Abstract
Adults of the sulfur butterfly Colias erate poliographus Motschulsky showed sexual dimorphism in their epicuticular composition, with octadecanal and hexyl myristate, palmitate, and stearate identified as male-specific compounds in 3-day-old adults. Since males of the closely related North American species Colias philodice Godart also have the 3 hexyl esters and utilize them as aphrodisiac pheromones toward conspecific females, these substances are likely to serve as pheromones for C. erate. These male-specific compounds were more abundant in wings, especially forewings, than in the body. Male wings lacked androconia but possessed characteristic intermembranous cells. These cells were present behind the base of the socket of ordinary scales and distributed from the basal to discal areas in the forewing costal region and hind wing inner-marginal region. The intermembranous cells were morphologically similar to the male-specific secretory organs of C. philodice and seemed to be the source of the male-specific compounds of C. erate. However, in the wing areas including these cells, the wing scales contained larger amounts of male-specific compounds than the wing membrane, suggesting that the male-specific compounds produced in the intermembranous cells were transferred to and disseminated from the wing scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Androconia are specialized wing scales exclusively present in adult males of certain butterfly species. In some butterfly families, including Pieridae, Lycaenidae, Danaidae, and Nymphalidae, there is a wide variety of forms and distribution patterns of androconia (Kristensen and Simonsen 2003). The morphological features of androconia are often useful as taxonomic criteria for several species (e.g., Hall and Harvey 2002; Ômura et al. 2015). Because their forms are distinct from ordinary wing scales, androconia are believed to be involved in the storage and emanation of scent substances. Indeed, in many butterfly species, various volatile substances have been extracted from male wings containing androconia, and several of them serve as sex pheromones in close-range courtship displays (Vane-Wright and Boppré 1993). In Pieridae, adult males of the genus Pieris (Pierinae) have characteristic alar androconia (Warren 1961), and Pieris rapae (L.), P. napi (L.), and P. brassicae (L.) adult males emit male-specific scents, including sex pheromones (Andersson et al. 2003, 2007; Yildizhan et al. 2009).
Despite lacking characteristic external organs such as androconia, several butterfly species emit male-specific scents from their wings. North American sulfur butterflies Colias philodice Godart and C. eurytheme Boisduval (Pieridae, Coliadinae) possess characteristic secretory cells, instead of androconia, behind the ordinary wing scales in the costal region on the dorsal surface of the hind wings (Rutowski 1980; Sielberglied and Taylor 1978). Such intermembranous cells are specific to males and regarded as the secretory (scent-producing) organs based on their histological features (Rutowski 1980). Chemical analyses and behavioral investigations have confirmed that hexyl myristate, palmitate, and stearate serve as aphrodisiac sex pheromones in C. philodice, while 13-methylheptacosane, heptacosane, and nonacosane serve that role in C. eurytheme (Grula et al. 1980; Sappington and Taylor 1990a, b). Despite their low volatility, these compounds elicited clear electroantennographic (EAG) responses, indicating that these sulfur butterflies receive them as olfactory cues for mating (Grula et al. 1980).
The genus Colias has more than 80 species and is a large group of sulfur butterflies inhabiting North and South America, Eurasia, and Africa (Savela 2014). Since Colias butterflies often have a sympatric distribution with closely related species, this genus has been useful for studying the mechanisms of sexual selection and species recognition. Ultraviolet patterns on the wings are thought to be essential for precopulatory isolation and have been intensively studied for many Colias species (Brunton 1998; Rutowski et al. 2007; Sielberglied and Taylor 1973). On the other hand, the involvement of chemical signals in mating selection has been investigated in only 2 species, C. philodice and C. eurytheme. It remains unclear whether the 3 chemical traits found in previous studies, i.e., sexual dimorphism in epicuticular composition, species specificity of male-specific components, and the presence of secretory cells in the hind wing membrane, are widely conserved in other Colias species. Many comparative studies are needed to better understand the significance of chemical signals in the precopulatory isolation of Colias butterflies. The present study was conducted on the Japanese subspecies of the eastern pale clouded yellow Colias erate poliographus Motschulsky to examine (1) the presence of male-specific components, (2) quantitative changes in the components with aging, and (3) the distribution of the components and possible secretory cells of male-specific components.
