Abstract
Aedes albopictus is an important arbovirus vector, including dengue. Currently, there is no specific treatment for dengue. Its prevention solely depends on effective vector control measures. In this study, silver nanoparticles (AgNPs) were biosynthesized using a cheap leaf extract of Berberis tinctoria as reducing and stabilizing agent and tested against Ae. albopictus and two mosquito natural enemies. AgNPs were characterized by using UV–vis spectrophotometry, X-ray diffraction, and scanning electron microscopy. In laboratory conditions, the toxicity of AgNPs was evaluated on larvae and pupae of Ae. albopictus. Suitability Index/Predator Safety Factor was assessed on Toxorhynchites splendens and Mesocyclops thermocyclopoides. The leaf extract of B. tinctoria was toxic against larval instars (I–IV) and pupae of Ae. albopictus; LC50 was 182.72 ppm (I instar), 230.99 ppm (II), 269.65 ppm (III), 321.75 ppm (IV), and 359.71 ppm (pupa). B. tinctoria-synthesized AgNPs were highly effective, with LC50 of 4.97 ppm (I instar), 5.97 ppm (II), 7.60 ppm (III), 9.65 ppm (IV), and 14.87 ppm (pupa). Both the leaf extract and AgNPs showed reduced toxicity against the mosquito natural enemies M. thermocyclopoides and T. splendens. Overall, this study firstly shed light on effectiveness of B. tinctoria-synthesized AgNPs as an eco-friendly nanopesticide, highlighting the concrete possibility to employ this newer and safer tool in arbovirus vector control programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mosquitoes (Diptera: Culicidae) represent a key threat for millions of people worldwide, since they act as vectors for devastating parasites and pathogens, including malaria, yellow fever, dengue, West Nile, chikungunya, and filariasis (Mehlhorn et al. 2012; Benelli 2015a). Aedes albopictus (Skuse), commonly known as the Asian tiger mosquito, is currently retained the most invasive mosquito species in the world, since it is able to rapidly adapt to different anthropogenic environments, thanks to its ecological and physiological plasticity. Recently, the Asian tiger mosquito has invaded many countries, spreading rapidly to Europe, North and South America, the Caribbean, Africa, and the Middle East (Caminade et al. 2012; Murugan et al. 2016a). Ae. albopictus is both a nuisance and a disease vector. Its medical importance is mainly due to the aggressive daytime human-biting behavior and to its ability to transmit many diseases. It works as a vector for many viruses, including dengue, yellow fever, West Nile, Japanese encephalitis, St. Louis, encephalitis virus (Flaviridae, genus Flavivirus), chikungunya, eastern equine encephalitis, Venezuelan equine encephalitis, western equine encephalitis, Ross River, Sindbis, Mayaro, Getah (Togaviridae, genus Alphavirus), Potosi, San Angelo, La Crosse, Jamestown Canyon (Bunyaviridae, genus Bunyavirus), Rift Valley fever (Bunyaviridae, genus Phlebovirus), and Orungo virus (Reoviridae, genus Orbivirus). Ae. albopictus is also the vector of different filariases, such as Dirofilaria immitis Leidy, D. repens Railliet and Henry, and Setaria labiatopapillosa Perroncito (Paupy et al. 2009; Benelli 2015b).
Mosquito young instars are usually targeted using organophosphates, insect growth regulators, and microbial control agents. Indoors residual spraying and insecticide-treated bed nets are also employed to reduce transmission of malaria in tropical countries (Benelli 2015a). However, these chemicals have strong negative effects on human health and/or the environment and induce resistance in a number of mosquito species (Hemingway and Ranson 2000). On this basis, eco-friendly tools have been recently implemented to enhance control of mosquito vectors. Particularly, important efforts have been carried out investigating the efficacy of botanical products against mosquito vectors. Many plant-borne compounds have been reported as excellent toxics against Culicidae, acting as ovicides, larvicides, pupicides, adulticides, oviposition deterrents, adult repellents, growth and/or reproduction inhibitors (Amer and Mehlhorn 2006a, b, c, d; Pavela 2008, 2009; see Benelli 2015c; Pavela 2015 and Benelli et al. 2015 for recent reviews).
