Abstract
The development of reliable antibiotics in the fight against multidrug resistant bacteria is a crucial challenge nowadays. Here, we describe a green chemistry approach for the synthesis of antimicrobial silver nanoparticles (AgNPs) employing the leaf extract of the orchid Anoectochilus elatus. Synthesized Ag NPs were studied by UV–visible and ATR-FT-IR spectroscopy, as well as by XRD, SEM and TEM. The UV–Visible spectra of AgNPs exhibited a surface plasmon resonance peak at 420 nm. XRD analysis showed that peaks at 38.09°, 44.59°, 64.67° and 77.54° confirmed the crystalline nature of AgNPs. TEM revealed spherical shapes with an average particle size of 20 nm. Synthesized AgNPs were screened to evaluate their antimicrobial activity against five Gram-negative and two Gram-positive human bacterial pathogens. AgNPs showed effective antibacterial activity against all tested bacterial strains at the concentration of 30–50 μL. Furthermore, the bactericidal efficacy of AgNPs can be useful to develop new eco-friendly antibacterial products to be used for photocatalytic, textile fabric applications, as well as to co-formulate detergents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biophysical characteristics of nanomaterials are largely dependent to their size and shape. In addition, the high surface to volume ratio of nanomaterials has been largely documented in nanoscience [1–5], showing its importance for the extraordinary optical and bioactivity properties of nanoparticles. Notably, nanoparticle research has played a central role in some of the most significant discoveries in material and biomedical science. Within the last two decades, 4d transition metal nanoparticles have been used for broad spectrum of applications such as sensors [6], catalytic [7], X-ray biomedical [8], anticancer [9], antioxidant [10], larvicidal [11–21] and antibacterial products [22]. Some of the metal nanoparticles are non toxic as well as bio-compatible. Conversely, others, including palladium (Pd) and ruthenium (Ru) ones are toxic to human health and the environment. Nowadays, most of the nanoparticles currently marketed are fabricated using physical and chemical methods. These methods are highly hazardous, costly and energy consuming processes [21–23]. Currently, the so-called “green chemistry” approach is receiving attractiveness for nanoparticle synthesis using plant extract-based reducing routes. Different plant materials have been proposed, including the leaf extracts of Gloriosa superba [24], Terminalia arjuna [23], Pterocarpus santalinus [25], Malva sylvestris [26], Bauhinia variegata [27], Berberis tinctoria [28], Mimusops elengi [29] and Melia azedarach [5]. Other examples include the gum extract of Cochlospermum gossypium [30] and C. religiosum [31], the fruit extract of Rubus glaucus [32], Nothapodytes nimmoniana [33], Abelmoschus esculentus [34], Citrus limon, C. reticulata and C. sinensis [35], as well as the seed extract of T. chebula [36] and Cuminum cyminum [37]. Generally, medical plant extracts contain different phytoconstituents helping the capping and reduction process for formation of nanoparticles. Recently, the orchid leaf extract mediated biosynthesis of Ag and Au NPs showed antagonistic activity on human microbial pathogens [38].
Anoectochilus elatus Lindl.(Orchidaceae) is an endangered monopodial terrestrial also known as “South Indian Jewel Orchid”. It is distributed Kolli hills of Eastern Ghats and Tirunelveli hills of Southern Western Ghats of Tamil Nadu and Kerala [39]. It is used for treatment of chest and abdominal pains as well as to treat snakebites. Phytochemical screening of this plant leaves showed the presence of bioactive flavonoids and reducing sugars [40]. In addition, the Anoectochilus-derived bioactive compound kinsenoside has been tested to treat diabetes, hyperliposis and breast cancer [41].
In this research, we focused on the green synthesis of Ag NPs the A. elatus leaf extract. Synthesized Ag NPs were analyzed by UV–Visible, ATR-FT-IR, XRD, SEM and TEM analyses. Synthesized Ag NPs were screened to evaluate their antimicrobial activity against two Gram-positive and five Gram-negative human bacterial pathogens.
Materials and Methods
Collection of Orchid Material
Healthy leaves of A. elatus were gathered from the Valparai hills, Southern Western Ghats, India, which are located at 10.37°N 6.97°E (Fig. 1).
Orchid-Mediated Nanosynthesis and Characterization of Nanoparticles
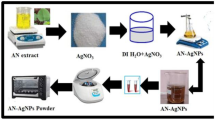
10 g of A. elatus fresh leaves were cut into 2–3 cm. The finely cut leaves were carefully boiled in 100 mL of double distilled water for 15 min. After that, the leaf extract was filtered through Whatman filter paper no. 1, and stored at room temperature for further use. The suspension was prepared in 250 mL conical flask which contains 100 mL of 1mM AgNO3 solution and 10 mL of orchid leaf extract, which were stirred well for 60 min. Then, the color less mixture changed to brown color showing the fabrication of Ag NPs.