Materials and methods
Insects
Adults of C. erate were obtained from our laboratory stock cultures originated from females collected in the field at Higashihiroshima (34.23ºN, 132.42ºE, Hiroshima Prefecture). Gravid females were placed in plastic containers (35 × 50 × 25 cm) to allow laying of eggs on fresh leaves of Trifolium repens (white clover) under incandescent lamps. Larvae were reared on potted plants of white clover at 25 °C under a photoregime of 16L:8D. Pupae were placed in rearing cages (30 × 30 × 50 cm) until adult emergence. Within 24 h of eclosion, adults were sexed and kept individually in plastic containers (9 cm height, 12 cm inner diameter). They were gently held between pure papers with fingers, their proboscis made to extend using forceps, and were fed with 15 % aqueous sucrose once daily. Several males and females were placed together in mesh cages (40 × 60 × 40 cm) to allow mating.
Extraction
Adult butterflies were frozen to death at −20 °C and subjected to 3-min extractions with purified dichloromethane at room temperature. We conducted the following 5 extractions. (1) At 3 days after eclosion, 13 males and 13 females were individually extracted with 2 ml of the solvent (whole-individual extracts). (2) At 1, 3, and 7 days, 5 adults of each sex were extracted together using 10 ml of the solvent (extracts for age-dependent variation). (3) Six 3-day-old males were divided into 3 parts (forewings, hind wings, and body), and each divided part of each male was individually extracted with 0.6 ml of the solvent (divided-individual extracts). (4) Forewings and hind wings of 3-day-old males were divided into 8 regions (see Fig. 1), and each region of 5 males was individually extracted with 0.6 ml of the solvent (divided-wing extracts). For each wing region, a total of 2 samples were prepared from 10 males. (5) The wing regions of F3 and H1 (see Fig. 1) were dissected from 3 males that were 3 days old. Using cotton wool washed with dichloromethane, all scales were separated from the wing membrane. The membranes and scales of each wing region were individually extracted with 0.6 ml of the solvent (extracts of wing membranes and scales).
These crude extracts were subsequently filtered, concentrated to 100 μl under a nitrogen stream at 10 °C, and stored at −20 °C until the chemical analyses.
Chemical analyses
Gas chromatography–mass spectrometry (GC–MS) analyses were performed using a Shimadzu QP5000 mass spectrometer coupled with a Shimadzu GC-17A gas chromatograph. The samples were analyzed using an Agilent J&W DB-1 capillary column (0.25 mm ID × 15 m, 0.25 μm film thickness) by using a splitless injector at 280 °C, with an oven temperature program of 50 °C (1 min isothermal) and an increase of 10 °C/min to 280 °C. Compounds were identified by comparing the retention times and mass spectra with those of authentic samples. Quantitative estimates of individual compounds were obtained by comparing the peak intensities with that of pentacosane.
Authentic samples
All chemicals, including authentic samples, were commercial products from Tokyo Chemical Industry (TCI). Hexyl myristate, palmitate, and stearate were synthesized by esterification of 1-hexanol (purity 98 %) and the corresponding carboxylic acid (>97 %) using sulfuric acid as catalyst (Shimizu et al. 2012). 13-Methylheptacosane was synthesized by Wittig reaction of n-tetradecyltriphenylphosphonium bromide (97 %) and 2-tetradecanone (97 %), and catalytic reduction of the resulting methylalkenes with Pd/C and hydrogen (Guedót et al. 2009). Octadecanal was synthesized by PCC oxidation of 1-octadecanol (98 %) (Ginzel et al. 2006). These synthetic products were purified by flash chromatography using silica gel and identified by GC–MS.
Microscopy
Microscopy was conducted for forewings and hind wings dissected from 3-day-old males and females. The forms and distribution patterns of wing scales were examined using an Olympus SZX-7 stereomicroscope. For scale-removed wings, the location of possible secretory organs was investigated using a Keyence BZ-9000 fluorescent microscope under bright conditions and digital Z-stacking mode. Scanning electron microscopy (SEM) was performed using a Jeol JSM-5400 scanning electron microscope. The preparations for SEM were mounted on a stage with adhesive tape and sputter-coated with gold. To describe the location of sex-specific microstructures on wings, the wing veins and cells were labelled as in the Comstock-Needham system (Miller 1969).