In a biological control perspective, mosquito young instar populations may be controlled by a number of aquatic predators, including odonate nymphs, water bugs, tadpoles, fishes, and copepods (Bowatte et al. 2013; Kalimuthu et al. 2014; Murugan et al. 2015a, b, c, d). Copepods are small aquatic crustaceans, most of them are omnivorous and can prey on immature mosquitoes, especially first instar larvae (Hurlbut 1938; Marten et al. 1989; Rawlins et al. 1997; Manrique-Saide et al. 1998; Williamson 1999). Mesocyclops thermocyclopoides is a common copepod species in tropical and subtropical areas, evaluated as a biocontrol agent against Aedes mosquitoes (Mahesh Kumar et al. 2012). This species feeds on the first and second instars of mosquito larvae, fatally wounding about seven individuals per day (Schaper and Hernández 1998).
Interestingly, there are also some Culicidae species that contribute to the control of mosquito vectors of public health importance. Toxorhynchites, also called “elephant mosquito” or “mosquito eater,” is a cosmopolitan genus of mosquitoes. The genus includes the largest known species of mosquito, and it is among the few kinds of mosquito that do not consume blood. The adults subsist on carbohydrate-rich materials, such as honeydew, or saps and juices from damaged plants, refuse, fruit, and nectar. The larvae of Toxorhynchites prey on the larvae of other mosquitoes and similar nektonic prey. In this respect, they contrast with blood-sucking species of mosquitoes. Toxorhynchites larvae live on a protein- and fat-rich diet of aquatic animals such as mosquito larvae. They have no need to risk their lives sucking blood in adulthood, having already accumulated the necessary materials for oogenesis and vitellogenesis. Most species occur in forests. The larvae of one jungle variety, Toxorhynchites splendens, consume larvae of other mosquito species occurring in tree crevices, particularly Aedes ones. The adults of these mosquitoes are larger than Aedes and are harmless to humans. The larvae of Toxorhynchites splendens are cannibalistic only if other preys are unavailable (Steffan 1975; Steffan and Evenhuis 1981; Focks 1985). Overall, studies on T. splendens as biocontrol agents for larval mosquito control conducted in several countries reported promising results (Focks 1985; Rawlins et al. 1991; Aditya et al. 2006).
The development of green processes for the biosynthesis of nanoparticles is evolving into an important branch of nanotechnology (Shin et al. 2007). Plants and microbes are currently used for nanoparticle synthesis. The use of plants to fabricate nanoparticles is rapid, low cost, eco-friendly, and a single-step method for biosynthesis process (Huang et al. 2007; Kumar and Yadav 2009). Recently, silver nanoparticles have been biosynthesized using various plant extracts (e.g., Aloe vera (Dinesh et al. 2015), Phyllanthus niruri (Suresh et al. 2015), Moringa oleifera (Sujitha et al. 2015), Caulerpa scalpelliformis (Murugan et al. 2015a)) and have been found highly effective against important mosquito vectors, even if tested at low doses (see Benelli 2016 for a review).
Berberis tinctoria Lesch. is an evergreen erect shrub with yellow wood belonging to Berberidaceae. The leaves of B. tinctoria have been evaluated for hepatoprotective activity and antioxidant activity (Murugesh et al. 2005). They contain berberine, an important alkaloid, which is effective for various infectious diseases and possess antibacterial property (Sasikumar et al. 2007). The use of berberine has been described in Indian and Chinese medicine for the treatment of diarrhea and intestinal parasitic infections (Saha et al. 2011). In this research, we investigated the mosquitocidal properties of B. tinctoria leaf extract and green-fabricated silver nanoparticles. The biosynthesized silver nanoparticles were characterized by UV–vis spectrophotometry, scanning electron microscopy (SEM), and X-ray diffraction (XRD). Mosquitocidal properties were assessed in laboratory against larvae (I–IV instar) and pupae of the arbovirus vector Ae. albopictus. Green-synthesized AgNPs and B. tinctoria leaf extract were tested against the two mosquito predators T. splendens and M. thermocyclopoides, and the Suitability Index/Predator Safety Factor was calculated.
Materials and methods
Plant material and preparation of the extract
B. tinctoria was collected from Kattabettu (11° 24' 44.8" N 76° 48' 47.6" E; Kothagiri, Tamil Nadu, India). Leaves were identified at the Department of Botany, Bharathiar University (Coimbatore, India). Voucher specimens (ID: BERTIN1-3) were stored in our laboratories and are available upon request. The plant leaves were washed with distilled water and shade-dried for 2 days at 28 °C. Ten grams of washed and finely cut leaves were stored in a 300-ml Erlenmeyer flask filled with 100 ml of sterile distilled water. The mixture was boiled for 5 min before finally decanted it. The aqueous extract was stored at 4 °C and used within 5 days.