Orchid extract and synthesized AgNPs were analyzed by UV–Visible spectroscopy (UV-1601 pc Shimadzu spectrophotometer), ATR-FT-IR spectrometer (Thermo Nicolet 380), the crystallinity size of the synthesized nanoparticles was analyzed using PANalytical X-PERT PRO equipped with Cu Kα radiation (wavelength: 0.15,406 nm) as the X-ray source. The surface morphology of the Ag NPs was confirmed by Tescan scanning electron microscope equipped with Vega3sem software. The particle size was analyzed by Tecnai 20 G2 (FEI make) transmission electron microscope (TEM).
Antibacterial Activity
The bactericidal property of the green-synthesized Ag NPs was investigated against two Gram-positive (Bacillus subtilis, Streptococcus pneumoniae) and five Gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, and Shigella dysenteriae) by disc diffusion method was evaluated following Gopinath et al. [24]. Tested bacteria were grown in nutrient broth at 37 °C until the bacterial suspension reached 1.5 × 108 CFU/mL, then separate sterile cotton swabs were dipped into the overnight bacterial broth cultures. 10, 30 and 50 µL/disc of Ag NPs were placed on the inoculated plates and pressed firmly into the agar with sterile forceps [24].
Statistical Analysis
We provided all data as mean ± SE. We employed ANOVA followed by Tukey’s HSD test (P ≤ 0.05) to analyze the data from antibacterial assays [20]. SPSS software package 16.0 version was used for all analyses.
Results and Discussion
UV–Visible Spectroscopy
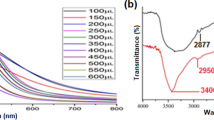
The orchid leaf extract and biosynthesized Ag NPs were examined by UV–Visible spectroscopy at different time intervals such as 10, 20, 30, 40, 50 and 60 min. The orchid extract did not show any absorbance peak at in the range of 400–700 nm, while the Ag NP spectra clearly showed notable absorbance peak at 420 nm (Fig. 2). This hump gradually increases at 420 nm in the tested time intervals, which indicates Surface Plasmon Resonance (SPR) of Ag NPs linked with reduction of Ag+ to Ag0. Presumably, SPR peaks was linked to the particles size of Ag NPs. When SPR peak appeared blue shift indicates decreasing particles size and red shift indicates increasing particles size, according to the Mie theory. We concluded the average particle size was 20 nm, which well matched with previous reports [24].
ATR-FT-IR, XRD, SEM and TEM
ATR-FTIR analysis revealed several functional groups potentially responsible for Ag NPs reduction and stabilization. FTIR spectrum of plant extract and synthesized Ag NPs showed a number of bands observed at 3415, 2926, 1600, 1377 and 1073 cm−1, which corresponded to OH stretching, aliphatic C–H stretching, C=C stretching, NO2 stretching, and –C–O–C stretching mode (Fig. 3), linked to the presence of flavonoids and reducing sugars that can be helpful to reduce and stabilize Ag NPs [40]. Notably, two weak bands recorded at 920 and 607 cm−1 could correspond to aromatic single bond –CH and C–Cl stretching, which rise may be due to the reduction of AgNO3 to Ag NPs.
X-ray powder diffraction was used to identify the crystallinity and structure of Ag NPs. The XRD patterns showed angles (2θ) of 38.09, 44.59, 64.67 and 77.54 corresponding to (111), (200) (220) and (311) planes of the Ag NPs (Fig. 4). All reflections can be indexed to Face-Centered-Cubic (FCC) nature of synthesized Ag NPs, in good agreement with standard data (JCPDS Card No: 03-0921). From the XRD data, the calculated the average crystallite size was estimated 14.02 nm.
Lastly, the surface topology of A. elatus-fabricated Ag NPs was investigated by SEM analysis. SEM micrographs displayed an assembly of spherical shapes (Fig. 5a). Furthermore, TEM of Ag NPs clearly showed spherical morphology in the range from 10 to 45 nm with the average particle size of 20 nm (Fig. 5b).
Antibacterial Activity
Here we evaluated the antibacterial activity of the orchid-fabricated Ag NPs, showing that 30–50 μL of Ag NPs reduced the growth of the seven microbial pathogens (Fig. 6). Dose-dependent response was noted (Table 1), while the orchid extract alone did not affected the growth of all tested bacteria species. Hitherto, the precise mechanisms leading to antibacterial activity of Ag NPs is not clearly understood. It has been argued that synthesized Ag NPs bind with cytoplasmic membrane by the electrostatic attraction leading to cell wall damage [25]. Ag NPs entering in the bacterial cytosol can impede protein synthesis and interfere with cell signaling pathways. Also this action can cause lethal effect on bacterial cells [24]. In agreement with our results, green synthesized Ag NPs showed bactericidal activity on Vibrio parahaemolyticus. Beneficially, it is did not affect the non target organism of Artemia nauplii [42]. The present results suggested that efficacy of synthesized Ag NPs on human pathogens, highlighting their promising potential for pharmaceutical drug development.