Results
Sexual differences in chemical composition of whole-individual extracts
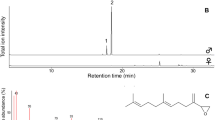
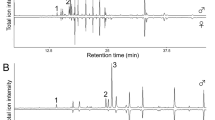
The whole-individual extracts of males and females displayed remarkably different patterns of total ion chromatograms (Fig. 2). The extracts contained few highly volatile components but were abundant in less volatile components, in which we identified 19 compounds: 10 linear alkanes, 3 hexyl esters, 2 higher fatty acids, 1 branched alkane, 1 aliphatic aldehyde, 1 acyclic triterpene, and 1 cholesterol (Table 1). The male and female extracts had 4 major components with average amounts of more than 1 μg per individual. Hexyl palmitate was the predominant component in males, but it was present at less than 200 ng in females. In contrast, tricosane was the only major component in females. Pentacosane, heptacosane, and nonacosane were the major components shared by both sexes. With respect to sex differences in the quantity of each compound, 8 linear alkanes and octadecanoic acid were significantly more abundant in females than in males (p < 0.05 or 0.01, Welch’s t-test), while 3 hexyl esters and octadecanal were significantly more abundant in males than in females (p < 0.001, Welch’s t-test).
Typical total ion chromatograms obtained from the whole-individual extracts of Colias erate males (upper) and females (lower). Chromatograms were run on an Agilent J&W DB-1 capillary column (0.25 mm ID × 15 m), programmed from 50 °C (1 min isothermal) to 280 °C at 10 °C/min. Peak numbers correspond to compound numbers in Table 1
Age-dependent variation in amounts of designated compounds
To evaluate age-dependent quantitative changes, the average amounts of 9 designated compounds were calculated for either sex at days 1, 3, and 7. These compounds were 4 male-specific compounds (octadecanal and 3 hexyl esters), 4 linear alkanes as the major components (tricosane, pentacosane, heptacosane, and nonacosane), and 13-methylheptacosane. Among them, 3 hexyl esters, 13-methylheptacosane, heptacosane, and nonacosane were the sex pheromones of 2 North American Colias males (Grula et al. 1980; Sappington and Taylor 1990a, b). In both sexes, the amounts of the 4 linear alkanes increased after eclosion and reached a plateau at day 3 (Table 2). In contrast, the amounts of octadecanal, 3 hexyl esters, and 13-methylheptacosane in males increased until day 7. Although females contained small amounts of hexyl palmitate and 13-methylheptacosane at day 3, these quantities increased until day 7.
Location of male-specific compounds in the insect body
The relative abundance of each of the 19 compounds in the forewings, hind wings, and body of males was compared using divided-individual extracts (Table 3). Most compounds were distributed in all parts, with each of the 3 parts having at least 13 % of the total amount of compound. However, octadecanal, 3 hexyl esters, and 13-methylheptacosane had a characteristic distribution pattern; these compounds were less abundant in bodies (less than 8 %), but were concentrated in the wings, especially the forewings. This suggests that the possible secretory and storage organs of these compounds are localized on male wings.
Using divided-wing extracts, 8 wing regions were compared for their relative abundance of hexyl palmitate, the predominant compound in males (Table 4). This compound was 3 times more abundant in the forewings (F1–F4) than the hind wings (H1–H4). Whereas the abundance in forewings was approximately constant among the 4 regions, that in the hind wings was biased toward the H1 and H2 regions.
Microscopy of possible secretory organs
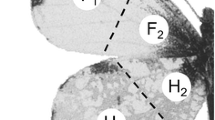
Stereomicroscopic observation of the wing surface confirmed that both sexes were identical in the forms and distribution pattern of wing scales, indicating that C. erate males lack characteristic structures such as androconia and sex brands. However, digital microscopy using the Z-stacking mode for the scale-removed wings revealed that males, but not females, had many rows of stain-like spots on the surface of the wing membrane (Figs. 3a, b, 4a, b). These spots were more conspicuous in the forewings than hind wings, and were attributed to sac-like intermembranous cells lying under the ordinary scale sockets, which had associated integumental swelling structures (Figs. 3c–e, 4c–e). These cells were exclusively distributed from basal to discal areas on the ventral surface of forewing cells 1A, 2A, and Cu2 and on the dorsal surface of hind wing cell Sc+R1 (Figs. 3f, 4f).