Biosynthesis and characterization of silver nanoparticles
To reduce Ag+ ions to Ag0, 10 ml of plant aqueous extract were added to 190 ml of aqueous AgNO3 (1 mM). The effect of reaction time on synthesis rate and size of AgNPs was studied by carrying out the reaction in a water bath at 95 °C with reflux (elapsed time, from 10 min to 4 h). AgNPs were subjected to repeated centrifugation at 15,000 rpm for 20 min followed by re-dispersion of the pellet in de-ionized water. UV–vis spectra were recorded as a function of reaction time on a UV-3600 Shimadzu spectrophotometer operated at a resolution of 1 nm. After freeze-drying of the purified AgNP, the structure and composition were analyzed by 10 kV Ultra High Resolution SEM (FEI QUANTA-200 SEM). XRD using Cukα radiation (PAN anlyticalX’pert Pro MPD diffractometer) was used to determine the crystalline structure of AgNPs. In addition, X-ray analysis on dried AgNPs was carried out using a Philips Model PW 1050/37 diffractometer, operating at 40 kV and 30 mA, with a step size of 0.02° (2θ) (Suresh et al. 2015).
Tested concentrations
From the aqueous extract, the concentrations were made (50, 150, 250, 350, and 450 ppm), and 2 ml of B. tinctoria-synthesized AgNPs was diluted in 100 ml of distilled water for the preparation of 2 % (v:v) stock solution. Then, the experimental concentrations (i.e., 2.5, 5, 10, 20, and 40 ppm) were prepared by subsequent dilution of stock solution in distilled water. All stocks and dilutions were kept refrigerated at −4 °C and tested within 8 weeks.
Ae. albopictus rearing
Experiments were conducted using laboratory-reared pathogen-free strains of Ae. albopictus colonies that were originally established as described by Subramaniam et al. (2015). Batches of 100–110 eggs were transferred to 18 cm l × 13 cm W × 4 cm D enamel trays containing 500 ml of water where they were allowed to hatch in laboratory conditions (27 ± 2 °C and 75–85 % R.H.;14:10 (L:D)) photoperiod. Mosquito larvae were fed daily with 0.5 g of ground dog biscuit (Pedigree, USA) and brewer’s yeast (Sigma-Aldrich, Germany) in a 3:2 (w:w) ratio. Ae. albopictus larvae and pupae were used for acute toxicity experiments. Furthermore, each container was placed inside a cubic chiffon cage (90 × 90 × 90 cm) to wait for adult emergence. Adults of both species were fed on 10 % (w:v) sucrose solution. Five days after emergence, Ae. albopictus adults were deprived of sugar feeding for 12 h and then supplied with artificial blood feeding. The blood meal was furnished, by means of a professional heating blood (lamb blood), at fixed temperature of 38 °C and provided with a membrane of cow gut. After 30 min, the blood meal was removed, due to blood drying phenomena, and gut membrane was substituted with a new fresh one for the following utilization (Nicoletti et al. 2012).
Larvicidal and pupicidal activity against Ae. albopictus
Following the methods reported by Murugan et al. (2015a, b), 25 Ae. albopictus larvae (I, II, III, or IV instar) or pupae were exposed for 24 h in a glass beaker filled with 250 ml of de-chlorinated water plus B. tinctoria aqueous extract (i.e., 50, 150, 250, 350, and 450 ppm) and B. tinctoria-synthesized AgNPs (i.e., 2.5, 5, 10, 20, and 40 ppm). Larval food was provided for each tested concentration. Each concentration was replicated five times against all instars. In the control, for both species, 25 larvae or pupae were transferred in 250 ml of de-chlorinated water. No mortality was observed in the control. Percentage mortality was calculated as follows:
LC50 and LC90 were calculated by probit analysis, following the method by Finney (1971). SPSS software package 16.0 version was used for all analyses.
Toxicity against mosquito natural enemies
Following the method by Sivagnaname and Kalyanasundaram (2004), here, the effect of B. tinctoria leaf extract and AgNPs was tested on two non-target mosquito natural enemies, T. splendens (IV instar larvae) and M. thermocyclopoids (adults). Both species were collected from rural ponds in Coimbatore (Tamil Nadu, India) and maintained at 27 ± 3 °C and R.H. 85 % in cement tanks (120-cm diameter, 60-cm depth) filled with de-chlorinated water (Murugan et al. 2015c). Leaf extracts of B. tinctoria and green-synthesized AgNPs were evaluated at a concentration of five times higher than the LC50 dose of mosquito larvae. Five replicates were performed for each concentration, along with negative controls. The tested organisms were observed for mortality and other abnormalities such as sluggishness and reduced swimming activity after 48 h of exposure. The exposed predators were also observed continuously for 10 days to understand the post-treatment effect of this extract on survival and swimming activity. LC50 and LC90 values were obtained by probit analysis, and the Suitability Index/Predator Safety Factor was calculated for each tested species using the following formula (Deo et al. 1998).