Conclusions
In summary, here Ag NPs have been synthesized using the Anoectochilus elatus orchid leaf extract. Ag NPs were characterized by UV–Visible and ATR-FT-IR spectroscopy, as well as by XRD, SEM and TEM analyses. The UV–Visible spectra of Ag NPs showed a surface plasmon resonance peak at 420 nm. ATR-FT-IR spectrum indicated that A. elatus leaf extract had the ability to perform dual functions of reduction and capping of Ag NPs. XRD analysis showed peaks at 38.09°, 44.59°, 64.67° and 77.54° confirming the crystalline nature of Ag NPs. TEM image revealed spherical shapes and particle size of 20 nm, whereas others are in the range from 10 to 45 nm. Synthesized Ag NPs were screened to evaluate their antimicrobial activity against two Gram-positive and five Gram-negative human bacterial pathogens. Ag NPs showed effective antibacterial activity against all tested bacterial strains at the concentration of 30–50 μL. Furthermore, the bactericidal efficacy of Ag NPs can be useful to develop new eco-friendly antibacterial products to be used for photocatalytic, textile fabric applications, as well as to co-formulate detergents.
References
V. Gopinath, D. MubarakAli, S. Priyadarshini, N. M. Priyadharsshini, N. Thajuddin, and P. Velusamy (2012). Colloids Surf. B 96, 69–74.
U. Muthukumaran, M. Govindarajan, and M. Rajeswary (2015). Parasitol. Res. 114, 989–999.
U. Muthukumaran, M. Govindarajan, and M. Rajeswary (2015). Parasitol. Res. 114, 1817.
G. Benelli (2016). Enzyme Microb. Technol. 95, 58–68.
K. Bhakyaraj, S. Kumaraguru, K. Gopinath, V. Sabitha, P. R. Kaleeswarran, V. Karthika, A. Sudha, U. Muthukumaran, K. Jayakumar, S. Mohan, and A. Arumugam (2016). J. Clust. Sci. doi:10.1007/s10876-016-1114-8.
H. Wang, H. Wang, T. Li, J. Ma, K. Li, and X. Zuo (2017). Sensor. Actuat. B-Chem. 239, 1205–1212.
M. Li, Y. Gong, W. Wang, G. Xu, Y. Liu, and J. Guo (2017). Appl. Surf. Sci. 394, 351–357.
F. Matte, J. Vedelago, F. Malano, C. Gomez, M. C. Strumia, and M. Valente (2017). Radiat. Phys. Chem. 130, 442–450.
S. Kummara, M. B. Patil, and T. Uriah (2016). Biomed. Pharmacother. 84, 10–21.
J. K. Patra, G. Das, and K. H. Baek (2016). J. Photochem. Photobiol. B 161, 200–210.
S. Marimuthu, A. A. Rahuman, G. Rajakumar, T. Santhoshkumar, A. V. Kirthi, C. Jayaseelan, A. Bagavan, A. A. Zahir, G. Elango, and C. Kamaraj (2011). Parasitol. Res. 108, 1541.
K. Veerekumar, M. Govindarajan, and M. Rajeswary (2013). Parasitol. Res. 112, 4073.
K. Veerakumar, M. Govindarajan, M. Rajeswary, and U. Muthukumaran (2014). Parasitol. Res. 113, 1775.
P. Madhiyazhagan, K. Murugan, A. Naresh Kumar, T. Nataraj, D. Dinesh, C. Panneerselvam, J. Subramaniam, P. Mahesh Kumar, U. Suresh, M. Roni, M. Nicoletti, A. A. Alarfaj, A. Higuchi, A. Murugan, and G. Benelli (2015). Parasitol. Res. 114, 4305.
M. Govindarajan, M. Rajeswary, K. Veerakumar, U. Muthukumaran, S. L. Hoti, and G. Benelli (2016). Exp. Parasitol. 161, 40–47.
M. Govindarajan, M. Nicoletti, and G. Benelli (2016). J. Clust. Sci. 27, 745–761.
M. Govindarajan, S. L. Hoti, and G. Benelli (2016). Enzyme Microb. Technol. 95, 155–163.
M. Govindarajan, M. Rajeswary, U. Muthukumaran, S. L. Hoti, H. F. Khater, and G. Benelli (2016). J. Photochem. Photobiol. B 161, 482–489.
