Abstract
The present study was focused on green synthesis of silver nanoparticles against pathogens of both gram-positive and gram-negative classes. Green synthesis of silver nanoparticles was successfully performed using Ziziana latifolia (ZL) Husk extract with the catalyst serving as sunlight. In making and stabilizing silver nanoparticles, the biomolecules present in the husk extract played the role of reducing and stabilizing agents. The resultant silver nanoparticles were characterized using UV–vis analysis, FTIR, HRTEM, SAD, EDAX, Zeta potential, and particle size analysis. In a DAPI study using the NIH 3T3 fibroblast cell line, ZL-AgNPS demonstrated outstanding stability and great biocompatibility. In vivo zebrafish toxicity and hemocompatibility experiments further indicated that the ZL-AgNPS were extremely biocompatible and safe. The novelty of this study is the synthesis of very stable silver nanoparticles in the aqueous phase by a single step reaction in sunlight without the use of any external stabilizing agent and reduction agent that the existing protocols do not require. The results showed that produced AgNPs are efficient against both microorganisms when the antibacterial activity of silver nanoparticles was compared to that of the pathogenic bacteria B. subtilis and E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
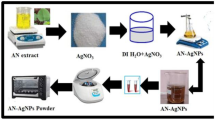
In the current material science research, most of the study is on the synthesis of metal nanoparticles; given their applications in medicine, cosmetics, biomedical devices, and so on, future uses and special features physical, chemical, and biological properties, silver nanoparticles are excitingly of greater interest. Several studies have used plant resources in the green production of silver nanoparticles. Additionally, it is necessary to assess the most dependable and straightforward technique for producing ecologically friendly, economically stable, and commercially viable silver nanoparticles using a variety of natural sources, primarily plant-based resources. The use of plant-based products and the biosynthesis of silver nanoparticles have both been studied recently [1]. Structures between 1 and 100 nm in size are called nanoparticles. Nanoparticles have distinctive physical and chemical characteristics because of their large surface area and small size. Silver nitrate was traditionally used for wound care and instrument cleaning in the medical community, and silver formulations for wound infection and burn care were developed [2]. Silver nanoparticles also have exhibited a broad spectrum of antibacterial and antifungal activities against multidrug-resistant bacteria [3]. Conventional synthesis of silver nanoparticles used mechanical using a plant extract that is free from noxious smells, and the cost of the process is less than that of using the synthetic method [4]. There is currently a greater focus on plant-based synthesis because it is eco-friendly, readily available, widely distributed, and safer to handle. Rice husk from Ziziana latifolia is reported to offer a wide range of bioactivities, including anticancer, immunomodulatory, anti-inflammatory, and antioxidant potential due to its probable radical scavenging of numerous phytochemicals [5,6,7,8]. The silver nanoparticles were successfully synthesized by using Z. latifolia aqueous husk extract as a reducing agent and aqueous silver nitrate as the precursor. Nowadays, physical and chemical methods are widely used for the synthesis of nanoparticles, but unfortunately, these methods are expensive and not at all eco-friendly. This study highlights the cost effective, ecofriendly, and sunlight act as a important role for catalyst quickly reduced green synthesis of nanoparticles and also analyses the anti-bacterial effects of the synthesized nanoparticles (Scheme 1), and characterization effects on 3T3 cells biocompatibility, zebrafish toxicity, and hemocompatibility experiments indicated that the ZL-AgNPS non-toxic and potential utilization as an anti-microbial effect.
2 Materials and methods
2.1 Media and chemicals
Analytical-grade silver nitrate (AgNO3) and all media components and analytical reagents were purchased from Hi-Media Laboratories Pvt Ltd (Mumbai, India). NIH 3T3 fibroblast cell lines were obtained from the National Centre for Cell Sciences (NCCS), India.