Results and discussion
Biosynthesis and characterization of silver nanoparticles
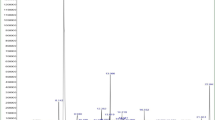
When the B. tinctoria leaf extract was added to the AgNO3 aqueous solution, the color changed from pale yellow to reddish-brown (Fig. 1a), indicating the reduction from Ag+ to Ag0, thus the formation of AgNPs. The darker color could be due to the excitation of surface plasmon vibrations, which is typical of AgNPs (Ahmad et al. 2003; Krishnaraj et al. 2010). UV–vis spectrophotometry can be used to examine size and shape-controlled nanoparticles in aqueous suspensions (see Shrivastava and Dash 2010; Govindarajan et al. 2016). The absorption spectrum of B. tinctoria-synthesized AgNPs showed a maximum absorption peak at 420 nm after 120 min (Fig. 1b), broadening of the peak indicated that the particles are poly-dispersed (Prasad and Elumalai 2011). In agreement with our results, Sujitha et al. (2015) reported that the synthesis of AgNPs using M. oleifera leads to a maximum absorbance peak at 450 nm. The B. tinctoria leaf extract without AgNO3 did not show any change in color over time for at least 4 weeks.
SEM analysis showed that B. tinctoria-synthesized AgNPs were predominantly spherical in shape (Fig. 2), with a mean size of 65–70 nm. In XRD trials, Bragg reflections corresponding to the 111, 200, 220, 311, and 222 sets of lattice planes were observed (Fig. 3). The XRD pattern showed that the AgNPs formed by the reduction of AgNO3 by B. tinctoria leaf extract were crystalline in nature. Notably, these sharp Bragg peaks might be due to the capping agents stabilizing AgNPs. XRD results also suggest that crystallization of the bioorganic phase occurs on the surface of the AgNPs. Similarly, Sathyavathi et al. (2010) reported diffraction peaks at 44.50°, 52.20°, and 76.7° 2θ, which correspond to the 111, 200, and 220 facets of the face-centered cubic crystal structure.
Larvicidal and pupicidal activity against Ae. albopictus
In our laboratory assays, the leaf extract of B. tinctoria was toxic against larval instars (I–IV) and pupae of the arbovirus vector Ae. albopictus. LC50 values were 182.72 ppm (I instar larvae), 230.99 ppm (II), 269.65 ppm (III), 321.75 ppm (IV), and 359.71 ppm (pupae), respectively (Table 1). AgNPs synthesized from the leaf extract of B. tinctoria were highly effective against Ae. albopictus young instars, with LC50 of 4.97 ppm (I), 5.97 ppm (II), 7.60 ppm (III), 9.65 ppm (IV), and 14.87 ppm (pupae) (Table 2). A dose-dependent effect was found, in agreement with a number of previously reported plant-borne pesticides (e.g., Amer and Mehlhorn 2006c, d; Bagavan et al. 2009; Benelli 2015c). Recently, a growing number of green-synthesized AgNPs showed comparable larvicidal and pupicidal toxicity against different mosquito vectors (Santhoshkumar et al. 2011; Subramaniam et al. 2015; Benelli 2016; Murugan et al. 2016b). For example, Suresh et al. (2015) highlighted that AgNPs synthesized using the aqueous extract of P. niruri are highly effective against larvae and pupae of Ae. aegypti, with LC50 values ranging from 3.90 ppm (I) to 13.04 ppm (pupae). Low doses of C. scalpelliformis-synthesized AgNPs are highly toxic also against the filariasis vector Culex quinquefasciatus, with LC50 values ranging from 3.08 ppm (I) to 7.33 ppm (pupae) (Murugan et al. 2015a). We hypothesize that the toxicity of AgNPs against arbovirus vectors may be enabled by the small size of these nanoparticles, which allows passage through the insect cuticle and into individual cells where they interfere with molting and other physiological processes (Murugan et al. 2015e, f). Further research on the impact of sub-lethal doses of green-fabricated AgNPs on mosquito fecundity and longevity is urgently needed (Roni et al. 2015).