Distribution and morphology of scale sockets on the ventral surface of Colias erate forewings. a–d Male (a, c) and female (b, d) socket arrangement from basal to discal areas in the forewing cells 1A, 2A, and Cu2 in the Comstock-Needham system. Sac-like intermembranous cells (black arrows) are located at the bottom of several sockets of males (c). e Scanning electron micrograph of the socket together with integumental swelling (white arrow). f Distribution of intermembranous cells in the male forewing (white area)
Distribution and morphology of scale sockets on the dorsal surface of Colias erate hind wings. a–d Male (a, c) and female (b, d) socket arrangement from basal to discal areas in the hind wing cell Sc+R1 in the Comstock-Needham system. Sac-like intermembranous cells (black arrows) are located at the bottom of several sockets of males (c). e Scanning electron micrograph of the socket together with integumental swelling (white arrow). f Distribution of intermembranous cells in the male hind wing (white area)
Distribution of male-specific compounds between wing membranes and scales
The wing membranes and scales in the F3 and H1 wing regions, including intermembranous cells, were compared for their relative abundance of 9 designated compounds (Table 5). Among them, 4 linear alkanes were more abundant in the wing membranes, while octadecanal, 3 hexyl esters, and 13-methylheptacosane were more abundant in the wing scales.
Discussion
C. erate adults had a distinct sexual dimorphism in the chemical composition of epicuticular substances. Hexyl palmitate was the predominant component in males, and its quantity was approximately 40-fold higher than that in females. Hexyl myristate and stearate were detected in small amounts in males, but were absent in females. With regard to the overall chemical composition and the predominance of hexyl esters, C. erate males were identical to C. philodice males, which utilize 3 hexyl esters as aphrodisiac pheromones toward conspecific females (Grula et al. 1980). Although phylogenetic relationships within the genus Colias remain unclear, C. erate is in a distant clade from C. philodice and C. eurytheme (Brunton 1998; Pollock et al. 1998; Wheat and Watt 2008; Wheat et al. 2005). Since C. erate and C. philodice are biogeographically and phylogenetically distant, the male chemical profile characterized by these hexyl esters seems to be widely preserved within the genus Colias.
Among 10 identified linear alkanes with carbon numbers from 21 to 31, pentacosane, heptacosane, and nonacosane were the principal components. Although these hydrocarbons commonly occurred in both sexes, 8 linear alkanes were significantly more abundant in females than in males at 3 days old. These alkanes are omnipresent cuticular hydrocarbons in many insects (Blomquist 2010). In addition to linear alkanes, 13-methylheptacosane was also present as a minor component in both sexes. This branched alkane, together with heptacosane and nonacosane, is known to act as a sex pheromone in C. eurytheme males (Sappington et al. 1990a, b). It is noteworthy that 13-methylheptacosane was present together with hexyl palmitate in C. erate males. In a previous study, 3 hexyl esters were specific to C. philodice, while 13-methylheptacosane was exclusively present in C. eurytheme (Grula et al. 1980). Since biosynthetic capabilities leading to either hexyl esters or 13-methylheptacosane coexist in C. erate, this Eurasian Colias species might preserve more ancestral traits than the 2 North American species.
Hexadecanoic and octadecanoic acids, octadecanal, squalene, and cholesterol are often identified as cuticular lipid components in many insects (Buckner 1993; Canavoso et al. 2001; Juárez and Bromquist 1993). Among them, C. erate males contained significantly larger amounts of octadecanal than females, while octadecanoic acid was significantly more abundant in females than in males. The presence of octadecanal and octadecanoic acid has not been reported in C. philodice and C. eurytheme (Grula et al. 1980; Sappington et al. 1990a, b).
Adult butterflies frequently showed age-dependent changes in their chemical composition. Especially, quantitative changes in sex pheromone components synchronize the timing of sexual maturation and have a great impact on copulation success (Nieberding et al. 2012). In the present study, the amounts of octadecanal, hexyl esters, and 13-methylheptacosane in males increased during 1–7 days after eclosion, while those of 4 linear alkanes reached a plateau at day 3. Similar age-dependent increments within 7 days after eclosion have also been reported for the sex pheromones of C. eurytheme males (Sappington et al. 1990a). Interestingly, hexyl palmitate and 13-methylheptacosane were detectable in females and increased until day 7, indicating that C. erate females also have a small amount of these synthases.