2.2 Preparation of husk extract
Adopting the method of Femi-Adepoju et al. [9], the aqueous extract was prepared. AG Z. latifolia husk was removed, and husk was soaked in distilled water in the ratio of 1:2 w/v for overnight, then filtered using the Whatman filter paper [9]. It was then stored at 4 °C, and the extract’s final filtrate was used to reduce Ag0. About 25 to 500 µl of the Z. latifolia husk extract was taken in different vials, and 1 mM of AgNO3 solution was added to these and kept under sunlight for 20 min, and the colour change was observed. The mixture was centrifuged at 15,000 rpm for 15 min at 4 °C. The pellets were collected and filtered using nylon cloth. Then, pellets were stored in refrigerator for further use.
2.3 Characterization methods
Primary characterization of AgNPs was carried out by FTIR, HR-TEM, particle size, and zeta potential analysis method. UV–visible spectrophotometer was used for the preliminary characterisation of AgNPs (CARY 100 Conc), FTIR (JASCO FTIR- 4700), HRTEM, XRD (Rigaku mini flex-II desktop), EDAX (Thermofisher Scientific Quanta200), Zeta potential & Particle size analysis method (Microtrac Zetatrac analyser by laser Doppler velocimetry) and the antibacterial action against both gram-positive and gram-negative bacteria were performed using the conventional disc method [10, 11]. The AgNPs were loaded into a well with a diameter of 8 mm and, utilising the Streptomycin as control, all the plates were incubated at 37 °C for 24 h. The zone of inhibition (ZOI) was calculated by subtracting the inhibition zone from the total diameter. Diffusion method media and glassware were autoclaved at 121 °C for 15 min.
2.3.1 UV–visible spectrophotometer
The analysis of metal nanoparticles can be done using the UV–Vis spectrophotometer, which is a very helpful, simple, and sensitive method. Using a Shimadzo Double Beam Spectrophotometer, AgNPs’ Surface Plasmon Resonance (SPR) was examined [12]. After administering plant extract into the silver nitrate solution for 0 min, three absorbance readings of the reduction process were taken at different wavelength intervals (300–550 nm), first of the mixture when the reduction process had just started; second, after 15 min when the reduction of AgNO3 had started; and third, when the reduction of AgNO3 had completed and AgNPs were completely formed. This could be confirmed by the change in colour of the mixture.
2.3.2 Fourier transform infrared spectroscopy
The Shimadzu IR Spirit model was used for the FTIR characterization to find the possible phytoconstituents in Z. latifolia husk extract that are involved in the formation of AgNPs. Dry powdered KBr was uniformly blended with dried husk extract or dried AgNPs in a ratio of 1:100. Transferring the prepared sample into the IR sample cell allowed the spectra to be run between 4000 and 400 cm−1.
2.3.3 High resolution-transmission electron microscopy (HR-TEM)
For this analysis, samples of synthesized AgNPs using Z. latifolia husk extract were dispersed in isopropyl alcohol through ultrasound. Immediately after, a small amount was placed in a carbon-coated copper grid to dry under environmental conditions and then examined under transmission electron microscopy [13].
2.3.4 Particle size
A particle size analyser was used to determine the nanoparticle’ particle size range and polydispersity (90 Plus Particle Size Analyser, Brookhaven Instruments Corporation). The particle size was determined by monitoring Brownian motion nanoparticles’ time-dependent fluctuation of laser light scattering.
2.3.5 Zeta potential
A zeta potential analyser was used to measure the zeta potential of the produced nanoparticles (90 Plus Particle Size Analyzer, Brookhaven Instruments Corporation, using Zeta Plus software) at pH around 5.5 by suspending the nanoparticle-containing broth in potassium chloride solution with ionic strength 10–3 M. The direction and velocity of particles moving under the influence of a known electric field are used to calculate the zeta potential.
2.4 X-ray diffraction
An X-ray diffractometer was used to investigate the diffraction (D2-Phaser, Bruker). The AgNPs sample was ground in an agate mortar and then transferred to a quartz plaque for Cu K- radiation exposure at a wavelength of 1.5406 nm. The diffractometer ran at 30 kV, 10 mA, with a step size of 0.02° and a count duration of 1 s per step in the range of 2 de 25–75°.