Toxicity against mosquito natural enemies
B. tinctoria was tested against the non-target mosquito predators T. splendens and M. thermocyclopoids, with LC50 values of 552.28 and 480.92 ppm, respectively (Tables 3 and 4, respectively). Experiments conducted testing AgNPs on T. splendens and M. thermocyclopoids lead to LC50 values of 234.48 and 218.16 ppm, respectively. The Safety Index/Predator Safety Factor calculated for the leaf extract of B. tinctoria was 3.02 and 2.63 for T. splendens and M. thermocyclopoids, respectively, while for AgNPs, it was 47.1 and 43.8, respectively. Currently, moderate knowledge is available about the acute toxicity towards aquatic non-target species (Benelli 2016). Pergularia rubra- and Pergularia daemia-synthesized AgNPs did not exhibit any evident toxicity effect against Poecilia reticulata fishes, after 48 h of exposure to LC50 and LC90 values calculated on IV instar larvae of Ae. aegypti and Anopheles stephensi (Patil et al. 2012a, b). Subarani et al. (2013) did not reported toxicity effects of Vinca rosea-synthesized AgNPs against P. reticulata, after 72 h of exposure to dosages toxic against An. stephensi and C. quinquefasciatus. Similarly, Haldar et al. (2013) did not detected toxicity of AgNPs produced using dried green fruits of Drypetes roxburghii against P. reticulata, after 48 h of exposure to LC50 of IV instar larvae of An. stephensi and C. quinquefasciatus. Rawani et al. (2013) showed that mosquitocidal AgNPs synthesized using Solanum nigrum berry extracts were not toxic against two mosquito predators, Toxorhynchites larvae and Diplonychus annulatum, and Chironomus circumdatus larvae, exposed to lethal concentrations of dry nanoparticles calculated on An. stephensi and C. quinquefasciatus larvae. AgNPs biosynthesized using the 2,7.bis[2-[diethylamino]-ethoxy]fluorence isolate from the Melia azedarach leaves did not show acute toxicity against Mesocyclops pehpeiensis copepods (Ramanibai and Velayutham 2015).
Conclusions
Overall, we biosynthesized AgNPs using a cheap aqueous extract of B. tinctoria leaves as a reducing and stabilizing agent. Bio-fabricated AgNPs were mostly spherical in shape, crystalline in nature, with face-centered cubic geometry, and their mean size was 65–70 nm. This study highlighted that B. tinctoria-synthesized AgNPs are easy to produce, stable over time, and may be employed at low dosages to strongly reduce young instar populations of arbovirus mosquito vectors, with little impact on vector natural enemies such as copepods and Toxorhynchites predaceous larvae. Further research is ongoing to shed light on the toxicity mechanism(s) of AgNPs against mosquito predators and their potential long-term toxicity effects on arthropod longevity and fecundity.
References
Aditya G, Ash A, Saha GK (2006) Predatory activity of Rhantus sikkimensis and larvae of Toxorhynchites splendens on mosquito larvae in Darjeeling, India. J Vector Borne Dis 43:66–72
Ahmad A, Mukherjee P, Mandal D, Senapati S, Khan MI, Kumar R, Sastry M (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium zoxysporum. Colloids Surf B: Biointerfaces 28:313–318
Amer A, Mehlhorn H (2006a) Repellency effect of forty-one essential oils against Aedes, Anopheles and Culex mosquitoes. Parasitol Res 99:478–490
Amer A, Mehlhorn H (2006b) The sensilla of Aedes and Anopheles mosquitoes and their importance in repellency. Parasitol Res 99:491–499
Amer A, Mehlhorn H (2006c) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res 99:466–472
Amer A, Mehlhorn H (2006d) Persistency of larvicidal effects of plant oil extracts under different storage conditions. Parasitol Res 99:473–477
Bagavan A, Kamaraj C, Elango G, AbduzZahir A, Abdul Rahuman A (2009) Adulticidal and larvicidal efficacy of some medicinal plant extracts against tick, fluke and mosquitoes. Vet Parasitol 166:286–292
Benelli G (2015a) Research in mosquito control: current challenges for a brighter future. Parasitol Res 114:2801–2805
Benelli G (2015b) The best time to have sex: mating behaviour and effect of daylight time on male sexual competitiveness in the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae). Parasitol Res 114:887–894
Benelli G (2015c) Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol Res 114:3201–3212