In 3-day-old males, the wings contained more octadecanal, 3 hexyl esters, and 13-methylheptacosane than the body. These compounds were especially more abundant in the forewings than in the hind wings. The abundance of hexyl palmitate was approximately equal among the 4 regions of forewings but was greater in the 2 costal regions than the 2 inner marginal regions of hind wings. These results strongly reflect in the possible location of secretory and storage organs of the male-specific compounds. Microscopic surveys revealed that C. erate males possess characteristic intermembranous cells from basal to discal areas in the forewing cells 1A, 2A, and Cu2 and the hind wing cell Sc+R1. Since these cells were absent in the same areas of female wings and morphologically identical to the secretory (scent-producing) organs of C. philodice males (Rutowski 1980), these were regarded as the sources of male-specific compounds in C. erate. In general, adult male coliadine butterflies have a patch of secretory cells, which often forms a sex brand, in the friction area of the ventral surface of the forewing or the dorsal surface of the hind wing (Vetter and Rutowski 1978). However, the distribution pattern of secretory cells differs among sulfur butterfly species; C. philodice, C. cesonia, and Nathalis iole have them on the hind wing (Vetter and Rutowski 1978; Grula et al. 1980), while Eureme lisa and E. nicippe possess them on the forewing (Rutowski 1977; Vetter and Rutowski 1978). C. erate males uniquely possess a patch of secretory cells on both the forewings and hind wings.
In the F3 and H1 regions of male wings, including secretory cells, 4 linear alkanes were more abundant in the membranes than in the scales. Octadecanal, 3 hexyl esters, and 13-methylheptacosane, in contrast, showed larger amounts in the wing scales, although these secretory cells were present in the wing membranes. The disagreement between the origin and the presence of male-specific compounds implies a possible dissemination mechanism such that these compounds originate from the intermembranous cells and are transferred to the wing scales in the F3 and H1 regions. Then, these compounds would be spread from these wing regions to other regions by the contact of wing surfaces (Rutowski 1977). C. erate males lack androconia in these wing regions, the scales of which were not differentiated from those found in other regions of male wings and in the same regions of female wings. So far, morphologically specialized scales (androconia) have not been discovered in adult male coliadine butterflies (Vetter and Rutowski 1978). These findings demonstrate that sulfur butterflies have evolved to use their ordinary wing scales as dissemination devices for male-specific compounds.
We clarified the chemical natures, quantitative changes by aging, and origins of male-specific compounds of C. erate. Further studies are needed to investigate the pheromonal activities of these compounds.
References
Andersson J, Borg-Karlson A-K, Wiklund C (2003) Antiaphrodisiacs in pierid butterflies: a theme with variation! J Chem Ecol 29:1489–1499
Andersson J, Borg-Karlson A-K, Vongvanich N, Wiklund C (2007) Male sex pheromone release and female mate choice in a butterfly. J Exp Biol 210:964–970
Blomquist GJ (2010) Structure and analysis of insect hydrocarbons. In: Blomquist BJ, Bagnères A-G (eds) Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge University Press, Cambridge, pp 19–34
Brunton CFA (1998) The evolution of ultraviolet patterns in European Colias butterflies (Lepidoptera: Pieridae): a phylogeny using mitochondrial DNA. Heredity 80:611–616
Buckner JS (1993) Cuticular polar lipids of insects. In: Stanley-Samuelson DW, Nelson DR (eds) Insect lipids: chemistry, biochemistry and biology. University of Nebraska Press, Lincoln, pp 227–270
Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA (2001) Fat metabolism in insects. Ann Rev Nutr 21:23–46
Ginzel MD, Moreira JA, Ray AM, Millar JG, Hanks LM (2006) (Z)-9-Nonacosene—major component of the contact sex pheromone of the beetle Megacyllene caryae. J Chem Ecol 32:435–451
Grula JW, McChesney JD, Taylor OR Jr (1980) Aphrodisiac pheromones of the sulfur butterflies Colias eurytheme and C. philodice (Lepidoptera, Pieridae). J Chem Ecol 6:241–256
