press, reprinted edition 1989 by Princeton University Press.
Cane, D. E. 1999. Isoprenoid biosynthesis: Overview, pp. 1–13, in D. Barton, K. Nakanishi, O. Meth-Cohn, D. E. Cane (eds.). Comprehensive Natural Products Chemistry Vol. 2. Elsevier, Amsterdam.
Clutton-Block, T. and Langley, P. 1997. Persistent courtship reduces male and female longevity in captive tsetse flies Glossina morsitans morsitans Westwood (Diptera: Glossinidae). Behav. Ecol. 8:392–395.
Conner, W. E., Boada, R., Schroeder, F. C., Gonzalez, A., Meinwald, J., and Eisner, T. 2000. Chemical defense: Bestowal of a nuptial alkaloidal garment by a male moth on its mate. Proc. Natl. Acad. Sci. USA 97:14406–14411.
Cook, S. E., Vernon, J. G., Bateson, M., and Guilford, T. 1994. Mate choice in the polymorphic African swallowtail butterfly, Papilio dardanus: male-like female may avoid sexual harassment. Anim. Behav. 47:389–397.
Danci, A., Gries, R., Schaefer, P. W., and Gries, G. 2006. Evidence for four-component close-range sex pheromone in the parasitic wasp Glyptapanteles flavicoxis. J. Chem. Ecol. 32:1539–1554.
Dickschat, J. S., Bode, H. B., Mahmud, T., Müller, R., and Schulz, S. 2005. A novel type of geosmin biosynthesis in myxobacteria. J. Org. Chem. 70:5174–5182.
Drutu, I., Krygowski, E. S., and Wood, J. L. 2001. Reactive enols in synthesis 2. Synthesis of (+)-latifolic acid and (+)-latifoline. J. Org. Chem. 66:7025–7029.
Eisenreich, W., Bacher, A., Arigoni, D., and Rohdich, F. 2004. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell. Mol. Life Sci. 61:1401–1426.
Eltringham, M. A. 1925. On the abdominal glands in Heliconius (Lepidoptera). Trans. Entomol. Soc. Lond. 269–275.
Emsley, M. G. 1963. A morphological study of image Heliconiinae (Lep.: Nymphalidae) with a consideration of the evolutionary relationships within the group. Zoologica 48:85–131.
Engler-Chaouat, H. S. and Gilbert, L. E. 2007. De novo synthesis vs. sequestration: Negatively correlated metabolic traits and the evolution of host plant specialization in cyanogenic butterflies. J. Chem. Ecol. 33:25–42.
Estrada, C. and Jiggins, C. D. 2002. Patterns of pollen feeding and habitat preference among Heliconius species. Ecol. Entomol. 27:448–456.
Franklin, C. L., Li, H., and Martin, S. F. 2003. Design, Synthesis, and Evaluation of water-soluble phospholipid analogues as inhibitors of phospholipase C from Bacillus cereus. J. Org. Chem. 68:7298–7307.
Gilbert, L. E. 1976. Postmating female odor in Heliconius butterflies: A male-contributed antiaphrodisiac? Science 193:419–420.
Happ, G. 1969. Multiple sex pheromones of the mealworm beetle, Tenebrio molitor L. Nature 222:180–181.
Jetz, W., Rowe, C., and Guilford, T. 2001. Non-warning odors trigger innate color aversions—as long as they are novel. Behav. Ecol. 12:134–139.
Jiggins, C. D., Estrada, C., and Rodrigues, A. 2004. Mimicry and the evolution of premating isolation in Heliconius melpomene Linnaeus. J. Evol. Biol. 17:680–691.
Kaye, H., Mackintosch, N. J., Rothschild, M., and Moore, B. P. 1989. Odour of pyrazine potentiates an association between environmental cues and unpalatable taste. Anim. Behav. 37:1–6.
Kukuk, P. 1985. Evidence for an antiaphrodisiac in the sweat bee Lasioglossum (Dialictus) zephyrum. Science 227:656–657.
Lindström, L., Rowe, C., and Guilford, T. 2001. Pyrazine odour makes visually conspicuous prey aversive. Proc. R. Soc. Lond B. 268:159–162.
Matsushita, H. and Negishi, E. 1982. Palladium-catalyzed reactions of allylic electrophiles with organometallic reagents. A regioselective 1,4-elimination and a regio- and stereoselective reduction of allylic derivatives. J. Org. Chem. 47:4161–4165.
Miyakado, M., Meinwald, J., and Gilbert, L. E. 1989. (R)-(Z,E)-9,11-Octadecadien-13-olide: An intriguing lactone from Heliconius pachinus (Lepidoptera). Experientia 45:1006–1008.
Moore, B. P., Brown, W. V., and Rothschild, M. 1990. Methylalkylpyrazines in aposematic insects, their hostplants and mimics. Chemoecology 1:43–51.