Benelli G (2016) Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res. doi:10.1007/s00436-015-4800-9
Benelli G, Murugan K, Panneerselvam C, Madhiyazhagan P, Conti B, Nicoletti M (2015) Old ingredients for a new recipe? Neem cake, a low-cost botanical by-product in the fight against mosquito-borne diseases. Parasitol Res 114:391–397
Bowatte G, Perera P, Senevirathne G, Meegaskumbura S, Meegaskumbura M (2013) Tadpoles as dengue mosquito (Aedes aegypti) egg predators. Biol Control 67:469–47
Caminade C, Medlock JM, Ducheyne E, Mc Intryre KM, Leach S, Baylis M, Morse A (2012) Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface 9:2708–2717
Deo PG, Hasan SB, Majumdar SK (1998) Toxicity and suitability of some insecticides for household use. Int Pest Control 30:118–129
Dinesh D, Murugan K, Madhiyazhagan P, Panneerselvam C, Nicoletti M, Jiang W, Benelli G, Chandramohan B, Suresh U (2015) Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi? Parasitol Res 114:1519–1529
Finney DJ (1971) Probit analysis. Cambridge University Press, London, pp 68–78
Focks DA (1985) Toxorhynchites. In: H.C Chapman (ed.), Biological control of mosquitoes. J Am Mosq Control Assoc Bull 6:42-45
Govindarajan M, Rajeswary M, Veerakumar K, Muthukumaran U, Hoti SL, Mehlhorn H, Barnard DR, Benelli G (2016) Novel synthesis of silver nanoparticles using Bauhinia variegata: a recent eco-friendly approach for mosquito control. Parasitol Res. doi:10.1007/s00436-015-4794-3
Haldar KM, Haldar B, Chandra G (2013) Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii (Wall.). Parasitol Res 112:1451–1459
Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45:371–391
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 18:105104–105115
Hurlbut HS (1938) Copepod observed preying on first instar larva of Anopheles quadrimaculatus. J Parasitol 24:281
Kalimuthu K, Lin SM, Tseng LC, Murugan K, Hwang JS (2014) Bio-efficacy potential of seaweed Gracilaria firma with copepod, Megacyclops formosanus for the control larvae of dengue vector Aedes aegypti. Hydrobiology 741:113–123
Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N (2010) Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B 76:50–56
Kumar V, Yadav SK (2009) Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 84:151–157
Mahesh Kumar P, Murugan K, Kovendan K, Panneerselvam C, Prasanna Kumar K, Amerasan D, Subramaniam J, Kalimuthu K, Nataraj T (2012) Mosquitocidal activity of Solanum xanthocarpum fruit extract and copepod Mesocyclops thermocyclopoides for the control of dengue vector Aedes aegypti. Parasitol Res 111:609–618
Manrique-Saide P, Ibanez-Bernal S, Delfin-Gonzalez H, Parra Tabla V (1998) Mesocyclops longisetus effects on survivorship of Aedes aegypti immature stages in car tyres. Med Vet Entomol 12:386–390
Marten GG, Astaiza R, Suarez MF, Monje C, Reid JW (1989) Natural control of larval Anopheles albuminus (Diptera: Culicidae) by the predator Mesocyclops (Copepoda: Cyclopoida). J Med Entomol 26:624–662
Mehlhorn H, Al-Rasheid KAS, Al-Quraishy S, Abdel-Ghaffar F (2012) Research and increase of expertise in arachno-entomology are urgently needed. Parasitol Res 110:259–265
Murugan K, Benelli G, Ayyappan S, Dinesh D, Panneerselvam C, Nicoletti M, Hwang JS, Mahesh Kumar P, Subramaniam J, Suresh U (2015a) Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasitol Res 114:2243–2253
Murugan K, Benelli G, Panneerselvam C, Subramaniam J, Jeyalalitha T, Dinesh D, Nicoletti M, Hwang JS, Suresh U, Madhiyazhagan P (2015b) Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp Parasitol 153:129–138
Murugan K, Priyanka V, Dinesh D, Madhiyazhagan P, Panneerselvam C, Subramaniam J, Suresh U, Chandramohan B, Roni M, Nicoletti M, Alarfaj AA, Higuchi A, Munusamy MA, Khater HF, Messing RH, Benelli G (2015c) Enhanced predation by Asian bullfrog tadpoles, Hoplobatrachus tigerinus, against the dengue vector Aedes aegypti in an aquatic environment treated with mosquitocidal nanoparticles. Parasitol Res. doi:10.1007/s00436-015-4582-0
Murugan K, Venus JSE, Panneerselvam C, Bedini S, Conti B, Nicoletti M, Kumar Sarkar S, Hwang JS, Subramaniam J, Madhiyazhagan P, Mahesh Kumar P, Dinesh D, Suresh U, Benelli G (2015d) Biosynthesis, mosquitocidal and antibacterial properties of Toddalia asiatica-synthesized silver nanoparticles: do they impact predation of guppy Poecilia reticulata against the filariasis mosquito Culex quinquefasciatus? Environ Sci Pollut Res. doi:10.1007/s11356-015-4920-x