2.5 Antimicrobial study of the silver nanoparticles
Pathogens such as gram-poitive strains (Bacillus subtilis and Staphylococcus aureus) and gram-negative strains (Pseudomonas aeruginosa and Escherichia coli) were used as a model test. Strains for determining the antimicrobial activity of the developed nanoparticles. The AgNPs were loaded into a well with a diameter of 8 mm and, using the Streptomycin as control, all the plates were incubated at 37 °C for 24 h [14]. The zone of inhibition (ZOI) was calculated by subtracting the inhibition zone from the total diameter.
2.6 Cell viability assay
The following investigations were carried out to determine the cytotoxicity of ZL- Ag-NPs. In 96-well tissue culture plates, confluent monolayers of 3T3 cells were generated. Cells were treated with various concentrations of ZL-Ag-NPs. The mitochondrial enzyme succinate dehydrogenase’s ability to break the tetrazolium salt MTT [3-(4, 5-dimethylthiazol-2 ol) 2, 5-diphenyl tetrazolium bromide), Sigma Chem. Co. St. Louis, USA] forming a formazan crystal was then examined. Each concentration was assessed three times. The test drug concentration required for a 50% reduction in cell viability was calculated using the 50% inhibitory concentration (IC50). The per cent viability was computed using the OD value and formula.
2.7 DAPI staining
The 3T3 cells were collected, washed in PBS (pH 7.2), and stained with 1 ml EtBr. In addition, 3, 6-Diamidino-2-phenylindole dihydrochloride (DAPI) Nuclear Stain was used on 3T3 cells. Before washing with PBS, the cells were washed and fixed with methanol/acetic acid (3:1; v/v). The cells were washed and stained for 20 min in the dark with 1 mg/ml DAPI. The PBS was used twice to wash the cells (5 min each) after incubation for 2 min and visualized under a fluorescence microscope (Nikon Eclipse, Inc, Japan) at 40 × magnifications with a 480 nm excitation filter. A fluorescent microscope with the appropriate excitation filter was used to capture stained images.
2.8 Hemolytic assay
Thirty mL RBC suspension was added to Eppendorf tubes containing ZL-AgNPS (5, 10, 15, 20, and 25 mM) in the heparinized buffer. For about 2 h, samples were incubated at 37 °C. After the incubation period, samples were centrifuged for 10 min at 4 °C at 18,000 rpm. The absorbance at 540 nm was used to determine the free haemoglobin in the supernatant. Negative controls included samples containing RBC suspension in heparinized buffer, while positive controls included samples containing RBC suspension in distilled water [15]. The percentage of haemolysis was calculated using the formula provided.
2.9 In vivo toxicity study in zebrafish embryos
Zebrafish embryos were gathered within 2 h of spawning, and fertilized eggs were acquired by natural mating of adult zebrafish. Six healthy embryos of approximately 2 h post-fertilization (HPF) were transferred from newly fertilized eggs to each well of a 24 well plate with duplicates, along with 1 ml of E3 medium (To produce a stock solution, use E3 medium (34.8 g NaCl, 1.6 g KCl, 5.8 g CaCl2. 2H2O, 9.78 g MgCl2. 6H2O), a traditional medium for working with zebrafish embryos (34.8 g NaCl, 1.6 g KCl, 5.8 g CaCl2. 2H2O, 9.78 g MgCl2. 6H2O). In 2 L of water, dissolve the specified amount of salts. Autoclave the solution after adjusting the pH to 7.2 using NaOH. Dilute 16.5 ml of the 60 × Stock solution to 1 L with sterile water to make 1 medium) and different concentrations of optimized AgNPs, incubate for 4 days at room temperature [16]. To assess the toxicity of AgNPs, a stereomicroscope (Nikon Eclipse TS 100) was used to observe the hatching rate, death, and malformations.
2.10 Statistical analysis
Data were expressed as mean ± SD. Statistical significance was determined by one way ANOVA. Statistical analyses were performed using Graph Pad Prism 5.01 software. Statistical significance was determined by p-values of < 0.05 (p < 0.05; p < 0.001).