Guedót C, Millar JG, Horton DR, Landolt PJ (2009) Identification of a sex attractant pheromone for male winterform pear psylla, Cacopsylla pyricola. J Chem Ecol 35:1437–1447
Hall JPW, Harvey DJ (2002) A survey of androconial organs in the Riodinidae (Lepidoptera). Zool J Linn Soc 136:171–197
Juárez P, Bromquist GJ (1993) Cuticular hydrocarbons of Triatoma infestans and T. mazzotti. Comp Biochem Physiol B 106:667–674
Kristensen NP, Simonsen TJ (2003) ‘Hairs’ and cells. In: Kristensen NP, Simonsen TJ (eds) Lepidoptera, moths and butterflies vol. 2: morphology, physiology, and development. Handbuch der Zoologie/Handbook of Zoology IV/36. Walter de Gruyter, Berlin and New York, pp 9–22
Miller LD (1969) Nomenclature of wing veins and cells. J Res Lepid 8:37–48
Nieberding CM, Fischer K, Saastamoinen M, Allen CE, Wallin EA, Hedenström E, Brakefield PM (2012) Cracking the olfactory code of a butterfly: the scent of ageing. Ecol Lett 15:414–424
Ômura H, Itoh T, Wright DM, Pavulaan H, Schröder S (2015) A morphological study of alar androconia in Celastrina butterflies. Entomol Sci (in press)
Pollock DD, Watt WB, Rashbrook VK, Iyengar EV (1998) Molecular phylogeny for Colias butterflies and their relatives (Lepidoptera: Pieridae). Entomol Soc Am 91:524–531
Rutowski RL (1977) Chemical communication in the courtship of the small sulphur butterfly Eurema lisa (Lepidoptera, Pieridae). J Comp Physiol A 115:75–85
Rutowski RL (1980) Male scent-producing structures in Colias butterflies: function, localization, and adaptive features. J Chem Ecol 6:13–26
Rutowski RL, Macedonia JM, Merry JW, Morehouse NI, Yturralde K, Taylor-Taft L, Gaalema D, Kemp DJ, Papke RS (2007) Iridescent ultraviolet signal in the orange sulphur butterfly (Colias eurytheme): spatial, temporal and spectral properties. Biol J Linn Soc 90:349–364
Sappington TW, Taylor OR (1990a) Developmental and environmental sources of pheromone variation in Colias eurytheme butterflies. J Chem Ecol 16:2771–2786
Sappington TW, Taylor OR (1990b) Disruptive sexual selection in Colias eurytheme butterflies. Proc Natl Acad Sci USA 87:6132–6135
Savela M (2014) Lepidoptera and some other life forms. http://www.nic.funet.fi/pub/sci/bio/life/intro.html. Accessed 31 Oct 2014
Shimizu N, Kuwahara Y, Yakumaru R, Tanabe T (2012) n-Hexyl laurate and fourteen related fatty acid esters: new secretory compounds from the julid millipede, Anaulaciulus sp. J Chem Ecol 38:23–28
Sielberglied RE, Taylor OR (1973) Ultraviolet differences between the sulphur butterflies, Colias eurytheme and C. philodice, and a possible isolating mechanism. Nature 241:406–408
Sielberglied RE, Taylor OR Jr (1978) Ultraviolet reflection and its behavioral role in the courtship of the sulfur butterflies Colias eurytheme and C. philodice (Lepidoptera, Pieridae). Behav Ecol Sociobiol 3:203–243
Vane-Wright RI, Boppré M (1993) Visual and chemical signaling in butterflies: functional and phylogenetic perspectives. Phil Trans R Soc B 340:197–205
Vetter RS, Rutowski RL (1978) External sex brand morphology of three sulphur butterflies (Lepidoptera: Pieridae). Psyche 85:383–393
Warren BCS (1961) The androconial scales and their bearing on the question of speciation in the genus Pieris (Lepidoptera). Entomol Ts Arg 82:121–148
Wheat CW, Watt WB (2008) A mitochondrial-DNA-based phylogeny for some evolutionary-genetic model species of Colias butterflies (Lepidoptera, Pieridae). Mol Phyl Evol 47:893–902
Wheat CW, Watt WB, Boutwell CL (2005) A reconnaissance of population genetic variation in arctic and subarctic sulfur butterflies (Colias spp.; Lepidoptera, Pieridae). Can J Zool 83:1614–1623
Yildizhan S, van Loon J, Sramkova A, Ayasse M, Arsene C, ten Broeke C, Schulz S (2009) Aphrodisiac pheromones from the wings of the small cabbage white and large cabbage white butterflies, Pieris rapae and Pieris brassicae. Chem Bio Chem 10:1666–1677
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ômura, H., Yotsuzuka, S. Male-specific epicuticular compounds of the sulfur butterfly Colias erate poliographus (Lepidoptera: Pieridae). Appl Entomol Zool 50, 191–199 (2015). https://doi.org/10.1007/s13355-015-0321-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-015-0321-3