Murugan K, Sanoopa CP, Madhiyazhagan P, Dinesh D, Subramaniam J, Panneerselvam C, Roni M, Suresh U, Nicoletti M, Alarfaj AA, Munusamy MA, Higuchi A, Kumar S, Perumalsamy H, Ahn JY, Benelli G (2015e) Rapid biosynthesis of silver nanoparticles using Crotalaria verrucosa leaves against the dengue vector Aedes aegypti: what happens around? An analysis of dragonfly predatory behaviour after exposure at ultra-low doses. Nat Prod Res. doi:10.1080/14786419.2015.1074230
Murugan K, Dinesh D, Jenil Kumar P, Panneerselvam C, Subramaniam J, Madhiyazhagan P, Suresh U, Nicoletti M, Alarfaj AA, Munusamy MA, Higuchi A, Mehlhorn H, Benelli G (2015f) Datura metel-synthesized silver nanoparticles magnify predation of dragonfly nymphs against the malaria vector Anopheles stephensi. Parasitol Res. doi:10.1007/s00436-015-4710-x
Murugan K, Vadivalagan C, Karthika P, Panneerselvam C, Paulpandi M, Subramaniam J, Wei H, Al Thabiani A, Saleh Alsalhi M, Devanesan S, Nicoletti M, Paramasivan R, Parajulee MN, Benelli G (2016a) DNA barcoding and molecular evolution of mosquito vectors of medical and veterinary importance. Parasitol Res. doi:10.1007/s00436-015-4726-2
Murugan K, Aruna P, Panneerselvam C, Madhiyazhagan P, Paulpandi M, Subramaniam J, Rajaganesh R, Wei H, Saleh Alsalhi M, Devanesan S, Nicoletti M, Syuhei B, Canale A, Benelli G (2016b) Fighting arboviral diseases: low toxicity on mammalian cells, dengue growth inhibition (in vitro) and mosquitocidal activity of Centroceras clavulatum-synthesized silver nanoparticles. Parasitol Res. doi:10.1007/s00436-015-4783-6
Murugesh KS, Yeligar VC, Maiti BC, Maity TK (2005) Hepatoprotective and antioxidant activity role of Berberis tinctoria Lesch.leaves on paracetamol induced hepatic damage in rats. Iran J Pharmacol Ther 4:64–69
Nicoletti M, Mariani S, Maccioni O, Coccioletti T, Murugan K (2012) Neem cake: chemical composition and larvicidal activity on Asian tiger mosquito. Parasitol Res 111:205–2013
Patil CD, Patil SV, Borase HP, Salunke BK, Salunkhe RB (2012a) Larvicidal activity of silver nanoparticles synthesized using Plumeria rubra plant latex against Aedes aegypti and Anopheles stephensi. Parasitol Res 110:1815–1822
Patil CD, Borase HP, Patil SV, Salunkhe RB, Salunke BK (2012b) Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex against Aedes aegypti and Anopheles stephensi and nontarget fish Poecillia reticulata. Parasitol Res 111:555–562
Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D (2009) Aedes albopictus, an arbovirus vector: from the darkness to light. Microb Infect 11:1177–1185
Pavela R (2008) Larvicidal effects of various Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol Res 102:555–559
Pavela R (2009) Larvicidal effects of some Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol Res 105:887–892
Pavela R (2015) Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind Crop Prod 76:174–187
Prasad TNVKV, Elumalai EK (2011) Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac J Trop Biomed 1:439–442
Ramanibai R, Velayutham K (2015) Bioactive compound synthesis of Ag nanoparticles from leaves of Melia azedarach and its control for mosquito larvae. Res Vet Sci 98:82–88
Rawani A, Ghosh A, Chandra G (2013) Mosquito larvicidal and anti-microbial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of Solanum nigrum L. (Solanaceae: Solanales). Acta Trop 128:613–622
Rawlins SC, Clark GG, Martinez R (1991) Effects of single introduction of Toxorhynchites moctezuma upon Aedes aegypti on a Caribbean island. J Am Mosq Control Assoc 7:7–10
Rawlins SC, Martinez R, Wiltshire S, Clarke D, Prabhaka P, Spinks M (1997) Evaluation of Caribbean strains of Macrocyclops and Mesocyclops (Cyclopoida: Cyclopidae) as biological control tools for the dengue vector, Aedes aegypti. J Am Mosq Control Assoc 13:18–23
Roni M, Murugan K, Panneerselvam C, Subramaniam J, Nicoletti M, Madhiyazhagan P, Dinesh D, Suresh U, Khater HF, Wei H, Canale A, Alarfaj AA, Munusamy MA, Higuchi A, Benelli G (2015) Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as potential eco-friendly control tool against Aedes aegypti and Plutella xylostella. Ecotoxicol Environ Saf 121:31–38