3 Results
3.1 UV–visible spectrophotometer
The UV–vis analysis is an indirect method used to study the formation of silver nanoparticles in an aqueous solution shows in Fig. 1a. As the aqueous husk extract of the black rice was blended with the colourless 1 mM of the AgNO3 solution, its colour changed to pale yellow colour instantly, changing to yellowish-brown after 10 min when kept under sunlight (Fig. 1a). The sharp absorbance peak of the synthesized silver nanoparticle was obtained at 421 nm.
3.2 Fourier transform infrared spectroscopy
Figure 1b shows the Fourier transform infra-red (FTIR) spectroscopy analysis of the husk extract and the AgNO3-treated husk extract in the range between 400 and 4000 cm−1 to pinpoint the functional groups responsible for capping as well as stabilizing the silver nanoparticles (Fig. 1b).
The primary peaks in aqueous husk extracts (Fig. 1b (i) were 2877, 2320, 1736, 1034, 690, and 587 cm−1, which represent the aromatic ring contained in the extract. The signal at 2904 cm−1 narrows in the FT-IR spectrum of AgNPs (Figure B(i) and (ii)), which might be attributable to the stretching vibration of phenolic hydroxyls (O–H bond), confirming the interactions of phenolic hydroxyls with AgNPs.
3.3 High resolution-transmission electron microscopy
High-resolution transmission electron microscopy (HRTEM) was used to find the nanoparticles’ size, shape, and morphology [17]. HRTEM images of AgNPs nanoparticles were nearly spherical, as shown in Fig. 2a. The synthesised nanoparticle’s electron diffraction field is chosen (SAED) pattern is shown in the inset of Fig. 2b. The rings designated 1, 2, 3, and 4 arise because of the reflections from (111), (200), (220), and (311) plane families of face-centred cubic structure. It was found that the distribution of the particle size is 50 nm, as shown in Fig. 2.
3.4 Particle size and zeta potential analysis
Figures 2c particle size and 2D zeta potential demonstrate that the electric potential in the interfacial double layer (DL) at the sliding plane versus a point in the bulk fluid away from the interface is zeta potential. It is the potential difference between the dispersion medium and the stationary layer of fluid linked to the dispersed particle, in other words. As illustrated in Fig. 2a, the average hydrodynamic diameter of ZL-AgNPs is 55 ± 3 nm with polydispersity index of 0.210. Zeta potential analysis shows a value of − 17.3 mV, calculated as the arbitrary number that differentiates high-charged and low-charged surfaces shows in Fig. 2c and d. The zeta potential is significant because its value can be linked to the stability of colloidal dispersions.
3.5 X-ray diffraction
X-ray powder diffraction (XRD) analysis was used to determine the crystal lattice and structure of the biosynthesized AgNPs. The X-ray diffraction tests revealed three well-resolved diffraction peaks at two angles of roughly 37.56, 43.25, and 64.10, which correspond to the crystallographic planes of silver nanoparticles 111, 200, 220, and (311) respectively (Fig. 2e).
3.6 Antimicrobial assay
The silver nanoparticles release the bacterial growth-inhibitory around the well, resulting in the inhibition zone formation shown in (Fig. 3). The inhibition region was 14 mm and 15 mm for gram-positive and gram-negative (Table 1). It shows that the synthesized silver nanoparticles have the potential to act against as both gram-positive and gram-negative classes.
3.7 Cell viability assay
We also used the MTT assay to examine the effect of ZL-AgNP on 3T3 cell lines. In vitro toxicity activity of ZL-AgNPs (10–50 µg/mL concentrations) against chosen 3T3 cell (Data not shown). The findings show that the compound-nanoparticle combination inhibits cell growth dose-dependent.
3.8 In vivo toxicity study in the zebrafish embryo
The zebrafish is an excellent model for investigating nanoparticle toxicity and drug testing. The embryos were used to test the toxicity of various concentrations of ZL-AgNPs (5 to 50 g/ml). In contrast to control, ZL-AgNPs were discovered to be non-toxic to the zebrafish embryo (saline). The hatching rate, percentage survival rate, and morphological behaviours.
3.9 Hemocompatibility
When RBC cells contact foreign materials, they swell, causing cell membranes to break and internal components, including haemoglobin, to leak out. As a result, hemolysis of blood cells is an important parameter to examine for materials used in vivo. Any biomaterial with less than 5% haemolysis was allowed.