Nahrstedt, A. and Davis, R. H. 1983. Occurrence, variation and biosynthesis of the cyanogenic glucosides linamarin and lotaustralin in species of the Heliconiini (Insecta: Lepidoptera). Comp. Biochem. Physiol. 75B:65–73.
Nahrstedt, A. and Davis, R. H. 1985. Biosynthesis and quantitative relationships of the cyanogenic glucosides, linamarin and lotaustralin, in genera of the Heliconiini (Insecta: Lepidoptera). Comp. Biochem. Physiol. 82B:745–749.
Pare, P. W. and Tumlinson, J. H. 1999. Plant volatiles as a defense against insect herbivores. Plant Physiol. 121:325–332.
Piel, J., Donath, J., Bandemer, K., and Boland, W. 1998. Mevalonate-independent biosynthesis of terpenoid volatiles in plants: induced and constitutive emission of volatiles. Angew. Chem. Int. Ed. 37:2478–2481.
Ross, G. N., Fales, H. M., Lloyd, H. A., Jones, T., Sokoloski, E. A., Marshall-Batty, K., and Blum, M. S. 2001. Novel chemistry of abdominal defensive glands of nymphalid butterfly Agraulis vanillae. J. Chem. Ecol. 27:1219–1228.
Schulz, S., Beccaloni, G., Nishida, R., Roisin, Y., Vane-Wright, R. I., and Mcneil, J. N. 1998. 2,5-Dialkyltetrahydrofurans, common components of the cuticular lipids of Lepidoptera. Z. Naturforsch. 53c:107–116.
Schulz, S., Beccaloni, G., Brown, K. S., Boppré, M., Freitas, A. V. L., Ockenfels, P., and Trigo, J. R. 2004. Semiochemicals derived from pyrrolizidine alkaloids in male ithomiine butterflies (Lepidoptera: Nymphalidae: Ithomiinae). Biochem. Syst. Ecol. 32:699–713.
Schulz, S., Yildizhan, S., Stritzke, K., Estrada, C., and Gilbert, L. E. 2007. Macrolides from the scent glands of the tropical butterflies Heliconius cydno and Heliconius pachinus. Org. Biomol. Chem. 5:3434–3441.
Scott, D. 1986. Sexual mimicry regulates the attractiveness of mated Drosophila melanogaster females. Proc. Natl. Acad. Sci. USA 83:8429–8433.
Simmons, L. W. 2001. Sperm Competition and Its Evolutionary Consequences in the Insects. Princeton University Press, Princeton and Oxford.
Simonsen, T. J. 2006. Glands, muscles and genitalia. Morphological and phylogenetic implications of histological characters in the male genitalia of fritillary butterflies (Lepidoptera: Nymphalidae: Argynnini). Zool. Scripta 35:231–241.
Sokal, R. R. and Rohlf, J. 1969. Biometry. W. H. Freeman and Company, San Francisco.
Stavenga, D. G. 2002. Reflections on colourful ommatidia of butterfly eyes. J. Exp. Biol. 205:1077–1085.
Swihart, C. A. 1972. The neural basis of color vision in the butterfly, Heliconius erato. J. Insect Physiol. 18:1015–1025.
Thornhill, R. and Alcock, J. 1983. The Evolution of Insect Mating Systems. Harvard University Press, Cambridge.
Tomalsky, M. D., Blum, M. S., Jones, T. H., Fales, H. M., Howard, D. F., and Passera, L. 1987. Chemistry and function of exocrine glands of the ants Tapinoma melanocephalum and T. erraticum. J. Chem. Ecol. 13:253–263.
Wedell, N. 2005. Female receptivity in butterflies and moths. J. Exp. Biol. 208:3433–3440.
Weller, S. J., Jacobson, N. L., and Conner, W. E. 1999. The evolution of chemical defenses and mating systems in tiger moths (Lepidoptera: Arctiidae). Biol. J. Linn. Soc. 68:557–578.
Williams, C. M. and Mander, L. N. 2001. Chromatography with silver nitrate. Tetrahedron 57:425–447.
Zaccardi, G., Kelber, A., Sison-Mangus, M. P., and Briscoe, A. D. 2006. Color discrimination in the red range with only one long-wavelength sensitive opsin. J. Exp. Biol. 209:1944–1955.
Acknowledgments
We thank A. Wartenberg, R. Watkins, S. Marout, and K. Busby for assisting rearing butterflies; the United States Department of Agriculture for import and rearing permits, and Costa Rica’s Ministerio del Ambiente y Energía for collection and exportation permits. This work was funded by the Deutsche Forschungsgemeinschaft and the University of Texas at Austin graduate program in Ecology, Evolution, and Behavior. This material is also based on work supported by the National Science Foundation and the Office of International Science and Engineering under grant No 0608167. Austin facilities were developed through grants from NSF and matching support from UT Austin to LEG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulz, S., Estrada, C., Yildizhan, S. et al. An Antiaphrodisiac in Heliconius melpomene Butterflies. J Chem Ecol 34, 82–93 (2008). https://doi.org/10.1007/s10886-007-9393-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9393-z