Saha P, Bhattacharjee S, Sarkar A, Manna A, Majumder S, et al. (2011) Berberine chloride mediates its anti-leishmanial activity via differential regulation of the mitogen activated protein kinase pathway in macrophages. PLoS ONE 6(4):e18467. doi:10.1371/journal.pone.0018467
Santhoshkumar T, Rahuman AA, Rajkumar G, Marimuthu S, Bagavan A, Jayaseelan C, Zahir AA, Elango G, Kamaraj C (2011) Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vector. Parasitol Res 108:693–702
Sasikumar JM, Thayumanavan THA, Subashkumar R, Janardhanan K, Lakshmana Perumalsamy P (2007) Antibacterial activity of some ethnomedicinal plants from the Nilgiris, Tamil Nadu, India. Nat Prod Rad 6:34–39
Sathyavathi R, Balamurali Krishna M, Venugopal Rao S, Saritha R, Narayana Rao D (2010) Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Adv Sci Lett 3:1–6
Schaper S, Hernández F (1998) La Luchacontrael dengue Mesocyclops thermocyclopoides: un posible control biológico para larvas de Aedes aegypti. Rev Cost Cienc Med 19:119–125
Shin SH, Ye MK, Kim HS (2007) The effects of nanosilver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int Immunol Pharmacol 7:1813–1818
Shrivastava S, Dash D (2010) Label-free colorimetric estimation of proteins using nanoparticles of silver. Nano-Micro Lett 2:164–168
Sivagnaname N, Kalyanasundaram M (2004) Laboratory evaluation of methanolic extract of Atlantia monophylla (Family: Rutaceae) against immature stages of mosquitoes and non-target organisms. Mem Inst Oswaldo Cruz 99:115–118
Steffan WA (1975) Systematics and biological control potential of Toxorhynchites (Diptera: Culicidae). Mosq Syst 7:59–67
Steffan WA, Evenhuis NL (1981) Biology of Toxorhynchites. Ann Rev Entomol 26:159–181
Subarani S, Sabhanayakam S, Kamaraj C (2013) Studies on the impact of biosynthesized silver nanoparticles (AgNPs) in relation to malaria and filariasis vector control against Anopheles stephensi Liston and Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 112:487–499
Subramaniam J, Murugan K, Panneerselvam C, Kovendan K, Madhiyazhagan P, Mahesh Kumar P, Dinesh D, Chandramohan B, Suresh U, Nicoletti M, Higuchi A, Hwang JS, Kumar S, Alarfaj AA, Munusamy MA, Messing RH, Benelli G (2015) Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: towards an integrative approach? Environ Sci Pollut Res. doi:10.1007/s11356-015-5253-5
Sujitha V, Murugan K, Paulpandi M, Panneerselvam C, Suresh U, Roni M, Nicoletti M, Higuchi A, Madhiyazhagan P, Subramaniam J, Dinesh D, Vadivalagan C, Chandramohan B, Alarfaj AA, Munusamy MA, Barnard DR, Benelli G (2015) Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol Res. doi:10.1007/s00436-015-4556-2
Suresh U, Murugan K, Benelli G, Nicoletti M, Barnard DR, Panneerselvam C, Mahesh Kumar P, Subramaniam J, Dinesh D, Chandramohan B (2015) Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol Res 114:1551–1562
Williamson CE (1999) Ecology and classification of North American freshwater invertebrates. Academic Press Inc., San Diego, pp 787–822
Acknowledgments
The authors would like to thank the financial support rendered by King Saud University, through Vice Deanship of Research Chairs. The authors are grateful to the UGC-MRP, New Delhi, India (No. F. No.36-250/2008 (SR) 24/03/2009) and the Department of Physics and Astronomy, King Saud University (project no. RGP-1435- 057) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international and national guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare no conflicts of interest. G. Benelli is an Editorial Board Member of Parasitology Research. This does not alter the author’s adherence to all the Parasitology Research policies on sharing data and materials.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kumar, P.M., Murugan, K., Madhiyazhagan, P. et al. Biosynthesis, characterization, and acute toxicity of Berberis tinctoria-fabricated silver nanoparticles against the Asian tiger mosquito, Aedes albopictus, and the mosquito predators Toxorhynchites splendens and Mesocyclops thermocyclopoides . Parasitol Res 115, 751–759 (2016). https://doi.org/10.1007/s00436-015-4799-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4799-y