3.10 DAPI staining
We analysed the ZL-AgNPs using the DAPI staining technique to further validate the compounds nuclear fragmentation on 3T3 cell lines. Figure 4 shows fluorescence microscopy of DAPI stained images after 24 h. Untreated cells showed no significant changes, whereas conjugate treated cells showed bright fetches, indicating condensed chromatins and nuclear fragmentations in the 3T3 cell lines.
4 Discussion
Medicinal herbs are frequently utilized to heal a variety of ailments. Medicinal plants produce secondary metabolites that have therapeutic potential. Extraction methods, plant sections, development phases, climatic conditions, and seasons all influenced the bioactive potential of medicinal plants. Vegetable eating has already been shown to reduce the risk of many diseases and disorders. Polyphenols, which are found in plants, can efficiently act as a stabilizing agent in the green synthesis of nanoparticles. Z. latifolia was used as a reducing agent for the green production of nanoparticles in this work. Unlike chemical approaches, plant-based green nanoparticle production is both cost-effective and environmentally beneficial. Nanoparticles made from plant sources have also proven to be more stable than those made from fungi and bacteria [18]. The synthesized silver nanoparticles was confirmed by colour formation from yellow to brown due to the reduction of silver salt by presence of reducing agents in aqueous extract of Z. latifolia, further the AgNPs formation was reveal UV-absorption spectra was taken from 200 to 800 nm. It was assumed that the tautomeric conversions of flavonoids from the enol-form to the keto-form might release an activated hydrogen atom that can reduce metal ions to form nanoparticles (yellow colour converted to wine red colour). The sharp absorbance peak of the synthesized silver nanoparticle was obtained at 421 nm. The maximum absorbance range was obtained at 300 µl concentration; beyond this range, the absorbance decreased gradually, as shown in Fig. 1a. The FTIR spectroscopy of nanoparticles synthesized in plants/plant extracts, it has been demonstrated that terpenoids are often associated with nanoparticles. Flavonoids contain various functional groups capable of nanoparticle formation [19]. The carbonyl groups of amino acids and the amide groups of flavonoids have a strong affinity for metal ions, and they may surround the nanoparticles in a protective coat-like shell, preventing further aggregation and stabilizing silver nanoparticles, according to FT-IR studies.
The size and shape of silver nanoparticles (ZL-AgNPs) were confirmed using a High Resolution-Transmission Electron Microscope [20]. The obtained silver nanoparticles were spherical in shape and formed as clean dispersals with few agglomerated. Figure 2a low polydispersity index indicates the uniformity of particle size distribution for ZL-AgNPs. The presence of Ag 111, 200, 220, and 311, planes of the facecentered cubic structured (FCC) silver nanoparticles obtained as shown in Fig. 2b was assigned to the presence of clear specific rings spotted by selected area electron diffraction (SAED), and it was confirmed that these are green synthesis of AgNPs.
The zeta potential describes how strongly nearby, similarly charged molecules and particles repel each other. A high zeta potential means the solution or distribution will be stable and resist aggregation [21]. The XRD pattern of dried silver nanoparticles is shown in Fig. 2e. The XRD peaks are broad, indicating nanoparticle production [22]. Four diffraction peaks can be attributed to the (111), (200), (220), and (311). Reflections of metallic face-centred cubic structure silver, indicating that the manufactured silver nanoparticles are made entirely of pure crystalline silver.