M. Govindarajan and G. Benelli (2016). RSC Adv. 6, 59021–59029.
G. Benelli (2017). J. Clust. Sci. doi:10.1007/s10876-016-1143-3.
G. Benelli, R. Pavela, F. Maggi, R. Petrelli, and M. Nicoletti (2017). J. Clust. Sci.. doi:10.1007/s10876-016-1131-7.
G. Liu, K. Li, Q. Luo, H. Wang, and Z. Zhang (2017). J. Colloid Interf. Sci. 490, 642–665.
K. Gopinath, K. S. Venkatesh, R. Ilangovan, K. Sankaranarayanan, and A. Arumugam (2013). Ind. Crop. Prod. 50, 737–742.
K. Gopinath, S. Kumaraguru, K. Bhakyaraj, S. Mohan, K. S. Venkatesh, M. Esakkirajan, P. Kaleeswarran, N. S. Alharbi, S. Kadaikunnan, M. Govindarajan, G. Benelli, and A. Arumugam (2016). Microbal. Pathog. 101, 1–11.
K. Gopinath, S. Gowri, and A. Arumuagm (2013). J. Nanostruct. Chem. 3, 68.
M. Govindarajan, S. L. Hoti, M. Rajeswary, and G. Benelli (2016). Parasitol. Res. 115, 2685–2695.
M. Govindarajan, M. Rajeswary, K. Veerakumar, U. Muthukumaran, S. L. Hoti, H. Mehlhorn, D. R. Barnard, and G. Benelli (2016). Parasitol. Res. 115, 723–733.
P. Mahesh Kumar, K. Murugan, P. Madhiyazhagan, K. Kovendan, D. Amerasan, B. Chandramohan, D. Dinesh, U. Suresh, M. Nicoletti, M. Saleh Alsalhi, S. Devanesan, H. Wei, K. Kalimuthu, J. S. Hwang, A. Lo Iacono, and G. Benelli (2016). Parasitol. Res. 115, 751–759.
J. Subramaniam, K. Murugan, C. Panneerselvam, K. Kovendan, P. Madhiyazhagan, P. Mahesh Kumar, D. Dinesh, B. Chandramohan, U. Suresh, M. Nicoletti, A. Higuchi, J. S. Hwang, S. Kumar, A. A. Alarfaj, M. A. Munusamy, R. H. Messing, and G. Benelli (2015). Environ. Sci. Pollut. Res. 22, 20067–20083.
V. T. P. Vinoda, P. Saravananb, B. Sreedharc, D. K. Devic, and R. B. Sashidhard (2011). Colloids Surf. B. 83, 291–298.
S. Maity, I. K. Sen, and S. S. Islam (2012). Phys. E 45, 130–134.
B. Kumar, K. Smita, L. Cumbal, and A. Debut (2017). Saudi J. Biol. Sci. 24, 45–50.
G. Mahendran and B. D. RanjithaKumari (2016). Food Sci. Hum. Wellness 5, 207–218.
C. Jayaseelan, R. Ramkumar, A. A. Rahuman, and P. Perumal (2013). Ind. Crop. Prod. 45, 423–429.
M. V. Sujitha and S. Kannan (2013). Spectrochim. Acta, Part A 102, 15–23.
K. M. Kumar, B. K. Mandal, M. Sinha, and V. Krishnakumar (2012). Spectrochim. Acta Part A 86, 490–494.
K. Sneha, M. Sathishkumar, S. Y. Lee, M. A. Bae, and Y. S. Yun (2011). J. Nanosci. Nanotechnol. 11, 1811–1814.
A. Kalaiarasan and R. Chinnappa (2015). Eur. J. Acad. Essays 2, 66–74.
N. Ahamed Sherif, J. H. Franklin Benjamin, S. Muthukrishnan, T. Senthil Kumar, and M. V. Rao (2012). Afr. J. Biotech. 11, 7549–7553.
M. Maridass, M. I. ZahirHussain, and G. Raju (2008). Ethnobot. Leaflets 12, 705–712.
N. Ahamed Sherif, T. Senthil Kumar, and M. V. Rao (2016). In Vitro Cell Dev. Biol. Plant 52, 72–80.
M. Latha, M. Priyanka, P. Rajasekar, R. Manikandan, and N. M. Prabhu (2016). Microb. Pathog 93, 88–94.
Acknowledgements
The authors extend their sincere appreciations to the Deanship of Scientific Research at King Saud University for its funding this Prolific Research Group (PRG-1437-36). K. Gopinath sincerely thanks the KRIND Institute of Research and Development, Trichy, for supporting antibacterial assays.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gopinath, K., Devi, N.P., Govindarajan, M. et al. One-Pot Green Synthesis of Silver Nanoparticles Using the Orchid Leaf Extracts of Anoectochilus elatus: Growth Inhibition Activity on Seven Microbial Pathogens. J Clust Sci 28, 1541–1550 (2017). https://doi.org/10.1007/s10876-017-1164-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1164-6