In the present study, it was identified that the gram-positive bacteria formed the higher inhibitory zones compared to the gram-negative strains [23]. This may be due to the physical attribute of the nanoparticle, i.e. larger surface area and small size of the AgNP’s. These nanostructures bind to the bacterial cell wall and then penetrate it, producing alterations in the cell membrane's structural integrity, such as cell permeability, which eventually leads to cell death (Fig. 3). Small hollow structures termed ‘pits’ may form on the cell surface, with some nanoparticle accumulation. Another process by which the cells perish could be the generation of free radicals by the silver nanoparticles. The ZL-AgNPs shows good antimicrobial activity against gram-positive (B. subtilis and S. aureus) and gram-negative strains (P. aeruginosa and E. coli). The effect of the antibacterial property of ZL-AgNPs was made versus two different gram positive and one gram negative wound infection causing pathogens. Among the evaluated pathogens with prepared ZL-AgNPs showed zone of inhibition of 13 ± 1.22 mm by B. subtilis followed by P. aeruginosa of 09 ± 1.5 mm, E. coli of 12 ± 0.9 and S. aureus of 15 ± 1 mm (Table 1). The MTT assay was used to evaluate the biocompatibility of the prepared ZL-AgNPs with the help of a 3T3 mouse fibroblast cell line (Data not shown). The proliferation of 3T3 mouse fibroblasts cells on the surface of silver nanoparticles was observed, indicating that the silver nanoparticles were not toxic to fibroblast cells [24]. The results revealed that the rate of cell proliferation was not affected in ZL-AgNPs followed by control group. On different concentration 10 µg/ml, 25 µg/ml, and 50 µg/ml, and ZL-AgNPs demonstrated a time-dependent increase in cell proliferation after seeding. Even though native ZL-AgNPs did not cause cytotoxicity in the treated cells, the ZL-AgNPs significantly increased cell proliferation when compared to the control, indicating the role of Z. latifolia husk extract in supporting the growth of fibroblasts cells in the ZL-AgNPs in fibroblast cell growth.
Figures 5 and 6 demonstrate this (1) (2), the hatching and survival rates are unaffected by the maximum concentration of ZL-AgNPs. Figure 5 and Fig. 6 illustrate the changes in morphological behaviours of zebra fish embryos observed using a stereomicroscope [25]. The total length of roots exposed to different concentrations of ZL-AgNPs was negligible. With varying concentrations of ZL-AgNPs (5 to 50 µg/ml), the tail length, body shape, heart, pericardial sac, and body pigmentation were observed, and no major malformations were observed in normal zebra fish development.
The amount of haemoglobin released during the assay is used to assess the effect of ZL-AgNPs on RBCs. It was clear from the findings that ZL-AgNPs were extremely hemocompatible, causing only about 2% haemolysis (Fig. 7a). It has been reported that green synthesis silver nanoparticles have been identified to be promising materials for improving the blood compatibility of biomaterials [26]. As a result, the presence of ZL (Z. latifolia) as a capping agent, which entirely covers the surface of the ZL-AgNPs as hemocompatible, can be attributed to the lack of substantial hemolysis for ZL-AgNPs (Fig. 7b).
The DAPI study was beneficial in identifying apoptotic condensed nuclei loss because it could form fluorescent complexes with nuclear material and active mitochondrial membranes of the cells. The photos reveal apoptotic nuclei shrinking in size (blue colour) and condensed chromatin forming at the nuclear membrane’s periphery [27]. In these cells, fractured nuclear bodies and mitochondrial membranes can also be seen. Furthermore, 3T3 mitochondrial location in cells treated with various doses of ZL-AgNP was studied (Fig. 4). The MTT assay and fluorescence microscopy analyses clearly show that ZL-AgNP can be employed as an efficient therapeutic agent.
5 Conclusion
To summarize the present study, silver nanoparticles were successfully processed by the green synthesis method with the aqueous extract of the Z. latifolia. The HR-TEM confirm the morphology with spherical, well-dispersed AgNPs with a narrow size distribution. The significant synergistic antimicrobial efficiency on E. coli and P. aeruginosa. Similarly, in vivo zebrafish toxicity studies revealed that ZL-AgNPs were safe. These results provide essential information for developing antimicrobial materials to observe the efficacy of antimicrobial agents against wound pathogens.
Change history
05 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13399-022-03493-y
Abbreviations
- ZL :
-
Zizania latifolia
- AgNPs :
-
Silver nanoparticles
- HR-TEM :
-
High resolution-transmission electron microscopy
- SAED :
-
Selected area electron diffraction
- FCC :
-
Facecentered cubic structured
- XRD :
-
X-ray powder diffraction
- MTT :
-
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide, DAPI: 4′, 6-diamidino-2-phenylindole
- FTIR :
-
Fourier transform infra-red
- EDAX :
-
Energy dispersive X-ray analysis
References
Mohamed JMM, Alqahtani A, Menaa F, Kayarohanam S, Fatease AA, Alqahtani T, Alamri A, El-Sherbiny M, Ramkanth S, Janakiraman AK (2022) In vitro physical characterizations and docking studies on carvedilol nanocrystals. Curr Comput-Aided Drug Des 12:988
Behravan M, Panahi AH, Naghizadeh A, Ziaee M, Mahdavi R, Mirzapour A (2019) Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol 124:148–154
Wu F, Chen B, Zou Q, Zhai C, Liu W, Chen J, Ni G (2019) Range estimation of horizontal stress of deep rock based on Mohr-Coulomb criterion. Results Phys 12:2107–2111
Das G, Patra JK, Debnath T, Ansari A, Shin H-S (2014) Investigation of antioxidant antibacterial antidiabetic and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L). PloS One. 14(8):e0220950
Jain S, Mehata MS (2017) Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep 7(1):1–13
Vickers NJ (2017) Animal communication: when i’m calling you, will you answer too? Curr Biol 27(14):R713–R715
Sharma HP, Chakraborty SK, Patel H (2020) Process parameter optimization for enzyme-aided juice extraction of wood apple (Feronia limonia). Agric Res 9(3):410–416
Alharbi FA, Alarfaj AA (2020) Green synthesis of silver nanoparticles from Neurada procumbens and its antibacterial activity against multi-drug resistant microbial pathogens. J King Saud Univ Sci 32(2):1346–1352
Femi-Adepoju AG, Dada AO, Otun KO, Adepoju AO, Fatoba OP (2019) Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia Pectinata (Willd) C Presl): characterization and antimicrobial studies. Heliyon. 5(4):e01543
Singh A, Gaud B, Jaybhaye S (2020) Optimization of synthesis parameters of silver nanoparticles and its antimicrobial activity. Mater Sci Technol 3:232–236
Mohamed JMM, Alqahtani A, Kumar TVA, Fatease AA, Alqahtani T, Krishnaraju V, Ahmad F, Menaa F, Alamri A, Muthumani R, Vijaya R (2021) Superfast synthesis of stabilized silver nanoparticles using aqueous Allium sativum (garlic) extract and isoniazid hydrazide conjugates: molecular docking and in-vitro characterizations. Mol 27(1):110
Afreen A, Ahmed R, Mehboob S, Tariq M, Alghamdi HA, Zahid AA, Ali I, Malik K, Hasan A (2020) Phytochemical-assisted biosynthesis of silver nanoparticles from Ajuga bracteosa for biomedical applications. Mater Res Express 7(7):75404
Bergal A, Matar GH, Andac M (2022) Olive and green tea leaf extracts mediated green synthesis of silver nanoparticles (AgNPs): comparison investigation on characterizations and antibacterial activity. BioNanoSci 12(2):307–321
Agnihotri S, Mukherjia S, Mukherji S (2014) Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv 4(8):3974–3983
Balachandar R, Gurumoorthy P, Karmegam N, Barabadi H, Subbaiya R, Anand K, Boomi P, Saravanan M (2019) Plant-mediated synthesis, characterization and bactericidal potential of emerging silver nanoparticles using stem extract of Phyllanthus pinnatus: a recent advance in phytonanotechnology. J Clust Sci 30(6):1481–1488
Mohamed JM, Alqahtani A, Ahmad F, Krishnaraju V, Kalpana K (2021) Pectin co-functionalized dual layered solid lipid nanoparticle made by soluble curcumin for the targeted potential treatment of colorectal cancer. Carbohydr Polym 252:117180
Souza VG, Pires JR, Rodrigues PF, Lopes AA, Fernandes FM, Duarte MP, Coelhoso IM, Fernando AL (2018) Bionanocomposites of chitosan/montmorillonite incorporated with Rosmarinus officinalis essential oil: development and physical characterization. Food Packag Shelf Life 16:148–156
Rolim WR, Pelegrino MT, de Araújo LB, Ferraz LS, Costa FN, Bernardes JS, Rodigues T, Brocchi M, Seabra AB (2019) Green tea extract mediated biogenic synthesis of silver nanoparticles: characterization, cytotoxicity evaluation and antibacterial activity. Appl Surf Sci 463:66–74
Alqahtani A, Raut B, Khan S, Mohamed JMM, Fatease AA, Alqahtani T, Alamri A, Ahmad F, Krishnaraju V (2022) The unique carboxymethyl Fenugreek gum gel loaded itraconazole self-emulsifying nanovesicles for topical onychomycosis treatment. Polymers (Basel) 14(2):325
Jain AS, Pawar PS, Sarkar A, Junnuthula V, Dyawanapelly S (2021) Bionanofactories for green synthesis of silver nanoparticles: toward antimicrobial applications. Int J Mol Sci 22(21):11993
Su D-l, Li P-j, Ning M, Li G-y, Shan Y (2019) Microwave assisted green synthesis of pectin based silver nanoparticles and their antibacterial and antifungal activities. Mater Lett 244:35–38
Sana SS, Badineni VR, Arla SK, Boya VKN (2015) Eco-friendly synthesis of silver nanoparticles using leaf extract of Grewia flaviscences and study of their antimicrobial activity. Mater Lett 145:347–350
Sana SS, Dogiparthi LK (2018) Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater Lett 226:47–51
Shkryl Y, Rusapetova T, Yugay Y, Egorova A, Silant’ev V, Grigorchuk V, Karabtsov A, Timofeeva Y, Vasyutkina E, Kudinova O, Ivanov V (2021) Biosynthesis and cytotoxic properties of Ag, Au, and bimetallic nanoparticles synthesized using Lithospermum erythrorhizon callus culture extract. Int. J. Mol. Sci. 22(17):9305
Kumar CS, Mahesh A, Antoniraj MG, Vaidevi S, Ruckmani K (2016) Ultrafast synthesis of stabilized gold nanoparticles using aqueous fruit extract of Limonia acidissima L and conjugated epirubicin: targeted drug delivery for treatment of breast cancer. RSC Adv. 6(32):26874–82
Devendiran RM, kumarChinnaiyan S, Yadav NK, Ramanathan G, Singaravelu S, Perumal PT, Sivagnanam UT (2016) Facile synthesis and evaluation of quercetin reduced and dextran sulphate stabilized gold nanoparticles decorated with folic acid for active targeting against breast cancer. RSC Adv. 6(39):32560–71
Senthilkumar C, Kannan PR, Balashanmugam P, Raghunandhakumar S, Sathiamurthi P, Sivakumar S, Arockiarajan A, Mary SA, Madhan B (2022) Collagen-Annona polysaccharide scaffolds with tetrahydrocurcumin loaded microspheres for antimicrobial wound dressing. Carbohydr Polym Tech Appl 3:100204
Acknowledgements
The authors are thankful to the Department of Environmental Biotechnology, Bharathidasan University, Tiruchirappalli-620 024, Tamil Nadu, India for providing the necessary lab facilities during the experimental study.
Funding
The work was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R199), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia and by the AlMaarefa Researchers Supporting program (MA-006), AlMaarefa University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
V. Satheesh and Jamal Moideen Muthu Mohamed conceived and designed research, V. Satheesh and Sivasudha Thilagar conducted experiments, Mohamed El-Sherbiny, Rasha Hamed Al-Serwi, and Gamal Othman analyzed data and wrote the manuscript, Sivasudha Thilagar supervision of the work. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Rasha Hamed Al‑Serwi should only be affiliated with Department of Basic Dental Sciences, College of Dentistry, Princess Nourah bint Abdulrahman University, P.O. Box 84428, Riyadh 11671, Saudi Arabia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Satheesh, V., Mohamed, J.M.M., El-Sherbiny, M. et al. Sunlight-assisted green synthesis of silver nanoparticles using Zizania latifolia extract: toward antimicrobial applications. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03363-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03363-7